Abstract
Introduction
Atopic dermatitis (AD) is a chronic skin disease that poses a significant burden on both patients and the society. AD causes the highest loss in disability-adjusted life years compared with other skin diseases. This study aimed to estimate the economic and humanistic burden of AD in adults and adolescents in seven countries in the Middle East and Africa region (Egypt, Lebanon, Saudi Arabia, Kuwait, Algeria, South Africa, and United Arab Emirates).
Methods
We conducted a literature review to identify country-specific data on this disease. Subsequently, meetings were organized with experts from each country to complete the missing data. The data were aggregated and calculation models were created to estimate the value of the humanistic and economic burden of the disease in each country. Finally, we conducted meetings with local experts to validate the results, and the necessary adjustments were made.
Results
On average, a patient with AD loses 0.19 quality-adjusted life years (QALYs) annually owing to this disease. The average annual healthcare cost per patient is highest in the United Arab Emirates, with an estimated value of US $3569 and a population-level indirect cost of US $112.5 million. The included countries allocated a range of 0.20–0.77% of their healthcare expenditure to AD-related healthcare services and technologies. The indirect cost of AD represents approximately 67% of the total disease cost and, on average, approximately 0.043% (range 0.022–0.059%) of the gross domestic product (GDP) of each country.
Conclusion
Although the humanistic and economic burdens differ from country to country, AD carries a significant socioeconomic burden in all countries. The quality of life is severely affected by the disease. If AD is controlled, the costs, especially indirect costs, could decrease and the disease burden could be alleviated significantly.
Plain Language Summary
Atopic dermatitis is a chronic condition characterized by inflamed and itchy skin. The prevalence and symptoms of atopic dermatitis are observed to increase in dry weather. Owing to its high prevalence in children, the majority of studies on atopic dermatitis are in children. Although it is also prevalent in adults and adolescents, its burden on adults has not been sufficiently studied, especially in Africa and the Middle East. This study quantified the burden of atopic dermatitis in adults and adolescents in seven countries in the Middle East and Africa. We estimated the economic and humanistic burden of this disease. We conducted a literature review and expert interviews to determine the effects on patients and caregivers. We created mathematical models to calculate the disease burden in each country, and local experts in each country validated the data. The study results showed that atopic dermatitis significantly affects the quality of life of patients. The direct medical costs of treatment in each country were calculated. The management of atopic dermatitis consumes around 0.20–0.77% of the healthcare expenditure in a country. The indirect cost of atopic dermatitis represents 0.022–0.059% of the gross domestic product (GDP) of a country. The country-specific burden data are essential to guide decision-makers in arriving at evidence-based decisions and efficiently allocating available resources. This study focused on the significant indirect economic burden of the disease, which can sometimes be underestimated because the disease is not fatal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The burden of atopic dermatitis has not been sufficiently quantified in Africa and the Middle East. |
The quality of life of patients and caregivers is severely affected by atopic dermatitis. |
Atopic dermatitis carries a significant socioeconomic burden worldwide. |
There is an opportunity to decrease the disease burden through proper management. |
By controlling diseases, the costs and quality of life loss burden can be alleviated significantly. |
Introduction
Atopic dermatitis (AD) is a chronic skin disease that significantly decreases the quality of life of patients [1]. It may also lead to economic losses for patients and societies, especially in a severe state [2]. AD is occasionally mistaken for a pediatric disease because it is very common in children; however, recent studies have shown that AD is also common in adults, with a prevalence ranging from 2.1% to 4.9% [3]. This disease creates a significant humanistic and economic burden for individual patients and society [4, 5]. The Global Burden of Disease study estimated that AD has the highest burden of disability-adjusted life years (DALYs) among skin diseases, exceeding that of psoriasis (75% higher), urticaria (82% higher), and scabies (more than 100% higher) [6]. Globally, the age-standardized rate of disability-adjusted life years is higher for AD than for other serious diseases, such as liver cirrhosis and alcohol-associated chronic liver diseases [6].
The treatments for AD include a wide range of topical and systemic agents, targeted therapies, and phototherapies. The treatment costs vary among these options, from inexpensive topical anti-inflammatory agents and emollients to expensive targeted therapies [7]. In addition to direct healthcare costs, AD also implies a hidden indirect cost that represents a considerable proportion of the total cost [8].
