Abstract
Atopic dermatitis (AD) is a common inflammatory skin disease characterized by intensely pruritic lesions. The prevalence of atopic dermatitis is increasing in developing regions, including Africa and the Middle East. However, these regions are underrepresented in the dermatology literature, and a better understanding of the growing burden of atopic dermatitis in Africa and the Middle East is necessary. Herein, we summarize current knowledge on atopic dermatitis epidemiology, disease burden, and treatment options in Africa and the Middle East, highlighting the unmet needs of patients in these regions. With these needs in mind, we provide clinical recommendations for appropriate management of atopic dermatitis in Africa and the Middle East.
Funding
Pfizer Inc.
Plain Language Summary
Plain language summary available for this article.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Plain Language Summary
This article reviews the epidemiology and disease burden of atopic dermatitis, an inflammatory skin disease, in Africa and the Middle East, and outlines appropriate treatment options for patients in these regions. Defining unmet needs in atopic dermatitis (AD), analyzing current treatment options, and exploring factors that contribute to AD burden in these understudied regions are important as AD is frequently encountered and difficult to manage.
Introduction
Atopic dermatitis (AD) is a common inflammatory skin disease characterized by the development of highly pruritic eczematous lesions [1, 2]. AD often begins in infancy and follows a chronic, relapsing course [1,2,3]. Diagnosis is based on patient and family history, as well as clinical characteristics of the disease, and is guided by several sets of diagnostic criteria, most of which derive from the 1980 Hanifin and Rajka criteria [3]. A 2003 update of the Hanifin and Rajka criteria by the American Academy of Dermatology (AAD) established essential, important, and associated features of AD [1, 2]. Essential features include pruritus and eczematous lesions that can be acute (erythema and vesicular eruptions), subacute (ill-defined weepy crusted plaques), or chronic (well-defined, dry lichenified plaques). Skin lesions often show age-specific morphology and distribution patterns [2]. Important features include early onset, dryness, and increased immunoglobulin E (IgE) level (atopy), although about 20% of patients with AD do not have elevated IgE levels [2]. Associated features assist in diagnosis and can include numerous secondary signs such as hyperlinear palms and atypical vascular responses [2]. A diagnosis of AD requires the exclusion of numerous other dermatologic conditions (e.g., scabies, seborrheic dermatitis, contact dermatitis, ichthyoses, cutaneous T cell lymphoma, psoriasis, photosensitivity dermatoses, immunodeficiency disorders, erythroderma of other causes) [2, 4]. Evidence of neuroimmune dysregulation (e.g., abnormal levels of interleukin [IL]-31, IL-4, IL-13, brain-derived natriuretic peptide) may further assist in differential diagnosis [5,6,7,8,9,10].
AD etiology is heterogeneous and complex, involving genetic, environmental, and neuroimmune factors that contribute to skin barrier dysfunction and inflammation [1]. Family history of AD is the strongest risk factor for the condition [1]. Known genetic risk factors include null mutations in the filaggrin (FLG) gene that encodes an epidermal structural protein and risk genes in susceptibility loci related to immune mechanisms of AD [1, 11]. Environmental factors such as climate, urban versus rural dwelling, diet, breastfeeding, obesity, and pollution also contribute to AD risk [12]. Cutaneous inflammation, a hallmark of AD, is characterized by processes that include CD4+ cell infiltration and elevated inflammatory cytokines [1]. Recently, neuroimmune risk factors including IL-4, IL-13, and IL-31 have been implicated in AD inflammation and pruritus [5, 6, 8, 13, 14]. IL-31 has also been shown to promote growth of itch-related sensory nerves [15].
Guidelines for AD treatment are similar globally (Table 1) [16,17,18,19,20,21]. Nonpharmacological treatment primarily focuses on adequate skin care, with regular moisturizer and emollient use recommended to prevent dryness and transepidermal water loss [16,17,18,19,20,21]. Other nonpharmacological treatments include behavioral interventions [18,19,20, 22] and stress reduction and psychological counseling [18, 19, 21, 23]. Common behavioral interventions involve identification and avoidance of triggers of itch, such as excessive sweating, wearing of wool fabrics, and food and environmental allergens [18,19,20, 22]. Topical corticosteroids (TCSs) remain the primary anti-inflammatory treatment option for AD [16, 18,19,20]. However, topical calcineurin inhibitors (TCIs) may be preferable for treatment of sensitive areas (face, neck, genitals), and they can be used in conjunction with or in place of TCSs for long-term use as steroid-sparing therapies [16, 18,19,20]. Other therapeutic approaches include ultraviolet therapy and systemic treatments such as antibiotics and broad immunosuppressants (e.g., cyclosporine A, azathioprine, methotrexate, mycophenolate mofetil) [17,18,19,20,21]. Recently developed anti-inflammatory medications such as dupilumab injection and crisaborole ointment are thus far approved for treatment of AD in the USA (dupilumab, crisaborole) [24, 25], the European Union (dupilumab) [25], Canada (dupilumab) [26], and Japan (dupilumab) [27]. Crisaborole ointment is a nonsteroidal phosphodiesterase 4 inhibitor for the treatment of mild to moderate AD [28]. Dupilumab is a biologic that targets the IL-4 receptor [24]. While recommendations for crisaborole are not yet included in clinical guidelines documents, dupilumab was recommended in the 2018 European Academy of Dermatology and Venerology (EADV) guidelines update for treatment of moderate to severe AD in patients whose AD is inadequately controlled by topical therapies and for whom other systemic therapy is inadvisable [21].
