Abstract
Introduction
The Understanding Psoriatic Disease Leveraging Insights for Treatment (UPLIFT) survey study was conducted globally in 2020 to understand how disease perceptions, including disease severity, treatment goals, and quality of life (QoL), have evolved recently, especially for mild-to-moderate psoriatic disease. Here, key findings from the UPLIFT survey based on respondents located in the US are presented. Leveraging results from the UPLIFT survey could lead to more effective interactions between patients and physicians and greater patient satisfaction.
Methods
UPLIFT was a multinational web-based survey of dermatologists, rheumatologists, and patients who self-reported a healthcare provider diagnosis of psoriasis (PsO) and/or psoriatic arthritis (PsA) conducted from March 2, 2020, to June 3, 2020.
Results
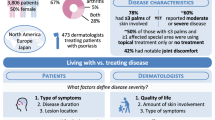
US respondents included 1006 patients (26.4% of global population; PsO only, n = 535; PsA only, n = 72; PsO and PsA, n = 399) and 216 physicians (dermatologists, n = 115; rheumatologists, n = 101). Most patients (66.4%) reported a body surface area (BSA; assessed by number of palms) of ≤ 3; of these, 56.2% rated their disease as moderate or severe. Most patients with PsO felt they were somewhat (40.1%) or very (49.3%) closely aligned with their dermatologists regarding treatment goals. Alternately, most patients with PsA felt that they were not too closely (32.1%) or not at all (59.3%) aligned with their rheumatologists. Most patients reported either a moderate (PsO, 35.5%; PsA, 31.8%) or strong (PsO, 47.7%; PsA, 53.9%) need for better treatments. Across BSA subgroups, most patients (60.8% to 86.1%) had a Dermatology Life Quality Index score ≥ 6, indicating at least a moderately impacted QoL.
Conclusions
Despite more treatment options, management of psoriatic disease remains suboptimal, with many patients reporting moderate-to-severe disease and impaired QoL, even with limited skin involvement. Results further suggest an unmet need for alignment between patients and physicians in the US to optimize the management of PsO and PsA.
Graphical Abstract

Plain Language Summary
The Understanding Psoriatic Disease Leveraging Insights for Treatment (UPLIFT) survey was an online survey conducted in 2020. The participants were patients who self-reported a healthcare provider diagnosis of psoriasis and/or psoriatic arthritis, dermatologists, and rheumatologists. The survey was distributed in several countries in North America, Europe, and Japan and a total of 3806 patients responded to the survey. Results from US patients and physicians are presented here.
UPLIFT was designed to understand current perceptions of patients and physicians relating to psoriasis and psoriatic arthritis, especially for mild-to-moderate disease. Participants were surveyed regarding treatments, severity of disease, impact on quality of life, treatment goals, and patient-physician interactions.
In the US, 1006 patients and 216 physicians completed the survey and were included in the analysis. Most patients had limited skin involvement but still rated their disease as moderate or severe. Regardless of whether patients had a small or large amount of skin involved, most reported at least a moderately impacted quality of life. The survey results suggested that there was disconnect between patients and physicians regarding treatment goals, treatment satisfaction, disease severity, and their recollection of what occurred during physician office visits. Despite new treatment options in recent years, the UPLIFT survey results show that US patients with psoriasis and psoriatic arthritis still experience a great disease burden and could benefit from better communication with physicians to optimize their treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
While there are several new treatment options for psoriasis and psoriatic arthritis, patient and physician perceptions of available treatments and of psoriatic disease have not been thoroughly explored |
The Understanding Psoriatic Disease Leveraging Insights for Treatment (UPLIFT) survey, with its focus on patients with mild-to-moderate disease, was designed to understand how disease severity, treatment goals, and treatment-related outcomes are perceived by patients and physicians |
Patient experience and physician assessment of disease severity and treatment satisfaction in the United States have not been assessed to determine how they compare to those of the global UPLIFT respondents or if they have evolved since the first Multinational Assessment of Psoriasis and Psoriatic Arthritis survey (2012 MAPP) |
What was learned from the study? |
Many surveyed US patients reported a significant burden of disease despite limited skin involvement, had special-area involvement, were dissatisfied with their current or past treatments, were not fully aligned with their physician, and had an impacted quality of life |
US results of the UPLIFT survey were similar to previously published global results and highlight the need for optimized treatment strategies for psoriatic disease |
Introduction