The prevalence of AD and its manifestations are affected by the climate. The disease tends to manifest more in dry weather [9, 10]; therefore, the burden may vary according to the climate of each country. The burden of AD in the Middle East and Africa has been discussed in a recent literature review [11], and other reviews have estimated its prevalence or burden in specific cities [12, 13]; however, to our knowledge, this is the first study to quantify the burden of the disease in adults and adolescents in specific countries in the region. Country-specific burden data are essential to allow decision-makers to make evidence-based decisions and efficiently allocate the available resources.
This study aimed to estimate the economic and humanistic burden of AD in adults and adolescents in seven countries in the Middle East and Africa region: Algeria, Egypt, Kuwait, Lebanon, Saudi Arabia (KSA), South Africa, and the United Arab Emirates (UAE).
Methods
Primary and secondary data were used to estimate the disease burden. We conducted a literature search and expert interviews to obtain and validate the data on humanistic and economic burdens in the seven selected countries. Additionally, calculation models were created using Microsoft Excel to quantify the burden in each country. We used a bottom-up approach to estimate the humanistic and economic burdens. The values of quality-adjusted life years (QALYs) lost, as well as the healthcare costs and indirect costs incurred by an average patient with AD, were multiplied by the number of patients with AD in a country to estimate the total burden. In general, this study had a conservative approach: if we could not find an accurate estimate of an input, its lower estimate was used; therefore, the actual burden is safely more than the estimate we have provided.
Prevalence
For the bottom-up calculation, the data on the number of adults and adolescents with AD in each country were required. These prevalence data should be stratified by age group because the quality of life and prevalence differ significantly among age groups. We used prevalence data estimates for the seven countries from the Global Burden of Disease study [14]. The 2019 prevalence data (latest reports) are presented in Table 1. The prevalence details by age and sex are shown in Table S1.
Humanistic Burden
To estimate the humanistic burden of AD in the seven selected countries, we multiplied the number of patients in each country by the average loss in quality of life annually (the value of utility lost per patient in 1 year).
There were no country-level data regarding the values of the annual utilities lost owing to AD; therefore, we opted to use data from international studies to calculate the age-standardized QALYs lost. We specifically searched for studies reporting the quality of life subgrouped by age because the utility loss differs among different age groups.
Beikert et al. [15] reported the quality-of-life values for patients with AD sub-grouped by age as EuroQoL 5-dimension (EQ-5D) visual analog scale values. To use these data to estimate the utility loss per age group, we converted the data into 0–1 utility values. There was no ready-made tool for this conversion; therefore, a regression model was built on the basis of five studies identified in the literature [16,17,18,19,20]. Each of these studies included EQ-5D index utility values and EQ-5D visual analog scale results for the same group of patients. We used these values to create a regression model and converted the EQ-5D visual analog scale values to EQ-5D index utility values.
Beikert et al. reported only values for patients aged ≥ 18 years; therefore, we used the data from another study (Ezzedine et al.) [21] to determine the quality of life for patients aged 10–18 years. Ezzedine et al. reported the utility values for patients aged 12–14 and 15–17 years. These values were used as proxies for the quality of life for those in the 10–14 and 15–19 age groups, respectively, to match the prevalence age structure grouping. In the study by Ezzedine et al., the quality-of-life values were reported on the basis of the children and adult versions of the Dermatology Life Quality Index (DLQI) questionnaire results, which were converted into EQ-5D index utility values through a specialized online tool [22].
After collecting the utility values for all patient age groups, we calculated the utility loss from the general population (the utility each patient with AD loses owing to the disease compared with the utility of the general population). The utility of the general population for each age group was reported by Janssen et al. [23] in 20 countries worldwide. We calculated the average utility for all countries, and assumed that this would be the baseline utility for each age group. The study reported values for those aged 18–75 years. We assumed that the patients in the 10–15 and 15–19 age groups would have the same quality of life as the 18–24 age subgroup.
Finally, to calculate the utility loss owing to AD, the utility value for a patient with AD in each subgroup was subtracted from that for the general population in the same subgroup. The humanistic burden in each country was calculated by multiplying the number of patients in each age group by the average utility lost for the same age group over 1 year. The product represents the QALYs lost per country per year owing to AD. The age-standardized utility loss per patient for each country was calculated by dividing the total QALYs lost by the number of patients with AD in each country. This value was calculated to allow comparability between countries.
To calculate the monetary value of QALYs lost owing to AD, the annual QALYs lost in the previous step were multiplied by the gross domestic product (GDP) per capita for each country in 2019 USD. To allow for comparability between countries, the total monetary value of QALYs lost was divided by each country’s GDP, and countries were compared by the monetary value of QALYs lost as a percentage of GDP. We obtained GDP and GDP per capita values from the 2018 World Health Organization Global Health Expenditure database [24].