AD is highly prevalent in developed countries, where prevalence rates seem to have plateaued at 10–20% [1]. However, AD prevalence is increasing in developing countries, including Africa and the Middle East [29, 30]. Although AD is becoming a pressing public health concern in Africa and the Middle East, these regions are underrepresented in the AD literature [31]. Treatment has been hindered by a lack of regionally appropriate diagnostic and research tools and by gaps in knowledge and reporting of AD [31]. An investigation of the nature and underlying causes of growing burden of AD in Africa and the Middle East can help address challenges to medical care in these regions and inform our understanding of AD. In this review, we will explore the epidemiology, etiology, and treatment of AD in the Middle East and Africa and provide recommendations for addressing unmet therapeutic needs in these regions.
Objective
We aim to (1) provide an overview of the burden of AD in Africa and the Middle East, (2) outline the unmet need for novel therapeutic options for AD in this part of the world, and (3) offer clinical recommendations for improving the management of atopic disease in Africa and the Middle East.
Methods
A literature search was conducted using PubMed and Web of Science for articles published in English within the previous 10 years. Search terms included “atopic dermatitis,” “Middle East,” “Africa,” “prevalence,” and “treatment.” Articles were manually reviewed for relevance, and additional searches were conducted to supplement the narrative where appropriate. Articles referenced throughout the current review include primary articles, clinical guidelines documents, and reviews, with primary articles preferentially referenced where possible. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Prevalence
The prevalence of AD varies significantly throughout the world [29]. Historically, the prevalence of AD in Africa and Middle Eastern countries has generally been lower than in Europe and North America, but recent time trends show increasing AD prevalence in the developing world [1, 31]. The most comprehensive global epidemiological data can be sourced from the International Study of Asthma and Allergies in Childhood (ISAAC) [32, 33]. Investigators from ISAAC phase 3, which surveyed more than 1 million schoolchildren throughout the world between 1999 and 2004, estimated the lifetime prevalence of AD in 6- to 7-year-old children to be relatively low in the eastern Mediterranean (7.2%) and high in Africa (23.3%) [32, 33]. The global prevalence for 6- to 7-year-old children was 14.2%. The investigators from ISAAC phase 3 estimated the lifetime prevalence of AD in 13- to 14-year-old children to be moderate in the eastern Mediterranean (10.8%) and in Africa (15.2%). The global prevalence for 13- to 14-year-old children was 12.8%. Prevalence differed between and within countries in the Middle East and Africa.
According to a 2012 systematic review, the prevalence of AD in Africa was increasing [29]. The lifetime prevalence of AD symptoms in 13- to 14-year-old children approximately doubled in Morocco, South Africa, and Kenya over an approximate 7-year period (1995–2001/2002). In contrast, the prevalence of AD symptoms in 13- to 14-year-old Nigerian children decreased during this period, although this trend may have been impacted by extremely high baseline prevalence. The same review did not find a clear time trend of change in Middle Eastern prevalence, citing studies that indicated increasing (Israel) and decreasing (Kuwait) lifetime prevalence. A 2008 comparison of ISAAC phases 1 (1992–1997) and 3 (1999–2004) found large increases in prevalence (at least two standard errors [SE]) in 13- to 14-year-old children in Kenya and Algeria [30]. Of nine African regions/countries surveyed in both phases, seven had higher lifetime prevalence in phase 3 than in phase 1 among 13–14 year olds. Of the six eastern Mediterranean regions/countries surveyed in both phases, four had higher phase 3 than phase 1 lifetime prevalence in this age group. All four eastern Mediterranean regions/countries surveyed for 6–7 year olds reported higher phase 3 than phase 1 lifetime prevalence.
Regional and ethnic diversity may play a significant role in variable rates of prevalence in the Middle East. For instance, prevalence differs between countries of Semitic (Qatar, Oman, Saudi Arabia) and Persian (Iran) origin, and between urban and rural or desert regions in Iran [34].
Impact of Social, Environmental, and Economic Factors on the Burden of Atopic Dermatitis
Many environmental, social, and economic factors relevant to Africa and the Middle East contribute to increasing prevalence and burden of AD (Table 2) [12, 19, 35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. Dwelling in urban environments compared with rural environments has been linked to AD in both the Middle East and Africa [34, 37]. In Iran, prevalence of AD is higher in Tehran (17.6%) and Kerman (9.1%) than in Shahr-e-Kord (2.1%), a western city where a greater proportion of citizens have rural lifestyles [34]. A similar pattern has been observed in Africa, with higher income and more urban countries such as South Africa showing large recent increases in AD prevalence [50]. Within individual African countries, AD risk often follows an urban–rural gradient, with a higher rate of AD in city dwellers than in rural dwellers [37]. Effects of increased socioeconomic status and urbanization on prevalence of AD are thought to stem from factors that include increased air pollution, sedentary lifestyle and diet, and smaller family size [12, 31, 50].