Psoriasis (PsO) is a chronic, systemic inflammatory disease that is associated with psoriatic arthritis (PsA). There are no cures for PsO and PsA, and both can significantly impair health-related quality of life (QoL) [1,2,3]. In the United States, PsO affects about 3.0% of adults, or approximately 7.5 million people, and PsA affects approximately 30% of them [4, 5]. The 2012 Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey demonstrated the high burden that psoriatic disease can have on patient quality of life (QoL), the potential for misalignment in perceptions between physicians and patients, and the need for more safe and effective therapies [6]. Despite the approval of new and effective treatments for psoriatic disease since the MAPP survey was conducted, patients with PsO and/or PsA still report high disease burden, especially when they have bothersome itch, musculoskeletal symptoms, or PsO in special areas (e.g., face, scalp) [6,7,8,9]. Even with limited skin involvement, these disease manifestations can impair QoL [6, 9]. In addition, patient and physician attitudes regarding psoriatic disease and treatments have shifted: with greater focus on the systemic inflammatory nature of psoriatic disease, the recognition that patient-reported outcomes can provide further insight to the burden of disease, and the fact that more patients are candidates for systemic treatment due to broader inclusion criteria, biologic, and nonbiologic treatments are being utilized more often [7, 10,11,12,13]. Many biologic therapies were approved by the US Food and Drug Administration (FDA) between 2012 and 2020, including tumor necrosis factor α inhibitors, interleukin (IL)-17 inhibitors, and IL-23 inhibitors [12]. The FDA approved oral phosphodiesterase 4 inhibitor apremilast for PsO treatment in 2014 [14]. Although the treatment landscape has broadened, certain barriers exist to access, including cost, safety, eligibility, patient preference, and the prescribing practices of physicians. The Understanding Psoriatic Disease Leveraging Insights for Treatment (UPLIFT) survey was developed to understand how patient and physician attitudes have evolved since the MAPP survey was conducted, especially regarding mild-to-moderate PsO and oligoarticular PsA, as well as the impact of disease on QoL. Global results of UPLIFT have been published [15]. Here, key findings from the UPLIFT survey based on data reported from respondents who were located in the US are reported, and patient and physician perceptions about disease severity and treatment are described.
Methods
Survey Design
As described in Lebwohl et al., the UPLIFT methodology and survey design were developed using an academic steering committee comprising international experts in rheumatology and dermatology, along with input from patients, physicians, and advocacy groups [15]. The survey was conducted between March 2, 2020, and June 3, 2020, by AplusA Bell Falla (Newark, NJ, USA) in accordance with the ethical principles that have their origin in the Declaration of Helsinki, was consistent with good clinical practice, and abided by applicable laws and regulations [15]. Informed consent was provided before commencement of survey procedures.
UPLIFT was a global, cross-sectional, quantitative online survey conducted in Canada, France, Germany, Italy, Japan, Spain, the UK, and the US. Here, we report on US respondents [15].
Pretests were conducted to assess the main survey’s ease of use, clarity, interview length, and participants’ understanding and interpretation of questions. The main survey consisted of screening questions, questions about experiences living with or treating PsO and/or PsA, and questions related to sociodemographics. The survey was designed to take approximately 25 min for patients to complete and about 30 min for physicians to complete.
Patients were recruited at random from a general population of an online panel of adults, and recruitment was stratified based on population demographics that included gender, age, and region. The online panel was sourced from loyalty panel recruitment (i.e., travel, entertainment, media, and retail programs); organic, open enrollment, and partnership recruitment (i.e., websites, social media influences, and mobile apps); and affiliate network recruitment (i.e., school and community websites). Dermatologists and rheumatologists were recruited at random, sourced from physician panels, and qualified based on a screening questionnaire. Due to low response rates among rheumatologists, primary care physicians who had a subspecialty in rheumatology were also recruited. The target sample size included 1000 patients, 100 dermatologists, and 100 rheumatologists from the US.
Eligibility Criteria
Eligible patients were adults aged ≥ 18 years who self-reported a healthcare provider (HCP)-diagnosis of PsO and/or PsA. Eligible physicians had a primary or secondary specialty in dermatology or rheumatology, reported that they spent ≥ 50% of their professional time directly treating patients, and saw ≥ 20 adult patients with PsO or PsA in a typical month. In addition, dermatologists were required to spend ≥ 50% of their office visits practicing medical dermatology.
Assessments
Patient assessments included respondent demographics and clinical characteristics; symptoms, disease burden, and impact of PsO and/or PsA; current and historical treatment, and treatment goals and recommendations; and presence of PsO overall and in special areas associated with high disease burden (areas that may be considered more sensitive or impactful; i.e., face, genitals, nails, scalp, and palms and/or soles) [15]. Assessment tools used were Psoriasis Epidemiology Screening Tool (PEST), Patient Health Questionnaire-2 (PHQ-2), Dermatology Life Quality Index (DLQI), European Alliance of Associations for Rheumatology Psoriatic Arthritis Impact of Disease 12-item questionnaire (PsAID-12), and the eight-item Health Assessment Questionnaire (HAQ-8). Patients self-reported skin involvement by PsO-involved body surface area (BSA) (assessed by number of palms) and self-rated their current disease severity as 1–3 (mild), 4–6 (moderate), or 7–10 (severe).
Topics assessed in the physician survey included their perceptions of disease burden and impact, classification of disease severity, treatment goals and patterns, and opinion on unmet needs and other disease challenges.