Economic Burden: Healthcare Costs
The healthcare costs items included outpatient visits, hospitalization, topical treatments, systematic treatments, targeted therapy, and phototherapy sessions. As the economic data are not transferable across countries, we collected the local data on the costs from each country. We conducted a series of structured interviews with experts from each country to estimate the healthcare costs of AD. The questionnaire used in the interview was based on a scoping review conducted to identify the relevant cost components related to the disease. This questionnaire was validated by a healthcare professional who recommended that the questionnaire should be stratified by severity levels (mild, moderate, and severe) because each level requires different interventions and, therefore, has different costs.
We conducted interviews with two or three healthcare professionals from each country. For each country, at least two experts were interviewed. If the results of the two estimates differed significantly (more than double the average), a third interview with a different expert was conducted. Among the three results, the lowest two results were chosen as per the conservative approach of the study.
The data collected during the interviews included the severity distribution among patients and the details of healthcare costs, such as healthcare resource utilization, outpatient visits, length of hospital stay, lab tests, and topical and systemic treatments for each severity level.
The public unit costs of treatments or services for patients with AD were collected for each country from online official price lists, online pharmacy prices, and hospital prices or expert interviews, if all the previous data were unavailable. The questionnaire template and details of each domain can be found in Tables S2 and S3. To allow for comparability between countries, the cost values were converted to 2019 USD using the annual average exchange rate from the World Bank database [25]. The values of healthcare costs for AD as a percentage of the total healthcare expenditure were calculated for each country to assess the relative healthcare cost burden. We obtained data on healthcare expenditures from the 2018 World Health Organization Global Health Expenditure database [24].
The questionnaire was sent to each healthcare professional to understand its structure, and an online structured 2-h interview was conducted with each healthcare professional to complete the questionnaire. The interviewers completed the questionnaires on the basis of the experts’ answers. A total of 17 clinical experts were interviewed. These experts were selected on the basis of a convenience sampling technique in each country, choosing accessible healthcare professionals who have experience in dermatology.
The questionnaires aimed to provide data on the annual average cost burden of AD per patient per country. To estimate the total healthcare cost per country, we multiplied the number of patients in each country by the average cost per patient (obtained from the questionnaire).
Not all patients with AD are diagnosed, and not all patients are treated [3]. The untreated population will, of course, incur no healthcare costs. Hanifin et al. estimated the percentage of AD cases diagnosed by a physician to be 37.1% [26]. Accordingly, the healthcare costs in our study were multiplied by 37.1% to adjust for the proportion of diagnosed and treated patients.
Economic Burden: Indirect Costs
On the basis of the literature search conducted, the indirect costs of AD are mainly related to productivity loss owing to absenteeism and presenteeism of patients and their caregivers. Absenteeism was defined as the number of days the patient was absent from work or school, and presenteeism was defined as the number of days the patient was at work or school, but was not productive [27].
The average annual presenteeism and absenteeism values for each patient with AD were calculated on the basis of a literature search of several studies that included numerical data on presenteeism and absenteeism owing to AD. A list of studies reporting absenteeism and presenteeism data is presented in Table S4. Few studies mentioned data on absenteeism for caregivers; most studies that included these data focused only on children. Therefore, because our study adopted a conservative approach and included adults and adolescents, the caregiver burden was excluded from our calculations. The reported presenteeism and absenteeism values were estimated on the basis of the weighted average of the AD severity.
The following example shows how presenteeism and absenteeism values were estimated from each study:
If patients with AD of mild severity represent 50% of the study population, and are absent for 5 days on average owing to AD, patients with moderate AD represent 35% and are absent for 15 days, and patients with severe AD represent 15% and are absent for 25 days, then the average absenteeism value would be calculated as 50% × 5 + 35% × 15 + 15% × 25 = 11.5 days of absenteeism annually for an average patient with AD.
The average productivity lost by patients in the literature was adapted to local settings, considering the prevalence of working age, employment rate, sex, and labor force participation rate (LFPR) [28,29,30]. These inputs were used to calculate the AD-related indirect costs owing to absenteeism and presenteeism.
To calculate the value of indirect costs for a whole country population, the approach was to multiply the number of patients in the working age group (age, 15–65 years) by the cost of 1 day of presenteeism or absenteeism, and the annual number of days lost. The cost of 1 day was calculated on the basis of the average salary in the country and number of working days per year. Simultaneously, the number of working patients was adjusted to the LFPR and unemployment rate by sex.