Findings in Africa support the “hygiene hypothesis,” which argues that increased prevalence of AD stems, at least in part, from decreased exposure to infection and microorganisms in early childhood [12]. In rural Ethiopia, higher rates of AD have been reported in children with access to piped drinking water than in children who drink river water, an effect attributed to reduced exposure to enteric infections [51]. Helminth (parasitic) infections, which are highly prevalent in Africa, have been shown to promote protection from atopic illness [31]. Results of an analysis of more than 2000 children in Uganda showed that maternal hookworm infection during pregnancy and early childhood infection with Trichuris trichiura or hookworm both were negatively associated with AD [37]. Protection may be mediated by regulatory T and B cells, parasite-induced expression of the anti-inflammatory cytokine IL-10, and reduced expression of atopy-associated genes in infected individuals [31]. However, a study in Ethiopian children showed a positive association between intestinal infection with the Trichuris parasite and risk for AD [51]. This study also showed a history of malaria to be a predictor of risk for AD [51]. Taken together, this underscores the need for additional research. Other hygiene-related factors associated with increased AD prevalence and burden include reduced pre- and postnatal exposure to farm animals and to dogs, antibiotic exposure, and reduced diversity of gut flora [12].
Climate conditions in Africa and the Middle East are also likely contributors to the burden of AD. In Iran and Saudi Arabia, desert conditions are associated with higher burden of AD because high temperatures and low humidity exacerbate dry skin [34, 52]. In Africa, high humidity and temperature promote growth of allergens, including mold [31].
Quality of Life
Most available data regarding the impact of AD on patient quality of life (QoL) in the Middle East is from Saudi Arabia. Numerous studies have reported that QoL is significantly affected in Saudi Arabian patients with dermatologic diseases, including AD [53,54,55,56]. Abolfotouh et al. reported data from 283 adult Saudi Arabian patients with skin disease who completed the Skindex-16 scale, which assesses QoL across symptom, emotional, and functional domains [53]. They used an Arabic version of Skindex-16 adapted for and validated in Saudi Arabian individuals. The authors found that, of the eight diagnoses represented among patients, eczematous dermatitis was associated with the highest mean symptom domain score, indicating a high physical burden of disease in patients with AD [53]. Another study by Ibrahim Al-Hoqail [54] of adult patients in outpatient dermatology treatment in Saudi Arabia used an Arabic version of the Dermatology Life Quality Index (DLQI), which has shown adequate validity and reliability in Moroccan patients with psoriasis but has not yet been validated for AD [57, 58]. The DLQI measures the effect of dermatologic disease on numerous aspects of daily life, including physical symptoms, feelings such as embarrassment, activities (social, recreational, work), and personal relationships. Higher scores are associated with poorer QoL. The author found that patients with eczematous dermatitis had the third highest mean DLQI score of eight skin diseases, with only patients who have papulosquamous disorders (not otherwise specified by the authors) and connective tissue/immunological disorders showing poorer QoL [54]. In another study of Saudi Arabian adult and teenaged dermatology outpatients, Ahmed et al. found that most patients with dermatologic disease (79%) experienced low QoL, as measured by the DLQI [56]. The most common dermatologic diagnoses among study patients were acne vulgaris (29.5%), AD (22.3%), vitiligo (20.7%), and psoriasis (6.4%).
Additional data indicate that family QoL is significantly affected when an infant or child has AD in Saudi Arabia [55, 59, 60]. A study of the families of 447 Saudi Arabian infants and children with AD found that only 15 families (3.4%) had normal QoL; most families (66.4%) experienced a moderate impact on QoL [60]. The authors measured QoL with an Arabic version of the Dermatitis Family Impact Questionnaire, which they tested for clarity and feasibility. Results of numerous studies have shown that degree of impairment in QoL of Saudi Arabian families is directly proportional to severity of child disease [55, 59, 60].
QoL data from the remainder of the Middle East are limited. A study conducted in the Sanandaj, Kurdistan province of Iran found that 1- to 6-year-old children with severe AD had significantly lower QoL than the same-age children with moderate AD [61]. The authors reported itching/scratching and sleep problems as the greatest contributors to poor QoL in patients with severe AD.
QoL data from Africa are limited but have been reported for South Africa and Uganda. A 2015 South African qualitative study involving interviews with ten parents of children aged 0–12 years who have AD helped identify detrimental physical, emotional, and social effects of AD to children and families [62]. Consistently reported factors with prominent impact on child QoL included physical discomfort from persistent itching and scratching, low self-esteem, and social isolation. Parents reported fatigue owing to care-related loss of sleep and change in daily routine, as well as emotional burden, including feelings of frustration, stress, and confusion. In another study, 81 Ugandan patients 1.5–75 years of age with pruritus owing to dermatoses, including AD, were surveyed, and the QoL of Ugandan patients was compared with that of German dermatology patients [63]. QoL was measured using a study-specific questionnaire translated by a physician. Ugandan patients were more likely than German patients to experience normal emotional reactions and were less likely to experience adverse emotional reactions in the form of aggressive behavior and loss of drive. The authors reported a trend toward higher frequency of pruritus affecting QoL and self-assessment in German patients than in Ugandan patients. Although this study did not account for disease severity, results suggest that the burden of AD on QoL may be lower in African patients than in European patients. However, additional research is necessary to make informed comparisons between African and European or North American patients.