Patients and physicians were asked to rank the top three contributing factors in relation to disease severity, treatment goals, and ideal treatments; their alignment in responses was assessed.
Analyses
Results of survey responses from patients and physicians were summarized using descriptive statistics without data imputation using SAS Enterprise Guide 7.15 HF9 software (SAS Institute Inc., Cary, NC, USA). Patient-rated disease severity, current treatment, and DLQI data were analyzed by level of BSA skin involvement (≤ 3 palms, 4–10 palms, or > 10 palms). Additional subgroup analyses included patients with limited skin involvement (BSA ≤ 3) and PsO in ≥ 1 special area (i.e., face, genitals, nails, scalp, palms, and/or soles) as well as patients with PsO and concomitant joint pain without a diagnosis of PsA.
Results
Prevalence of PsO and PsA
Of the 264,054 global responders (i.e., participants who clicked on the survey link), 1006 patients from the US met the inclusion criteria and self-reported an HCP diagnosis of PsO and/or PsA [15]. Reasons for exclusion included incomplete screening questionnaire, incomplete interview response, responding after the survey closed, or not meeting survey criteria. US patients accounted for 26.4% of the 3806 patients assessed globally. Of US patient responders, 53.2% had PsO only, 39.7% had PsO and PsA, and 7.1% had PsA only.
Patient Demographics and Physician Practice Characteristics
The mean age of patients was 46.3 years, and 51.3% were women (Table 1). The most common comorbidities were hypertension, arthritis (rheumatoid and/or osteoarthritis), and depression (Table 1). Of the 115 US-based dermatologist respondents, most practiced primarily in a community or office-based setting (85.2%), and 12.2% practiced at a major academic teaching hospital. Most of the 101 US-based rheumatologist respondents also practiced in a community or office-based setting (72.3%), and 17.8% practiced at a major academic teaching hospital.
Patient-Rated Disease Characteristics
Affected BSA was reported by 842 patients. Most reported a BSA of ≤ 3 palms (66.4%, n = 559). Of those patients, 56.2% rated their disease as moderate or severe (Fig. 1). PsO in special areas was also common among these patients; 74.2% of those with a BSA ≤ 3 reported PsO in ≥ 1 special area. Of the overall US patient population, scalp was the most commonly reported affected special area, with 45.6% of patients (n = 459) reporting scalp involvement. Most patients reported that they were currently experiencing PsO symptoms at the time of survey completion. Among the 934 patients with PsO (with or without PsA), the most common symptoms were itching, flaking, and redness.
Severity of current symptoms by BSA subgroups (patient-reported). aIn response to the following question: “On a scale of 1 to 10, where ‘1’ is ‘very mild’ and ‘10’ is ‘very severe,’ please tell us how severe is your psoriasis currently?” bIn response to the following question: “Based on the amount of psoriasis that could be covered by the palm of your hand (including fingers), how many palms of psoriasis would you say you currently have?” BSA, body surface area
Of patients with PsA only (n = 72), 52.8% reported involvement of ≤ 4 joints (consistent with oligoarthritis) and 47.2% reported involvement of > 4 joints (consistent with polyarthritis). A dual diagnosis of PsO and PsA was reported in 399 patients, and an additional 378 patients reported PsO and joint pain but no PsA diagnosis. Most patients with PsO and joint pain but without a diagnosis of PsA reported involvement of ≤ 4 joints (56.1%), whereas most patients with the dual diagnosis of PsO and PsA reported involvement of > 4 joints (69.4%). Small joints (i.e., of hands, feet, fingers, thumbs, or toes) were reported to have caused discomfort in 47.6% of patients with PsO and joint pain and in 57.1% of patients with PsO and PsA. Large joints (i.e., shoulders, hips, or knees) had caused discomfort in 78.0% of patients with PsO and joint pain and in 84.0% of patients with PsO and PsA. Overall, 51.9% of patients with PsO and joint pain had a PEST score ≥ 3 (n = 196), indicating that these patients should be referred to a rheumatologist for evaluation [16]. Of patients with a PEST score ≥ 3, 46.4% had oligoarthritis and 53.6% had polyarthritis. Patients with PsA (n = 471), with or without PsO, had a mean HAQ-8 of 0.83.
Patient-Reported Current Treatment
Overall, among all patients with BSA data (n = 842), 22.6% reported not using any current prescription treatment. Among patients with a BSA ≤ 3 (n = 559), the most frequently reported treatment was prescription topical therapy, which was being used by 23.8% of patients (Fig. 2); however, 27.4% of patients were not using any prescription treatment at the time of survey completion. Patients with a BSA of 4–10 or > 10 most often reported using oral therapies or biologic therapies, respectively (Fig. 2).