The following equation was created and used to calculate the productivity lost:
Validation Meetings
Our results are based on several sources. Local experts from each country validated the extracted and synthesized data. We conducted meetings with experts (payers and healthcare professionals) in the field to validate our results regarding the humanistic and economic burden in light of their local settings and culture. The healthcare professionals involved in the initial data collection did not contribute to validation.
Two research team members managed and coordinated each validation meeting (principal researcher and senior researcher). The meetings were conducted online with local experts who provided feedback about the results, recommended some changes, and provided better or more updated references for some data points. The meetings were recorded and transcribed, and all the key points of the validators were addressed. The research findings and calculations were updated after the validation meetings, and the estimates were adjusted on the basis of recommendations.
An example of the changes recommended by validators and applied to the results is using the unemployment rate reported by the Department of Statistics in South Africa [28] rather than another older estimate. Additionally, in South Africa experts recommended adding the average dispensing fee to drug prices instead of using the single exit price. In Lebanon, experts advised on using the average salary provided by the Salary Explorer website [31]. A summary of the results of the validation meetings and modifications can be found in Table S5.
Compliance with Ethics Guidelines This study is based on previously conducted research and does not include any new studies with human participants or animals performed by any of the authors.
Results
Humanistic Burden
The humanistic burden of AD is expressed as the utility loss per age group. The estimated utility value of an average patient with AD ranges from 0.54 to 0.77 (adjusted from Beikert et al. [15] and Ezzedine et al. [21]). Compared with the average population, the patients with AD are estimated to lose between 0.09 and 0.28 QALYs annually owing to AD. The details of the lost utility per patient are presented in Table 2.
At the country level, the aggregated QALY loss is higher in countries with larger populations. Egypt suffered the highest QALY loss, and Kuwait had the lowest QALY loss owing to AD. The aggregated AD humanistic burden is approximately 334,000 QALYs lost annually in the seven countries included in this study. The age-standardized utility loss per patient per country ranged from 0.185 to 0.189. The average utility loss per patient for the seven countries was estimated at 0.187. The details of humanistic burden including QALYs lost per country and utility lost per patient are shown in Fig. 1.
Healthcare Costs
The cost of AD per patient largely depends on the economic status and the prices of healthcare services of each country. The costs for each severity level were determined, and the weighted average was calculated to provide a single estimate for an average patient. The average annual healthcare cost was calculated for each country; the healthcare cost domains are detailed in Table S3.
In Algeria, the annual cost per patient is US $312. This cost is the lowest among the seven countries. The results showed that the UAE and Kuwait had a remarkably high average cost per patient compared with other countries in the region: US $3569 and US $2880 per patient, respectively. In most of the questionnaires conducted, the use of targeted therapies, with prices much higher than those of other topical or systemic interventions, was considered one of the main cost drivers. In countries where targeted therapies are more frequently used, the average cost per patient tends to be much higher than that in countries where targeted therapies are not commonly prescribed.
For country-level costs, the UAE also had the highest annual cost at US $112.5 million, followed by Saudi Arabia and Egypt with US $99.5 million and US $95.5 million, respectively. The lowest annual cost was in Lebanon at US $13.6 million. The total healthcare costs of the seven countries combined were estimated at more than US $460 million. The annual healthcare cost estimates are presented in Table 3.
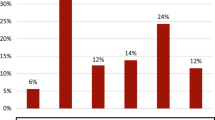
Using the absolute healthcare cost values for these countries, which do not share the same income level or healthcare expenditure, makes it difficult to compare the burdens of these countries. Therefore, we calculated the healthcare cost burden of AD as the ratio of the annual healthcare expenditure in each country. Egypt showed the highest cost for AD per healthcare expenditure at 0.77%, and South Africa and Saudi Arabia showed the lowest, at only 0.2%. On average, the healthcare cost of AD accounts for approximately 0.4% of the total health expenditure in these countries. The details are shown in Fig. 2.
Indirect Costs
The literature search showed an annual productivity loss of 6.1 days of absenteeism and 22.9 days of presenteeism owing to AD for an average patient (average of all severity-level patients). This means that, on average, each patient with AD loses approximately 28.9 days of productivity annually because of the disease.