Comorbidities
In the study measuring QoL of Saudi Arabian patients with skin diseases, Abolfotouh et al. found that 31.4% of patients had comorbidities, the most common being diabetes (43.8%) and hypertension (47.2%) [53]. About 20% of patients reported a comorbid psychiatric condition. Numerous additional studies support a relationship between dermatologic and psychiatric conditions in the Middle East. A 2016 study of 254 teenage and adult Saudi Arabian outpatients with dermatologic conditions, including AD, reported overall prevalence rates for depression, anxiety, and stress of 12.6%, 22.1%, and 7.5%, respectively [56]. The prevalence rates among the 56 patients with AD were similar: 10.7% (depression), 21.4% (anxiety), and 7.1% (stress). Dermatology patients with poor QoL were 3.5 times more likely to report depression, anxiety, or stress. Women were nearly three times more likely to report at least one negative emotional condition than men, although this was not statistically significant. Another study of 875 Saudi Arabian dermatology patients estimated high overall prevalence of anxiety (29%) and depression (14%) [64]. A cross-sectional study of Iranian dermatology patients reported psychiatric comorbidities in more than 50% of patients and showed higher rates of psychiatric comorbidities in patients with recurrent disease [65]. Collectively, these statistics suggest a potentially alarming pattern of psychological distress in Middle Eastern patients with dermatological conditions, including AD.
Although data are limited, comorbidities have also been noted in African patients. In the study in which QoL of Ugandan and German patients with pruritic conditions owing to dermatoses was compared, depressed mood was reported in 41% of Ugandan patients (vs 49% of German patients) [63]. Results of a survey of Senegalese infants and children with AD showed that AD significantly increased the risk for malarial infection in children older than 3.5 years of age and delayed development of clinical immunity [66].
Current Treatment Options and Unmet Needs
Although AD treatment guidelines are similar globally, in managing AD, Africa and the Middle East face challenges that contribute to disease burden. In Saudi Arabia, numerous AD medications are available over the counter, and medical consultation and treatment are free for citizens at government hospitals [67]. However, patients often prefer to see a specialist rather than a general practitioner, resulting in long wait lists and potentially delayed diagnosis. A case report from Saudi Arabia cites use of traditional medicine, general practitioner nonadherence to clinical guidelines, and lack of access to systemic therapies as contributing factors to disease severity and burden in a pediatric patient [52]. The author recommended numerous steps to improve local care for AD in Saudi Arabia, including adequate refresher training in dermatology for general practitioners; use of specialized nurses in dermatology; and enhanced coordination of primary, secondary, and tertiary care. The report also emphasized the need for further research into traditional Middle Eastern medicine and enhanced regulation of long-term prescription of potent topical steroids.
A qualitative report of parent impressions of AD management in the Gauteng district of South Africa identified dispensation of inappropriate medication or insufficient amounts of medication, inadequate primary care, financial obstacles, and lack of disease education for parents and health care providers as unmet needs in AD treatment [68]. The authors describe barriers of care general to South Africa. These include inadequate knowledge of allergic conditions among primary care providers and unavailability of treatment. Issues are amplified in rural settings, where lack of drugs and equipment, ill-informed health care providers, and financial obstacles add to the burden of AD. As in Saudi Arabia, a high proportion of South African patients report using alternative and traditional medicine.
AD research and care in Africa might also be affected by underreporting of the disease, stemming from limited access to health care and prioritization of infectious, highly virulent diseases such as malaria and tuberculosis [31]. Furthermore, many diagnostic tests were developed for use in European and North American individuals, and allergens inherent to tools such as the skin prick test for IgE immunoreactivity may not be relevant to African local environments. In regions with severe shortages of physicians such as sub-Saharan Africa, innovative tools to promote access to AD care such as the African Teledermatology Project, which connects sub-Saharan medical facilities to dermatologists in the USA, Europe, and Australia, are emerging [69]. However, the effectiveness of such tools in alleviating AD burden in Africa requires further research.
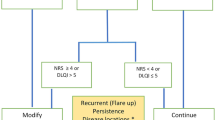
Taking into consideration the unmet needs of AD patients in Africa and the Middle East, we outlined treatment recommendations for management of AD in these patient populations in Table 3 based on literature [16, 20, 70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93] and our clinical experience. The goal of treatment should be threefold: to break the itch–rash cycle by controlling pruritus, to suppress inflammation, and to restore skin barrier function. When colonization or infection occurs, antiseptics, antibiotics, or antivirals are necessary [16,17,18, 20, 21]. The merits and drawbacks of therapeutic options should be weighed when developing a treatment plan. Furthermore, regional differences with respect to affordability and availability of AD therapies should be considered as these obstacles have been reported in Africa [68] and the Middle East [52].