Current treatment by level of BSA involvement. Oral plus biologic = oral Rx plus biologic or topical Rx plus oral Rx plus biologic; biologic = biologic only or biologic plus topical Rx; oral = oral Rx only or oral Rx plus topical Rx; topical only = topical Rx only; other = other only or phototherapy only or phototherapy plus other (i.e., anything other than prescription oral/biologic/topical therapy or phototherapy); no Rx treatment = no treatment other than oral OTC or topical OTC. BSA involvement based on patient response to the following question: “Based on the amount of psoriasis that could be covered by the palm of your hand (including fingers), how many palms of psoriasis would you say you currently have?” BSA, body surface area; OTC, over the counter; Rx, prescription
Of the patients who had PsA only (n = 72), 40.3% were not currently using treatment. Injectable or intravenous therapies and oral therapies were being used by a greater proportion of patients for PsA treatment than PsO treatment. Prescription oral medication had not been utilized by 20.4% of patients with PsA for PsA treatment or by 45.0% of patients with PsO for PsO treatment. At the time of survey completion, the most frequently reported current treatment for PsO was a prescription topical product (48.6%), whereas over-the-counter oral pain medication was the most commonly reported current treatment for PsA (48.1%) (Fig. S1).
Of the 934 patients with PsO, 392 provided reasons for not seeing an HCP for PsO in the past year. Several reasons were related to healthcare access: 20% indicated that they were unable to get an appointment or wait times were too long; 19% indicated lack of insurance or cost issues; 16% indicated transportation difficulty; and 14% indicated that no doctors or other HCPs were close to them. Of 471 patients with PsA, 392 provided reasons for not seeing an HCP for PsA in the past year. Similarly, many listed reasons related to healthcare access: 32% indicated lack of insurance or cost issues; 22% indicated that no doctors or other HCPs were close to them; 20% indicated that they were unable to get an appointment or wait times were too long; and 20% indicated transportation difficulty.
Patient-Reported QoL
Between 60.8% and 86.1% of patients had a DLQI score ≥ 6 across BSA subgroups, indicating at least a moderately impacted QoL (Fig. 3) [17]. Among patients who rated their disease as mild (1–3), 35.0% had a DLQI score ≥ 6. Conversely, an overwhelming majority (92.8%) of patients who rated their disease as severe (7–10) had a DLQI score ≥ 6 (Fig. 3).
Most patients with PsO in ≥ 1 special area had a DLQI score ≥ 6 (Fig. 4). The areas associated with the highest proportion of patients with a DLQI score ≥ 6 were face (75.9%) and palms and/or soles (73.0%). Overall, mean DLQI scores of individual questions were greater for patients with PsO in ≥ 1 special area than for patients without special-area involvement. Scores were highest among both groups of patients for the question, “How itchy, sore, painful or stinging has your skin been?” with mean scores of 1.7 for patients with special-area involvement and 1.4 for patients without special-area involvement (Fig. 5). Mean DLQI question scores for patients with special-area involvement are presented by area in Fig. S2.
The mean DLQI scores for patients with PsO without joint pain, PsO with joint pain, and PsO with PsA were 5.7, 9.9, and 14.3, respectively. The proportions of patients with DLQI score ≥ 6 across these groups are shown in Fig. 3.
Patients with PsA only had a mean total PsAID-12 score of 5.0; patients with PsO and PsA had a mean total PsAID-12 score of 5.8 (Fig. S3). A significant proportion of patients with both oligoarthritis (80.3%) and polyarthritis (82.1%) reported a PsAID-12 score ≥ 4, indicating unacceptable impact of PsA symptoms on the patient [18]. Discomfort, pain, and fatigue were the three symptoms with the highest proportion of patients reporting an individual PsAID score of ≥ 7 (Fig. S4).
Most patients with PsA with or without PsO had a PHQ-2 score ≥ 3, indicative of a positive depression screen [19]. The mean (SD) PHQ-2 score for all patients with PsO with or without PsA (n = 934) was 2.64 (1.90). Scores escalated with increasing joint involvement; mean (SD) scores were 2.25 (1.88) for patients with PsO only, 2.44 (1.86) for patients with PsO with joint pain, and 3.17 (1.79) for patients with PsO with PsA.
Analysis of Patients with Limited BSA and Involvement of ≥ 1 Special Area
Overall, 57.1% of patients with limited BSA (≤ 3) and PsO in ≥ 1 special area rated their disease as moderate to severe. Similarly, 58.8% of these patients had a DLQI score ≥ 6, indicative of at least a moderately impacted QoL [17]. A greater proportion of patients reporting limited BSA and PsO involving the face versus other special areas had a DLQI score ≥ 6. Of these patients with limited BSA (≤ 3) who perceived their disease as moderate to severe and had ≥ 1 involved special area, 61.3% who were being treated with topical therapy only had a DLQI ≥ 6.