Compared with the other countries included in this study, Saudi Arabia had the highest annual loss in indirect costs owing to AD (US $364 million), followed by the UAE (US $228 million) and South Africa (US $152 million). Kuwait, Egypt, Algeria, and Lebanon had much lower values, ranging from US $33 million in Lebanon (the lowest) to US $62 million in Kuwait. To show the relative effect of the disease on each country, these values were divided by the respective GDP of each country. The indirect cost of AD as a percentage of GDP was the highest in Lebanon (0.061%) and lowest in Egypt (0.022%). The average indirect cost, as a percentage of the national GDP for the seven countries, was 0.041%. The details of indirect costs are shown in Fig. 3.
Total Burden
The total burden of AD comprises the total economic burden (healthcare and indirect costs) and the monetary value of the QALYs lost owing to the disease.
The economic burden of countries owing to AD was calculated as the sum of healthcare and indirect costs of each country. The total economic burden of AD in Saudi Arabia was observed to be the highest, at US $463 million annually. The aggregated economic burden of the seven countries exceeds US $1.4 billion annually.
Indirect costs represented a significant portion of the total economic burden, ranging from 37% in Egypt to 79% in Saudi Arabia. On average, the indirect costs represented 67% of the total AD cost.
The monetary value of QALYs lost was calculated as the product of QALYs lost and GDP per capita for each country. The QALYs lost were translated into a monetary loss ranging from US $66.9 million in Lebanon to approximately US $1.5 billion in Saudi Arabia.
Table 4 presents a summary of the healthcare and indirect costs and their contribution to the total economic burden as a percentage as well as the monetary value of the QALYs lost. The sum of these values (total economic burden and monetary value of QALYs lost) provides an estimate of the total burden of AD in adults and adolescents in each country.
As the seven countries differ in their economic status and size, the relative burden of the disease was calculated by dividing the estimated values for each country by its GDP. The AD healthcare costs ranged from 0.013% to 0.038% of the GDP in these countries. The indirect costs ranged from 0.022% to 0.061%. The total economic burden ranges from 0.046% to 0.085%. The loss was much higher when including the humanistic burden in the calculation because each QALY lost owing to the disease was translated into monetary losses. The estimated monetary value of the QALYs lost ranged from 0.104% to 0.191% of each country's GDP. On the basis of this, the total burden of the disease ranges from 0.164% to 0.265% of the national GDP in these countries. The monetary value of QALYs lost represented a considerable share of this total burden, with the humanistic burden representing approximately 2.4 times the total economic burden in all countries. Details of the relative burden of AD are presented in Table 5.
Discussion
Our results show that AD in adults and adolescents causes a significant burden in all seven countries that were studied in the Middle East and Africa region. These results were obtained despite the heterogeneous age structures, income levels, and population sizes in these countries. The aggregated results show that, on average, patients with AD lose 19% of their health-related quality of life owing to their disease. This value is comparable to the utility decrements of more severe conditions, such as kidney transplantation [32]. The value of the total QALYs lost per country was associated with population size, with Egypt (most populous among the included countries) experiencing the greatest loss and Kuwait (least populous) experiencing the lowest loss.
The average healthcare cost per patient was highest in higher-income countries (the UAE and Kuwait). Medical interventions in these countries seem to be relatively more expensive, resulting in higher costs per patient. On the basis of the questionnaire results, more advanced treatments, such as targeted therapies and phototherapy, are more common in higher-income countries. The healthcare cost of AD represents 0.20–0.77% of the total healthcare expenditure in the countries studied here, with an unweighted average of 0.4%, which is comparable to other significant contributors to healthcare expenditure. For example, in Germany in 2019, screening programs represented 0.6% of the total healthcare expenditure and maternity services represented 0.3% [33]. For country-level healthcare costs, the calculated values were affected by the population size and income level. The UAE had the highest burden owing to its high GDP per capita, followed by Saudi Arabia, which has a lower GDP per capita, but a larger population, and Egypt, which has the largest population, but a lower GDP per capita.
The indirect costs are also related to income level and population size. Among the countries studied, Saudi Arabia had the highest indirect costs related to AD. This is probably owing to the fact that among the seven countries, Saudi Arabia is the only country that has a combination of a relatively large population and a high per capita GDP. Egypt, for example, has the largest population, but has a low average annual salary; therefore, the indirect costs were not high.
Presenteeism contributed more than absenteeism to indirect costs. The indirect costs represent a significantly greater portion of the total burden than healthcare costs in most countries, accounting for up to 79% of the total economic burden in Saudi Arabia. Only Algeria and Egypt had lower indirect costs than healthcare costs. However, the indirect costs of AD pose a substantial societal burden, representing an average of 61% of the economic burden.