Despite some deviations, our recommendations largely align with established international guidelines and comprise a multifaceted approach to treatment [16,17,18,19,20,21]. Emollients and moisturizers should be treatment mainstays. Topical corticosteroids are first-line therapies, but strength and treatment duration should be considered to avoid adverse events. Calcineurin inhibitors for AD are an important option, especially in children and for certain areas such as the face, neck, and intertriginous areas. Systemic therapies and phototherapy are useful for moderate to severe cases that remain uncontrolled by topical treatments. Behavioral interventions and disease state education should also be provided because these interventions can improve self-management and QoL, promote optimal sleep and dietary habits, and reduce physician visits and costs [21, 23].
As new treatments such as topical crisaborole and the biologic dupilumab become available in Africa and the Middle East, they should be added to AD management plans where indicated. However, there is a need for real-world efficacy, safety, and tolerability data for novel therapies in Africa and the Middle East because racial and ethnic differences in genetic and immune profiles relevant to AD pathophysiology have been documented [94, 95] and may impact treatment response. Furthermore, studies of the cost-effectiveness of these therapies will be especially important for regions of low socioeconomic status.
Treatment Effect on Qol
Available information is very limited regarding the effect of treatment on QoL of patients with AD in the Middle East and Africa. Results of an Iranian study showed that 6 weeks of dermatologic treatment with ultraviolet light, TCSs, and topical ointments produced a statistically significant improvement in QoL (57% change) as measured by a Persian version of the DLQI in patients with chronic dermatitis [96]. Statistically significant improvement after treatment was observed for all DLQI subdomains, but the greatest improvement was seen in the symptoms and feelings domain. Results of a study in Pretoria, South Africa, of 20 infants and children with severe AD showed a 33% improvement in mean Parent Index QoL (PIQoL) score following treatment with pimecrolimus [97]. The PIQoL questionnaire was translated from English as needed.
Conclusions
AD is a common, chronic inflammatory skin disease that is becoming increasingly prevalent in the developing world. Time trends in Africa and the Middle East suggest a growing regional burden of AD. Although data from Africa and the Middle East are limited, available information suggests relatively consistent increases in prevalence, with particularly large increases in Africa, and significant impact on QoL. Growing AD prevalence and burden is a serious public health problem in Africa and the Middle East because these regions face unique challenges in delivering care to patients with AD. Environmental factors contributing to AD prevalence that are specific to Africa and the Middle East should be considered for therapeutic strategies, education, and prevention.
Meaningful understanding of the burden and treatment of AD in the Middle East and Africa is impeded by significant gaps in data; therefore, it requires further investigation. Most region-specific data regarding AD in Africa and the Middle East are concentrated on epidemiology and risk factors associated with AD. Although many studies have been conducted to investigate the QoL of dermatology patients in the Middle East, most are from Saudi Arabia. Very limited information is available regarding QoL of patients with AD in Africa and regarding the effect of treatment on QoL in the Middle East and Africa. Furthermore, tools to effectively measure QoL are limited. An Arabic version of the Skindex-16 has been validated in patients with inflammatory dermatoses (psoriasis, vitiligo, eczematous dermatitis) [98]. Arabic and Persian versions of the DLQI have been validated in Moroccan and Iranian dermatology patients [57, 58, 99]. However, these versions of the DLQI were not specifically validated in AD patients, and no information is available regarding the validity of QoL instruments in other Middle Eastern ethnicities/cultures or in Africa. Available data do not adequately address the economic and academic/occupational impact of AD in Africa and the Middle East. There is also a lack of data regarding the region-specific symptom burden of AD (e.g., pruritus as a disease burden), which would necessitate tools such as a standardized itch questionnaire.
Data on treatment options and unmet needs are limited and are derived primarily from qualitative case studies and interviews, underscoring the need for further research on treatment options and effectiveness in Africa and the Middle East. These types of data are especially important because the primary diagnostic tools and treatments for AD were developed in European and North American patients. Additional studies are necessary to better understand differences in disease presentation in Africa and the Middle East compared with other regions and the potential implications of regional phenotypic differences on treatment effectiveness. Our clinical recommendations for improving management of AD in Africa and the Middle East outline a multifaceted approach of pharmacological and nonpharmacological topical treatments, systemic therapies, phototherapy, and behavioral interventions.
References
Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109–22.
Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338–51.
Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;60:44–7.
Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884–917.
Sonkoly E, Muller A, Lauerma AI, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117:411–7.
Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448–60.
Beck LA, Thaci D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–9.
Meng J, Moriyama M, Feld M, et al. New mechanism underlying IL-31-induced atopic dermatitis. J Allergy Clin Immunol. 2018;141(1677–1689):e8.
Simpson EL, Flohr C, Eichenfield LF, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol. 2018;78(863–871):e11.
Thijs JL, Strickland I, Bruijnzeel-Koomen C, et al. Moving toward endotypes in atopic dermatitis: identification of patient clusters based on serum biomarker analysis. J Allergy Clin Immunol. 2017;140:730–7.