Patient and Physician Perceptions of Disease Burden and Office Visits
Patients with PsO only (n = 535) ranked the most important factors that contributed to their disease severity as types of symptoms, locations of skin lesions, and the length of time they had suffered from PsO (Fig. S5). Dermatologists ranked the top three factors contributing to these PsO patients’ disease severity as amount of BSA involved, impact on overall QoL, and locations of skin lesions (Fig. S5). When asked what occurs at office visits with their dermatologists, 42.9% of patients with PsO only reported that their joints were examined at every visit; 27.7% reported that they discussed potential joint damage at every visit; 65.2% reported that only areas of skin that the patient pointed out were examined at every visit; 30.4% reported that they never discussed the impact of PsO on their emotional well-being; and 56.5% reported that they never discussed the impact of PsO on their sex life (Fig. S6). Alternately, when asked about their office visit habits, 50.4% of dermatologists reported performing a joint examination at every visit; 51.3% reported discussing potential damage at every visit; 84.4% reported performing a skin examination at every visit; 3.5% reported never discussing the emotional impact of PsO; and 16.5% reported never discussing the impact of PsO on a patient’s sex life (Fig. S6).
Patients with PsA with or without PsO (n = 471) ranked the top three factors contributing to their disease severity as joint pain, impacted QoL, and location of symptoms and/or joint discomfort (Fig. S7). Rheumatologists ranked joint erosion/deformity, the number of joints involved, and joint pain/stiffness as the most important determinants of disease severity (Fig. S7). Most patients reported that their rheumatologist examined their joints and asked them about disease-related pain at every visit (76.0% and 80.1%, respectively). Nearly all rheumatologists reported completing a joint examination (96.0%) and discussing PsA-related pain (94.1%) at every visit. Responses of patients and rheumatologists demonstrated the low frequency of discussions related to disease impact on patients’ sex lives, with 47.4% of patients and 33.7% of rheumatologists reporting that the topic was never discussed during office visits (Fig. S8). Regarding the impact of disease on emotional well-being, 20.5% of patients reported that this was never discussed with their rheumatologist, whereas only 2.0% of rheumatologists reported never discussing this topic (Fig. S8).
Patient and Physician Perceptions of Disease Burden and Treatment Goals and Attributes
The most important treatment goal for patients with PsO (n = 535) was itch reduction, followed by symptom control and total skin clearance (Fig. S5). Dermatologists ranked their treatment goals in relation to the severity of PsO; the top ranked goal across groups was to improve QoL, followed by achieving total skin clearance and achieving near total skin clearance (Fig. S5). Itch reduction was ranked eighth as a treatment goal among dermatologists. For patients with PsO only, the most important attributes for an ideal PsO therapy were to improve skin symptoms, provide significant skin clearance, and be safe for long-term use (Fig. S5). Dermatologists indicated that the most important attributes for an ideal PsO therapy based on disease severity were to have improved access and/or insurance coverage (moderate-to-severe), provide significant skin clearance (mild-to-severe), be safe for long-term use (mild-to-severe), have long-term efficacy (mild), and be convenient and/or easy to administer (mild). Rankings were the same for both moderate and severe disease. Overall, the most common responses for ideal attributes were that the treatment was safe for long-term use, had improved access and/or insurance coverage, and provided significant skin clearance (Fig. S5). Nearly half of patients who used topical or oral therapies felt that they were somewhat closely aligned with their dermatologists in treatment goals, and approximately a third felt that they were very closely aligned. Overall, most patients with PsO only felt that they were somewhat (40.1%) or very (49.3%) closely aligned with their dermatologists regarding their current treatment goals.
For patients with PsA (n = 471), the most important treatment goals were to reduce joint pain, reduce joint stiffness, and stop the progression of joint damage or joint erosion (Fig. S7). For rheumatologists, the top treatment goals for their patients with PsA were to inhibit the progression of joint damage or joint erosion, achieve disease remission or low disease activity, and reduce joint pain (Fig. S7). For patients, the top attributes of an ideal PsA therapy were that it reduces joint pain, be safe for long-term use, and have long-term efficacy (Fig. S7). Rheumatologists ranked their top attributes for an ideal therapy as having long-term efficacy, causing achievement of disease remission or low disease activity, and reducing joint pain (Fig. S7). In general, most patients with PsA reported that their perceived treatment goals were not aligned with those of their rheumatologist; 91.4% of patients reported that they were either not closely aligned or not at all aligned.
Patient and Physician Perceptions of Treatment Satisfaction
Most patients reported either a moderate (PsO, 35.5%; PsA, 31.8%) or strong (PsO, 47.7%; PsA, 53.9%) need for better treatments. Dermatologists were surveyed based on the severity of PsO; 42.6% believed there was a strong need for better treatments for severe PsO, 48.7% believed there was a moderate need for better treatments for moderate PsO, and 39.1% believed there was not much need for better treatments for mild PsO. Rheumatologists were surveyed based on oligoarticular or polyarticular PsA; a similar proportion of respondents felt that there was a moderate (oligoarticular, 43.6%; polyarticular, 41.6%) or strong (oligoarticular, 41.6%; polyarticular, 47.5%) need for better PsA treatment options. When patients were asked about their level of satisfaction with either current or past treatments, somewhat dissatisfied was the most common answer for effectiveness, safety, and convenience of prescription topical medications, prescription oral medications, and injectable or intravenous medications (Fig. S9).