The total burden was significantly affected when humanistic burden was translated into an economic figure. In the UAE and Egypt, the monetary value of QALYs lost exceeded three times the aggregated healthcare and indirect costs. The humanistic burden represented 2.4 times the total economic burden on average for all countries. This shows that AD is associated with a significant hidden burden that may be considered much higher than the direct, tangible burden.
Limitations
Owing to the scarcity of local data for the included countries, the age-standardized QALYs lost and lost productivity were calculated by adjusting the international data to local demographics. This approach may not have captured the exact local burden and, more importantly, may have ignored, to some extent, the differences in disease severity across countries. The estimated burden is probably an underestimation owing to the prevalence estimates from the Global Burden of Disease study, which are significantly lower than those of most other studies reporting the prevalence of AD. However, owing to the lack of age-stratified prevalence data in other studies, we used the best available estimates.
When we calculated the total economic burden, we assumed that the healthcare costs of AD were equal to the total direct costs, excluding other cost components that may contribute to direct costs, such as direct nonmedical costs.
On the basis of the experts’ opinions, other factors were not accounted for in the study, such as the effect on mental health, use of antidepressants, side effects of treatments, effect on career choice, and psychological effect on caregivers. However, these are partially accounted for in humanistic burden estimates.
Another factor confirming that our economic burden estimate for AD should be considered as a minimum estimate is the extra expense incurred by patients owing to the disease (e.g., personal care products and other informal costs). These expenses are usually difficult to calculate, but negatively affect a patient’s financial state.
For these reasons, further local studies are recommended to obtain a more accurate estimate of the burden of AD that considers the local healthcare system and various cultural aspects, specifically in terms of productivity loss and quality of life burden.
Conclusion
AD carries a considerable burden, mainly owing to the poor quality of life and significant productivity loss in patients. However, unlike diseases with high mortality, resource allocation is less prioritized for AD because the disease mainly affects the quality of life rather than the life years of the patients.
This study explored the humanistic and economic burdens of AD in adult and adolescent patients, combining the estimates of the minimum economic burden expected from healthcare and indirect costs related to the disease, which is significant in the geographic regions of the Middle East and Africa, as elsewhere. More evidence-based studies in the Middle East and Africa are needed for lobbying governments to allocate resources to help ease the burden of the disease. In addition, several interventions can be studied to alleviate this burden in these countries. These interventions should aim to optimize the treatment of AD to decrease the burden.
References
Talamonti M, Galluzzo M, Silvaggio D, Lombardo P, Tartaglia C, Bianchi L. Quality of life and psychological impact in patients with atopic dermatitis. J Clin Med Res. 2021;10(6):1298. https://doi.org/10.3390/jcm10061298.
Toron F, Neary MP, Smith TW, Gruben D, Romero W, Cha A, et al. Clinical and economic burden of mild-to-moderate atopic dermatitis in the UK: a propensity-score-matched case-control study. Dermatol Ther. 2021;11(3):907–28. https://doi.org/10.1007/s13555-021-00519-7.
Barbarot S, Auziere S, Gadkari A, Girolomoni G, Puig L, Simpson EL, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73(6):1284–93. https://doi.org/10.1111/all.13401.
Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(P3):340–7. https://doi.org/10.1016/j.anai.2018.07.006.
Ariëns LF, Van Nimwegen KJ, Shams M, de Bruin DT, Van der Schaft J, Van Os-Medendorp H, et al. Economic burden of adult patients with moderate to severe atopic dermatitis indicated for systemic treatment. Acta Derm Venereol. 2019;99(9):762–8. https://doi.org/10.2340/00015555-3212.
Laughter MR, Maymone MBC, Mashayekhi S, Arents BWM, Karimkhani C, Langan SM, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990–2017. Br J Dermatol. 2021;184(2):304–9. https://doi.org/10.1111/bjd.19580.
Howe, W. Treatment of atopic dermatitis (eczema). 2021. https://www.uptodate.com/contents/treatment-of-atopic-dermatitis-eczema#:~:text=Topical%20corticosteroids%20%E2%80%94%20For%20patients%20with,for%20two%20to%20four%20weeks/. Accessed 17 Dec 2021.
Druss BG, Rosenheck RA, Sledge WH. Health and disability costs of depressive illness in a major US corporation. Am J Psychiatry. 2000;157(8):1274–8. https://doi.org/10.1176/appi.ajp.157.8.1274.