Palmer CN, Irvine AD, Terron-Kwiatkowski A, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–6.
Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(Suppl 1):8–16.
Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171(217–228):e13.
Oh MH, Oh SY, Lu J, et al. TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. J Immunol. 2013;191:5371–82.
Feld M, Garcia R, Buddenkotte J, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. 2016;138(500–508):e24.
Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116–32.
Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327–49.
Schneider L, Tilles S, Lio P, et al. Atopic dermatitis: a practice parameter update 2012. J Allergy Clin Immunol. 2013;131(295–9):1–e27.
Sinclair W, Aboobaker J, Jordaan F, Modi D, Todd G. Management of atopic dermatitis in adolescents and adults in South Africa. S Afr Med J. 2008;98:303–19.
Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657–82.
Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850–78.
Hong J, Buddenkotte J, Berger TG, Steinhoff M. Management of itch in atopic dermatitis. Semin Cutan Med Surg. 2011;30:71–86.
LeBovidge JS, Elverson W, Timmons KG, et al. Multidisciplinary interventions in the management of atopic dermatitis. J Allergy Clin Immunol. 2016;138:325–34.
Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? J Allergy Clin Immunol. 2017;140:633–43.
Kaplon H, Reichert JM. Antibodies to watch in 2018. MAbs. 2018;10:183–203.
Sanofi Genzyme Corporation; Health Canada approves Dupixent™, the first targeted treatment for adults with moderate-to-severe atopic dermatitis, April 12, 2017; Toronto, ON.
Regeneron Pharmaceuticals Inc.; Regeneron announces approval of DUPIXENT® (dupilumab) in Japan for the treatment of atopic dermatitis, January 22, Tarrytown, NY; 2018.
Pfizer Labs; EUCRISA™ (crisaborole) ointment, 2%, for topical use. Pfizer Labs: New York, NY; 2017.
Deckers IA, McLean S, Linssen S, Mommers M, van Schayck CP, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS One. 2012;7:e39803.
Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR, International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121(947–54):e15.
Obeng BB, Hartgers F, Boakye D, Yazdanbakhsh M. Out of Africa: what can be learned from the studies of allergic disorders in Africa and Africans? Curr Opin Allergy Clin Immunol. 2008;8:391–7.
Mallol J, Crane J, von Mutius E, et al. The International Study of Asthma and Allergies in Childhood (ISAAC) phase three: a global synthesis. Allergol Immunopathol (Madr). 2013;41:73–85.
Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI, ISAAC Phase Three Study Group. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124(1251–8):e23.
Farajzadeh S, Esfandiarpour I, Sedaghatmanesh M, Saviz M. Epidemiology and clinical features of atopic dermatitis in Kerman, a desert area of Iran. Ann Dermatol. 2014;26:26–34.
Herrant M, Loucoubar C, Boufkhed S, et al. Risk factors associated with asthma, atopic dermatitis and rhinoconjunctivitis in a rural Senegalese cohort. Allergy Asthma Clin Immunol. 2015;11:24.
Kelbore AG, Alemu W, Shumye A, Getachew S. Magnitude and associated factors of atopic dermatitis among children in Ayder Referral Hospital, Mekelle, Ethiopia. BMC Dermatol. 2015;15:15.
Mpairwe H, Ndibazza J, Webb EL, et al. Maternal hookworm modifies risk factors for childhood eczema: results from a birth cohort in Uganda. Pediatr Allergy Immunol. 2014;25:481–8.
Flohr C, Tuyen LN, Lewis S, et al. Poor sanitation and helminth infection protect against skin sensitization in Vietnamese children: a cross-sectional study. J Allergy Clin Immunol. 2006;118:1305–11.
Dom S, Droste JH, Sariachvili MA, et al. Pre- and post-natal exposure to antibiotics and the development of eczema, recurrent wheezing and atopic sensitization in children up to the age of 4 years. Clin Exp Allergy. 2010;40:1378–87.
Sherriff A, Golding J, Alspac Study Team. Hygiene levels in a contemporary population cohort are associated with wheezing and atopic eczema in preschool infants. Arch Dis Child. 2002;87:26–9.
Tsakok T, McKeever TM, Yeo L, Flohr C. Does early life exposure to antibiotics increase the risk of eczema? A systematic review. Br J Dermatol. 2013;169:983–91.
Douwes J, Cheng S, Travier N, et al. Farm exposure in utero may protect against asthma, hay fever and eczema. Eur Respir J. 2008;32:603–11.
Langan SM, Flohr C, Williams HC. The role of furry pets in eczema: a systematic review. Arch Dermatol. 2007;143:1570–7.
Flohr C, Pascoe D, Williams HC. Atopic dermatitis and the ‘hygiene hypothesis’: too clean to be true? Br J Dermatol. 2005;152:202–16.
Wang M, Karlsson C, Olsson C, et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J Allergy Clin Immunol. 2008;121:129–34.
Bjorksten B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy. 1999;29:342–6.
Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001;108:516–20.