Regarding why patients considered their treatments burdensome, the most commonly reported reasons were the messiness of prescription topical treatments (56.0%), experience of side effects with prescription oral treatments (34.8%), and physical preparation for self-injection with injectable or intravenous treatments (26.4%) (Fig. S10).
Patients who reported having discontinued a prescription oral treatment (n = 366) ranked lack of effectiveness as the top reason for discontinuation (31.4%). Similarly, the most common reason for discontinuation among those who had discontinued an injectable or intravenous treatment (n = 251) was the lack or loss of effectiveness (19.9%; Fig. S11).
Discussion
The analysis of these data from US respondents to the UPLIFT survey allowed insight into how perceptions of PsO and PsA have evolved since the 2012 MAPP survey. Despite more treatments being available at the time of the UPLIFT survey versus the MAPP survey, UPLIFT responses demonstrate a continued unmet need for better disease management. In addition, differing perceptions among patients and physicians about treatment goals require additional research to understand how patients and physicians can collaboratively make management decisions to optimize overall care.
While there were certain differences between the global and US populations, overall disease perceptions were similar. The US population had a higher prevalence of overweight or obesity (58.6%) compared with the global population (49.2%); similarly, there were higher proportions of all comorbidities other than liver disease in the US population compared with the global population, suggesting a potentially greater disease burden for US patients [15]. The most common comorbidities were similar between the US and global populations [15]. A greater proportion of US respondents had PsO and PsA compared with the global population (39.7% versus 28% globally) [15]. A slightly lower percentage of US patients reported a BSA ≤ 3 (66.4% versus 78% globally), but a similar percentage of these patients ranked their symptoms as moderate to severe (56.2% versus 58% globally) [15]. Perceived treatment burden and reasons for treatment discontinuation were similar between the US and global populations for oral and topical therapies [15]. Similar to the US UPLIFT survey results presented here, a high proportion of respondents from the Japan UPLIFT survey subgroup perceived their symptoms to be moderate or severe irrespective of the level of skin involvement, suggesting a persistent unmet treatment need [20].
Nearly three-quarters of patients with PsO and a BSA ≤ 3 had special-area involvement, most rated their disease as moderate-to-severe, and most had at least moderately impacted QoL (DLQI score ≥ 6) [17]. These findings support the recent paradigm shift to evaluating disease severity and treatment candidacy not based primarily on BSA, but considering special-area involvement, disease burden, failure of topical therapy, and patient preference [11, 21,22,23]. Most patients with PsA with or without PsO had a positive depression screen [19], with mean PHQ-2 scores highest in patients with comorbid PsO and PsA, suggesting a greater burden in patients with a dual diagnosis. These results are supported by an analysis of surveys from the National Psoriasis Foundation (NPF), which indicated a larger QoL burden among patients with both PsO and PsA [9]. More than a third of patients with PsO had joint pain without a diagnosis of PsA; about half of those patients had a PEST score ≥ 3, indicating that they should be referred to a rheumatologist for evaluation [16]. Almost one-quarter of US patients were not currently receiving prescription treatment at the time of the survey, and patients were mostly dissatisfied with their current or past treatments. Many patients identified reasons associated with healthcare access for not having seen an HCP for PsO or PsA treatment within the past year. Although the lower rate of participation in that section of the survey makes it difficult to draw conclusions, trends indicated that patients with PsA were more impacted by lack of insurance or cost issues than patients with PsO. In another analysis of surveys from the NPF, undertreatment was similarly identified as a significant problem [24]. Responses from patients and physicians highlighted a potential disconnect regarding treatment goals, assessment of disease severity, ideal treatment, and expectations for office visits. Treatment goals identified by patients with PsO in the UPLIFT survey were similar to those expressed by patients through a survey from the NPF using the Patient Needs Questionnaire of the Patient Benefit Index; among top patient needs identified were having confidence in therapy, getting better skin quickly, and being free of itch [25]. Patients with PsO without PsA generally felt that their treatment goals were in alignment with their dermatologist, but most patients with PsA did not feel aligned with their rheumatologists. Similarly, in a multinational real-world survey of patients with PsO and their dermatologists conducted in 2015–2016, perceptions differed with respect to symptoms, disease severity, and control [26]. PsAID-12 results reaffirm the high impact of PsA on health-related QoL as well as the remaining unmet need for improved disease management [27]. Findings suggest that there is an opportunity for better communication between patients and their physicians to optimize treatment management and QoL.