Ibekwe PU, Ukonu BA. Impact of weather conditions on atopic dermatitis prevalence in Abuja, Nigeria. J Natl Med Assoc. 2019;111(1):88–93. https://doi.org/10.1016/j.jnma.2018.06.005.
Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133(7):1752–9. https://doi.org/10.1038/jid.2013.19.
Al-Afif KAM, Buraik MA, Buddenkotte J, Mounir M, Gerber R, Ahmed HM, et al. Understanding the burden of atopic dermatitis in Africa and the Middle East. Dermatol Ther. 2019;9(2):223–41. https://doi.org/10.1007/s13555-019-0285-2.
Alqahtani JM. Atopy and allergic diseases among Saudi young adults: a cross-sectional study. J Int Med Res. 2020;48(1):0300060519899760. https://doi.org/10.1177/0300060519899760.
Hossny E, Shousha G, Wassif GO, Hana S. A study of health-related quality of life in pediatric atopic dermatitis. Egypt J Pediatr Allergy Immunol. 2020;18(2):61–9. https://doi.org/10.21608/ejpa.2020.117838.
Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396(10258):1204–22. https://doi.org/10.1016/S0140-6736(20)30925-9.
Beikert FC, Langenbruch AK, Radtke MA, Kornek T, Purwins S, Augustin M. Willingness to pay and quality of life in patients with atopic dermatitis. Arch Dermatol Res. 2014;306(3):279–86. https://doi.org/10.1007/s00403-013-1402-1.
Andersen L, Nyeland ME, Nyberg F. Higher self-reported severity of atopic dermatitis in adults is associated with poorer self-reported health-related quality of life in France, Germany, the UK and the USA. Br J Dermatol. 2020;182(5):1176–83. https://doi.org/10.1111/bjd.1845.
Le PH, Vo TQ, Nguyen NH. Quality of life measurement alteration among Vietnamese: impact and treatment benefit related to eczema. J Pak Med Assoc. 2019;69(suppl 2):S49–56.
Lee SH, Lee SH, Lee SY, Lee B, Lee S, Park YL. Psychological health status and health-related quality of life in adults with atopic dermatitis: a nationwide cross-sectional study in South Korea. Acta Derm Venereol. 2018;98(1):89–97. https://doi.org/10.2340/00015555-2797.
Misery L, Seneschal J, Reguiai Z, Merhand S, Héas S, Huet F, et al. The impact of atopic dermatitis on sexual health. J Eur Acad Dermatol Venereol. 2019;33(2):428–32. https://doi.org/10.1111/jdv.15223.
Katoh N, Saeki H, Kataoka Y, Etoh T, Teramukai S, Takagi H, et al. Atopic dermatitis disease registry in Japanese adult patients with moderate to severe atopic dermatitis (ADDRESS-J): baseline characteristics, treatment history and disease burden. J Dermatol. 2019;46(4):290–300. https://doi.org/10.1111/1346-8138.14787.
Ezzedine K, Shourick J, Merhand S, Sampogna F, Taïeb C. Impact of atopic dermatitis in adolescents and their parents: a French study. Acta Derm Venereol. 2020;100:00015555–23653. https://doi.org/10.2340/00015555-3653.
DLQI to EQ-5D tool. Broadstreetheor.com. 2014. https://dlqi.broadstreetheor.com/. Accessed 23 Aug 2021.
Janssen MF, Szende A, Cabases J, Ramos-Goñi JM, Vilagut G, König HH. Population norms for the EQ-5D-3L: a cross-country analysis of population surveys for 20 countries. Eur J Health Econ. 2019;20(2):205–16. https://doi.org/10.1007/s10198-018-0955-5.
World Health Organization. Global Health Expenditure Database. 2018. https://apps.who.int/nha/database/Select/Indicators/en/. Accessed 20 Sep 2021.
Worldbank.org. Official exchange rate (LCU per US$, period average) | Data. 2021. https://data.worldbank.org/indicator/PA.NUS.FCRF. Accessed 14 Sep 2021.
Hanifin JM, Reed ML, Eczema Prevalence and Impact Working Group. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;18(2):82–91. https://doi.org/10.2310/6620.2007.06034.
Mitchell RJ, Bates P. Measuring health-related productivity loss. Popul Health Manag. 2011;14(2):93–8. https://doi.org/10.1089/pop.2010.0014.
Country Profiles. ILOSTAT. 2020. https://ilostat.ilo.org/data/country-profiles/. Accessed 31 Oct 2021.
General Authority of Statistics-Kingdom of Saudi Arabia. Labor Force. 2020 https://www.stats.gov.sa/en/814/. Accessed 24 Aug 2021.