Kalliomaki M, Kirjavainen P, Eerola E, et al. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107(1):129–34.
Watanabe S, Narisawa Y, Arase S, et al. Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J Allergy Clin Immunol. 2003;111:587–91.
Zar HJ, Ehrlich RI, Workman L, Weinberg EG. The changing prevalence of asthma, allergic rhinitis and atopic eczema in African adolescents from 1995 to 2002. Pediatr Allergy Immunol. 2007;18:560–5.
Haileamlak A, Dagoye D, Williams H, et al. Early life risk factors for atopic dermatitis in Ethiopian children. J Allergy Clin Immunol. 2005;115:370–6.
Yousuf MS. Managing childhood eczema in the Middle East. Wound Int. 2010;1:20–2.
Abolfotouh MA, Al-Khowailed MS, Suliman WE, Al-Turaif DA, Al-Bluwi E, Al-Kahtani HS. Quality of life in patients with skin diseases in central Saudi Arabia. Int J Gen Med. 2012;5:633–42.
Al-Hoqail IA. Impairment of quality of life among adults with skin disease in King Fahad Medical City, Saudi Arabia. J Fam Community Med. 2009;16:105–9.
Alzolibani AA. Impact of atopic dermatitis on the quality of life of Saudi children. Saudi Med J. 2014;35:391–6.
Ahmed AE, Al-Dahmash AM, Al-Boqami QT, Al-Tebainawi YF. Depression, anxiety and stress among Saudi Arabian dermatology patients: cross-sectional study. Sultan Qaboos Univ Med J. 2016;16:e217–23.
Ezzahrra LM. The Arabic version of the dermatology life quality index for Morocco: psychometric properties in psoriatics. J Am Acad Dermatol. 2010;62:AB138.
Khoudri I, Lamchahab FZ, Ismaili N, Senouci K, Hassam B, Abouqal R. Measuring quality of life in patients with psoriasis using the Arabic version for Morocco of the Dermatology Life Quality Index. Int J Dermatol. 2013;52:795–802.
Al Robaee AA, Shahzad M. Impairment quality of life in families of children with atopic dermatitis. Acta Dermatovenerol Croat. 2010;18:243–7.
Al Shobaili HA. The impact of childhood atopic dermatitis on the patients’ family. Pediatr Dermatol. 2010;27:618–23.
Shariat M, Kalmarzi RM, Hasani SA, et al. The impact of atopic dermatitis on the quality of life children in Sanandaj, Western Iran. Int J Pediatr. 2017;6:7003–11.
Meintjes KF, Nolte AG. Parents’ experience of childhood atopic eczema in the public health sector of Gauteng. Curationis. 2015;38:1–8.
Weisshaar E, Apfelbacher C, Jager G, et al. Pruritus as a leading symptom: clinical characteristics and quality of life in German and Ugandan patients. Br J Dermatol. 2006;155:957–64.
AlShahwan MA. The prevalence of anxiety and depression in Arab dermatology patients. J Cutan Med Surg. 2015;19:297–303.
Arbabi M, Zhand N, Samadi Z, Ghaninejad H, Golestan B. Psychiatric comorbidity and quality of life in patients with dermatologic diseases. Iran J Psychiatry. 2009;4:102–6.
Herrant M, Loucoubar C, Bassene H, et al. Asthma and atopic dermatitis are associated with increased risk of clinical Plasmodium falciparum malaria. BMJ Open. 2013;3:e002835.
Yousuf MS. The treatment of skin problems in Saudi Arabia. Wound Int. 2010;1(4):5.
Meintjes KF, Nolte AGW. Primary health care management challenges for childhood atopic eczema as experienced by the parents in a Gauteng district in South Africa. Health SA Gesondheid. 2016;21:315–22.
Weinberg J, Kaddu S, Gabler G, Kovarik C. The African Teledermatology Project: providing access to dermatologic care and education in sub-Saharan Africa. Pan Afr Med J. 2009;3:16.
Weber TM, Herndon JH Jr, Ewer M, et al. Efficacy and tolerability of steroid-free, over-the-counter treatment formulations in infants and children with atopic dermatitis. J Dermatol Nurses Assoc. 2015;7:17–24.
Grimalt R, Mengeaud V, Cambazard F, Study Investigators’ Group. The steroid-sparing effect of an emollient therapy in infants with atopic dermatitis: a randomized controlled study. Dermatology. 2007;214:61–7.
van Zuuren EJ, Fedorowicz Z, Christensen R, Lavrijsen A, Arents BWM. Emollients and moisturisers for eczema. Cochrane Database Syst Rev. 2017;2:CD012119.
Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess. 2000;4:1–191.
Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54:1–15.
Weinberg JM. Formulary review of therapeutic alternatives for atopic dermatitis: focus on pimecrolimus. J Manag Care Pharm. 2005;11:56–64.
Eichenfield LF, Lucky AW, Boguniewicz M, et al. Safety and efficacy of pimecrolimus (ASM 981) cream 1% in the treatment of mild and moderate atopic dermatitis in children and adolescents. J Am Acad Dermatol. 2002;46:495–504.