Some limitations of the survey included recall bias, participants being selected from an online panel that may not have been fully representative of the general population, some survey questions on the physician and patient survey not being equivalent, and the physician survey not including a representative number of other healthcare providers, such as nurse practitioners or physician assistants. In addition, the survey was conducted in part during the COVID-19 pandemic. This could have affected the burden of disease that patients experienced, patients’ ability to have an in-person office visit, and patient-reported outcomes, as well as responses from physicians and patients. A future publication will present UPLIFT results for the EU population.
Conclusion
The 2020 UPLIFT survey emphasized the consistent significant disease burden of US patients with PsO and/or PsA, even in those with localized disease. The findings were largely similar between the US and global populations [15]. Survey responses highlighted the need for treatment optimization as well as alignment between patients and HCPs. A large percentage of US patients had impaired QOL and a positive depression screen, suggesting a potential need for a mental health evaluation. In addition, most patients with PsO and joint pain may need a referral to a rheumatologist.
References
Elmets CA, Leonardi CL, Davis DMR, Gelfand JM, Lichten J, Mehta NN, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073–113.
Mrowietz U, Kragballe K, Reich K, Spuls P, Griffiths CE, Nast A, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1–10.
Nast A, Smith C, Spuls PI, Avila Valle G, Bata-Csörgö Z, Boonen H, et al. EuroGuiDerm Guideline on the systemic treatment of psoriasis vulgaris—Part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol. 2020;34:2461–98.
Armstrong AW, Mehta MD, Schupp CW, Gondo GC, Bell SJ, Griffiths CEM. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940–6.
Mease PJ, Gladman DD, Papp KA, Khraishi MM, Thaci D, Behrens F, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69:729–35.
Lebwohl MG, Bachelez H, Barker J, Girolomoni G, Kavanaugh A, Langley RG, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871–81.
Armstrong AW, Siegel MP, Bagel J, Boh EE, Buell M, Cooper KD, et al. From the Medical Board of the National Psoriasis Foundation: Treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290–8.
van de Kerkhof PCM, Reich K, Kavanaugh A, Bachelez H, Barker J, Girolomoni G, et al. Physician perspectives in the managment of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015;29:2002–10.
Edson-Heredia E, Zhu B, Guo J, Maeda-Chubachi T, Lebwohl M. Disease burden and quality of life in psoriasis patients with and without comorbid psoriatic arthritis: results from National Psoriasis Foundation panel surveys. Cutis. 2015;95:173–8.
Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol. 2020;182:840–8.
Strober B, Ryan C, van de Kerkhof P, van der Walt J, Kimball AB, Barker J, et al. Recategorization of psoriasis severity: Delphi consensus from the International Psoriasis Council. J Am Acad Dermatol. 2020;82:117–22.
Menter A, Strober BE, Kaplan DH, Kivelevitch D, Prater EF, Stoff B, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029–72.
Menter A, Gelfand JM, Connor C, Armstrong AW, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445–86.
Otezla [package insert]. Thousand Oaks: Amgen, Inc.; 2021.
Lebwohl M, Langley RG, Paul C, Puíg L, Reich K, van de Kerkhof P, et al. Evolution of patient perceptions of psoriatic disease: Results from the Understanding Psoriatic Disease Leveraging Insights for Treatment (UPLIFT) Survey. Dermatol Ther. 2022;12:61–78.
Ibrahim GH, Buch MH, Lawson C, Waxman R, Helliwell PS. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. 2009;27:469–74.
Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994–2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159:997–1035.
Gossec L, de Wit M, Kiltz U, Braun J, Kalyoncu U, Scrivo R, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis. 2014;73:1012–9.
Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345–59.
Torii H, Kishimoto M, Tanaka M, Noguchi H, Chaudhari S. Patient perceptions of psoriatic disease in Japan: results from the Japanese subgroup of the Understanding Psoriatic Disease Leveraging Insights for Treatment (UPLIFT) survey. J Dermatol. 2022;49:818–28.
Merola JF, Qureshi A, Husni ME. Underdiagnosed and undertreated psoriasis: nuances of treating psoriasis affecting the scalp, face, intertriginous areas, genitals, hands, feet, and nails. Dermatol Ther. 2018;31: e12589.
Augustin M, Langenbruch A, Gutknecht M, Reich K, Korber A, Maassen D, et al. Definition of psoriasis severity in routine clinical care: current guidelines fail to capture the complexity of long-term psoriasis management. Br J Dermatol. 2018;179:1385–91.
Mrowietz U, Augustin M. Using the upgrade criteria of the European Psoriasis Consensus is best practice care according to the people-centred healthcare concept of the World Health Organization. Br J Dermatol. 2022;187:1007–8.
Armstrong AW, Robertson AD, Wu J, Schupp C, Lebwohl MG. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA Dermatol. 2013;149:1180–5.
Armstrong A, Edson-Heredia E, Zhu B, Burge R, Bell S, Crowley JJ, et al. Treatment goals for psoriasis as measured by patient benefit index: results of a National Psoriasis Foundation Survey. Adv Ther. 2022;39:2657–67.