Africa S. More people participate in the South African labor market in the 4th quarter of 2020 | Statistics South Africa. Statssa.gov.za. 2021. http://www.statssa.gov.za/?p=14031#:~:text=The%20unemployment%20rate%20increased%20from,of%20the%20QLFS%20in%202008/ . Accessed 20 Sep 2021.
The Complete Guide. Salaryexplorer.com. Average Salary in Lebanon 2021. 2021. http://www.salaryexplorer.com/salary-survey.php?loc=119&loctype=1/. Accessed 20 Sep 2021.
Francis A, Didsbury MS, Van Zwieten A, Chen K, James LJ, Kim S, et al. Quality of life of children and adolescents with chronic kidney disease: a cross-sectional study. Arch Dis Child. 2019;104:134–40. https://doi.org/10.1136/archdischild-2018-314934.
Federal Statistical Office. Health expenditure by functions of health care. 2019. https://www.destatis.de/EN/Themes/Society-Environment/Health/Health-Expenditure/Tables/functions.html;jsessionid=748464D51403C94AD92824D49D338E4B.live741. Accessed 19 Oct 2021.
Global Health Data Exchange. GBD Results Tool| GHDx. 2021. http://ghdx.healthdata.org/gbd-results-tool/. Accessed 20 Aug 2021.
Acknowledgements
Funding
Abbvie funded this research and participated the review and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. Abbvie funded the journal’s rapid service.
Medical Writing, Editorial, and Other Assistance
The Authors would like to thank all contributors for their commitment and dedication to this publication. Editage provided English language editing to produce this manuscript using funding from AbbVie.
Author Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published. Zoltán Kaló, Sherif Abaza, Baher Elezbawy and Ahmad N Fasseeh conceptualized the study design. BE and ANF conducted the literature search. EF, BE and ANF conducted the interviews and validation meetings with the experts. Mohamed Tannira, Hala Dalle, Sandrine Aderian and Sherif Abaza facilitated the interviews and the validation meetings. Baher Elezbawy, Ahmad N Fasseeh and Zoltán Kaló conducted the analysis and drafted the manuscript. Laila Carolina Abu Esba, Hana Al Abdulkarim, Alfred Ammoury, Esraa Altawil, Abdulrahman Al Turaiki, Fatima Albreiki, Mohammed Al-Haddab, Atlal Al-Lafi, Maryam Alowayesh, Afaf Al-Sheikh, Mahira Elsayed, Amin Elshamy, Maysa Eshmawi, Assem Farag, Issam Hamadah, Meriem Hedibel, Suretha Kannenberg, Rita Karam, Mirna Metni, Noufal Raboobee, Martin Steinhoff, and Mohamed Farghaly revised the information presented and suggested edits related to their respective countries. All authors revised and approved the final version of the manuscript.
Disclosures
AbbVie sponsored the analysis and interpretation of Data; in reviewing and approval of the final version. Ahmad N Fasseeh, Sherif Abaza, Zoltán Kaló are shareholders in Syreon Middle East. Baher Elezbawy and Essam Fouly are employees at Syreon Middle East. Mohamed Tannira, Hala Dalle, and Sandrine Aderian are AbbVie employees and may hold AbbVie stock. For Laila Carolina Abu Esba, Hana Al Abdulkarim, Alfred Ammoury, Esraa Altawil, Abdulrahman Al Turaiki, Fatima Albreiki, Mohammed Al-Haddab, Atlal Al-Lafi, Maryam Alowayesh, Afaf Al-Sheikh, Mahira Elsayed, Amin Elshamy, Maysa Eshmawi, Assem Farag, Issam Hamadah, Meriem Hedibel, Suretha Kannenberg, Rita Karam, Mirna Metni, Noufal Raboobee, Martin Steinhoff, and Mohamed Farghaly, no conflict of interest and no authorship payments were done.
Compliance with Ethics Guidelines
This study is based on previously conducted research and does not contain any new studies with human participants or animals performed by any of the authors.
Prior Presentation
These data were previously presented at Virtual ISPOR Europe 2021 conference.
Data Availability
All data generated or analysed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Elezbawy, B., Fasseeh, A.N., Fouly, E. et al. Humanistic and Economic Burden of Atopic Dermatitis for Adults and Adolescents in the Middle East and Africa Region. Dermatol Ther (Heidelb) 13, 131–146 (2023). https://doi.org/10.1007/s13555-022-00857-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00857-0