Ho VC, Gupta A, Kaufmann R, et al. Safety and efficacy of nonsteroid pimecrolimus cream 1% in the treatment of atopic dermatitis in infants. J Pediatr. 2003;142:155–62.
Reitamo S, Wollenberg A, Schopf E, et al. Safety and efficacy of 1 year of tacrolimus ointment monotherapy in adults with atopic dermatitis. The European Tacrolimus Ointment Study Group. Arch Dermatol. 2000;136:999–1006.
Kang S, Paller A, Soter N, Satoi Y, Rico MJ, Hanifin JM. Safe treatment of head/neck AD with tacrolimus ointment. J Dermatolog Treat. 2003;14:86–94.
Wahn U, Bos JD, Goodfield M, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics. 2002;110:e2.
Valeant Pharmaceuticals North America LLC; ELIDEL® (pimecrolimus) Cream, 1% for topical use. Valeant Pharmaceuticals North America LLC: Bridgewater, NJ; 2017.
LEO Pharma Inc.; PROTOPIC® (tacrolimus) ointment 0.03%, ointment 0.1%. LEO Pharma Inc.: Madison, NJ; 2017.
Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75(494–503):e6.
Eichenfield LF, Call RS, Forsha DW, et al. Long-term safety of crisaborole ointment 2% in children and adults with mild to moderate atopic dermatitis. J Am Acad Dermatol. 2017;77(641–9):e5.
Eichenfield LF. Clinical advances in atopic dermatitis: novel therapies for improved patient outcomes. J Manag Care Med. 2017;20:5–9.
Simons FE. Prospective, long-term safety evaluation of the H1-receptor antagonist cetirizine in very young children with atopic dermatitis. ETAC Study Group Early. Treatment of the Atopic Child. J Allergy Clin Immunol. 1999;104:433–40.
Caufield M, Tom WL. Oral azathioprine for recalcitrant pediatric atopic dermatitis: clinical response and thiopurine monitoring. J Am Acad Dermatol. 2013;68:29–35.
Weatherhead SC, Wahie S, Reynolds NJ, Meggitt SJ. An open-label, dose-ranging study of methotrexate for moderate-to-severe adult atopic eczema. Br J Dermatol. 2007;156:346–51.
Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–48.
Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522–5.
Schmitt J, Buske-Kirschbaum A, Tesch F, et al. Increased attention-deficit/hyperactivity symptoms in atopic dermatitis are associated with history of antihistamine use. Allergy. 2018;73:615–26.
Kuznik A, Bego-Le-Bagousse G, Eckert L, et al. Economic evaluation of dupilumab for the treatment of moderate-to-severe atopic dermatitis in adults. Dermatol Ther (Heidelb). 2017;7:493–505.
Grundmann SA, Beissert S. Modern aspects of phototherapy for atopic dermatitis. J Allergy (Cairo). 2012;2012:121797.
Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups-variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340–57.
Noda S, Suarez-Farinas M, Ungar B, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136:1254–64.
Anvar M, Lohrasb MH, Javadpour A. Effect of convenient dermatologic intervention on quality of life in patients with chronic eczematous dermatitis. Iran J Psychiatry Behav Sci. 2010;4:47–50.
Kitchin O, Masekela R, Moodley T, et al. The value of pimecrolimus in improving quality of life of children with severe eczema - an open non-randomized study. SA Fam Pract. 2010;52(1):69–71.
AlGhamdi KM, AlShammari SA. Arabic version of Skindex-16: translation and cultural adaptation, with assessment of reliability and validity. Int J Dermatol. 2007;46(3):247–52.
Aghaei S, Sodaifi M, Jafari P, Mazharinia N, Finlay AY. DLQI scores in vitiligo: reliability and validity of the Persian version. BMC Dermatol. 2004;4:8.
Acknowledgements
Funding
This review and all article processing charges were funded by Pfizer Inc. All authors had full access to the articles reviewed in the manuscript and take complete responsibility for the integrity and accuracy of the manuscript.
Medical Writing
Editorial/medical writing support under the guidance of the authors was provided by Madeline L. Pfau, PhD, and Corey Mandel, PhD, at ApotheCom, New York, NY, and San Francisco, CA, USA, and was funded by Pfizer Inc., New York, NY, USA, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–464).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
Khalid Abdullah Mohammad Al-Afif, Mohamad Ali Buraik, Joerg Buddenkotte, and Martin Steinhoff have nothing to disclose. Mohamed Mounir was an employee of Pfizer at the time of the study. Haytham Mohamed Ahmed is an employee of Pfizer. Robert Gerber is an employee and shareholder of Pfizer. Anna M. Tallman was an employee of Pfizer at the time of the study. Anna M. Tallman is now an employee of Dermavant.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mohamed Mounir and Anna M. Tallman: Former Pfizer employees.
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7668206.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Al-Afif, K.A.M., Buraik, M.A., Buddenkotte, J. et al. Understanding the Burden of Atopic Dermatitis in Africa and the Middle East. Dermatol Ther (Heidelb) 9, 223–241 (2019). https://doi.org/10.1007/s13555-019-0285-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-019-0285-2