Griffiths CEM, Augustin M, Naldi L, Romiti R, Guevara-Sangines E, Howe T, et al. Patient-dermatologist agreement in psoriasis severity, symptoms and satisfaction: results from a real-world multinational survey. J Eur Acad Dermatol Venereol. 2018;32:1523–9.
Coates LC, Orbai AM, Azevedo VF, Cappelleri JC, Steinberg K, Lippe R, et al. Results of a global, patient-based survey assessing the impact of psoriatic arthritis discussed in the context of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire. Health Qual Life Outcomes. 2020;18:173.
Acknowledgements
We thank the patients and physicians who participated in the survey.
Funding
This survey was sponsored by Amgen Inc., Thousand Oaks, CA, USA. Amgen Inc. is funding the journal’s rapid service fees.
Medical Writing/Editorial Assistance
Writing support was funded by Amgen Inc. and provided by Christina Mulvihill, PharmD, of Peloton Advantage, LLC, an OPEN Health company, and Dawn Nicewarner, PhD, an employee of and stockholder in Amgen Inc.
Author Contributions
Joseph F. Merola, Alexis Ogdie, Alice B. Gottlieb, Linda Stein Gold, and Mark Lebwohl contributed to the study conception and design. Material preparation, data collection, and/or analysis were performed by Joseph F. Merola, Alexis Ogdie, Alice B. Gottlieb, Linda Stein Gold, Andrea Flower, Shauna Jardon, Yuri Klyachkin, and Mark Lebwohl. All authors contributed to the drafting of the manuscript and approved the final manuscript.
Prior Presentation
Merola JF, et al. Impact of Psoriasis in Special Areas on Patient Quality-of-Life Outcomes: Findings From the UPLIFT Survey in the United States. Presented at: The 2023 AAD Annual Meeting; March 17–21, 2023; New Orleans, LA, USA.
Disclosures
Joseph F. Merola: AbbVie, Amgen, Biogen, Bristol Myers Squibb, Dermavant, Eli Lilly, Janssen, LEO Pharma, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharmaceuticals, and UCB—consultant and/or investigator. Alexis Ogdie: AbbVie, Amgen Inc., Novartis, and Pfizer—grant/research support; AbbVie, Amgen Inc., Bristol Myers Squibb, Celgene, CorEvitas’ Psoriatic Arthritis/Spondyloarthritis Registry (formerly Corrona), Eli Lilly, Gilead, Janssen, Novartis, Pfizer, and UCB—consultant; royalties to husband from Novartis. Alice B. Gottlieb: Amgen, AnaptysBio, Avotres Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Dice Therapeutics, Dermavant, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi, Sun Pharma, UCB Pharma, and Xbiotech (stock options for an RA project)—honoraria as an advisory board member, non-promotional speaker or consultant; AnaptysBio, Janssen, Moonlake Therapeutics AG, Novartis, Ortho Dermatologics, Sun Pharma, Bristol Myers Squibb, and UCB Pharma—research/educational grants; all funds go to the Icahn School of Medicine at Mount Sinai. Linda Stein Gold: AbbVie, Amgen, Arcutis, Celgene Corporation, Dermira, Dermavant, Eli Lilly, Galderma, LEO Pharma, Novartis, Pfizer, Regeneron, Sanofi Genzyme, UCB, and Valeant—honoraria, grants, and/or research funding as a speaker, investigator, and/or advisory board member. Andrea Flower, Shauna Jardon, and Yuri Klyachkin: Amgen Inc.—employees and stockholders. Mark Lebwohl: Mount Sinai—employment; AbbVie, Amgen Inc., Arcutis, Boehringer Ingelheim, Dermavant, Eli Lilly, Incyte, Janssen, LEO Pharma, Ortho Dermatologics, Pfizer, and UCB—research funds; Aditum Bio, Allergan, Almirall, Arcutis, Avotres Therapeutics, BirchBioMed, BMD Skincare, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant Sciences, Evelo, Facilitate International Dermatologic Education, Foundation for Research and Education in Dermatology, Inozyme Pharma, Kyowa Kirin, LEO Pharma, Meiji Seika Pharma, Menlo, Mitsubishi, Neuroderm, Pfizer, Promius/Dr. Reddy’s Laboratories, Serono, Theravance, and Verrica—consultant.
Compliance with Ethics Guidelines
The study was approved by the relevant ethics committees and was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from patients before commencement of survey procedures.
Data Sharing
Qualified researchers may request data from Amgen clinical studies. Complete details are available at http://www.amgen.com/datasharing.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Merola, J.F., Ogdie, A., Gottlieb, A.B. et al. Patient and Physician Perceptions of Psoriatic Disease in the United States: Results from the UPLIFT Survey. Dermatol Ther (Heidelb) 13, 1329–1346 (2023). https://doi.org/10.1007/s13555-023-00929-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-023-00929-9