Abstract
Introduction
We aimed to evaluate US treatment patterns and, more specifically, switch patterns among patients with psoriasis (PsO) who initiated treatment with targeted therapy (TT) and subsequently switched to another therapy.
Methods
This retrospective study used IBM® MarketScan® Commercial and Medicare Databases (1/1/2006–3/31/2020) to evaluate treatment patterns in biologic- and apremilast-naive patients with PsO. TT included apremilast, adalimumab, etanercept, infliximab, ustekinumab, or other biologics (certolizumab pegol, secukinumab, brodalumab, ixekizumab, guselkumab, or tildrakizumab). Adults with ≥ 1 prescription for a TT, ≥ 2 PsO claims separated by ≥ 1 day on or before the index date (date of first TT prescription), and continuous medical and pharmacy enrollment for 1 year before and 2 years after the index date were eligible. Non-targeted therapy (NTT) was defined as non-targeted oral systemic treatment, topical treatment, phototherapy, or no treatment. Kaplan–Meier (KM) analysis was used to estimate time to reinitiation of TT (24-month continuous enrollment post-index was not required).
Results
A total of 11,526 patients with PsO were included; mean [standard deviation (SD)] age and Charlson Comorbidity Index score were 48.3 (12.8) years and 0.9 (1.43), respectively. During the follow-up, 69.2% of the patients were treated with NTT. Median time to first NTT, for those who received NTT, was 205 days (longest: adalimumab, 252 days). Among patients who switched to NTT after initiating treatment with TT, 52.6% reinitiated treatment with TT (least common: apremilast, 45.6%), with a median time to reinitiation of 106 days (longest: other biologics, 136 days). For all patients on NTT, the probability of reinitiating any TT was 60.7% at 24 months.
Conclusions
PsO treatment is often cyclical in nature. Patients frequently experience drug holidays or transition back to TT after using NTT. The consideration of real-world treatment patterns in future economic models may provide new insights into the clinical effectiveness and value of PsO treatments.
Plain Language Summary
Psoriasis is a chronic inflammatory skin disease that affects 3.0% of adults or an estimated 7.56 million Americans. The most common type of psoriasis is called plaque psoriasis because of its appearance with red patches and silvery scales on the skin. A major concern of medical providers is that not all patients continue their treatment as prescribed. Many patients discontinue, switch, and often restart treatment. To develop effective psoriasis treatment plans for shared decision-making among medical providers and patients, it is important to look at how treatments are used in the real world. This can be done by conducting studies using insurance claims data from healthcare insurance providers. In this study, we evaluated treatment patterns and, more specifically, patterns in changes of treatment in US patients who began their psoriasis treatment with a targeted therapy (biologics or apremilast) and then changed to another therapy. We found that patients often took drug holidays (days with no treatment) and returned back to using a targeted therapy after using a non-targeted therapy (e.g., other oral therapy, topical treatment, phototherapy, or no treatment). Findings from this real-world study may support future studies on the clinical effectiveness and value of current and future treatments for psoriasis—especially within these targeted to non-targeted transitions.
AbstractSection Graphical abstract
Similar content being viewed by others
Why carry out this study? |
Patients with moderate to severe psoriasis frequently discontinue or switch treatment. |
We aimed to evaluate treatment patterns and, more specifically, switch patterns among patients with moderate to severe psoriasis who initiated on targeted therapy and subsequently switched to another therapy. |
What was learned from the study? |
Among patients who switched to non-targeted therapy after initiating on targeted therapy, 52.6% reinitiated on targeted therapy, with a median time to reinitiation of 106 days. |
Patients frequently experience drug holidays or changes in treatment, and most transition back to any targeted therapy after using non-targeted therapy. |
The consideration of real-world treatment patterns in future models may provide new insights into the clinical effectiveness and value of psoriasis treatments. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.20269104.
Introduction
Psoriasis (PsO) is a chronic inflammatory skin disease [1] affecting 3.0% of adults or an estimated 7.56 million Americans [2]. Plaque PsO, the most common form [3], is commonly characterized by red plaques with silvery scales [1] that often present on extensors of extremities but may also affect nails and palmoplantar or intertriginous regions [3]. The systematic manifestation of PsO causes substantial morbidity in affected patients [3]. Comorbidities often associated with PsO include psoriatic arthritis, cardiometabolic diseases, psychological illnesses, and inflammatory bowel diseases [3].
Treatment selection for PsO is determined by many factors, including severity of disease [1], comorbid illness [4], patient preferences [1], and access to healthcare [5,6,7]. Patients with PsO are frequently categorized into groups: mild, moderate, and severe [8], depending on the clinical severity of the lesions. Mild PsO involves less than 3% of the body surface area (BSA), whereas involvement of 3% to 10% of the BSA is considered moderate and involvement of greater than 10% of the BSA is severe disease [1]. About 70%–80% of patients have mild plaque PsO, which may be sufficiently managed with topical therapies [5] such as corticosteroids, vitamin D analogues, calcineurin inhibitors, keratolytics, and targeted phototherapy [3]. For patients with moderate to severe PsO, systemic therapies are foundational, and the introduction of biologics was a significant clinical advancement [3]. Targeted therapies include four biologic classes: tumor necrosis factor inhibitors (etanercept, adalimumab, certolizumab pegol, infliximab), interleukin (IL)-17 inhibitors (secukinumab, ixekizumab, brodalumab), an IL-12/23 inhibitor (ustekinumab), IL-23 inhibitors (guselkumab, tildrakizumab, risankizumab), and an oral systemic treatment—a phosphodiesterase 4 (PDE-4) inhibitor (apremilast) [3, 5]. Non-targeted therapies (NTTs) include a mix of topical treatments, phototherapy, and oral non-targeted systemic treatments (e.g., methotrexate) [3].
Despite the availability of treatment options with established safety and efficacy for patients with moderate to severe psoriasis, adherence is low, and many patients discontinue or switch treatments [9,10,11,12]. Reported reasons for treatment discontinuation or switching vary by treatment [9, 10] and include side effects, development of additional morbidity, concern for safety, inconvenience, lack of treatment affordability, insurance denial, delay in obtaining refills, and the most cited reason—lack of treatment effectiveness [9, 10].
Because patient treatment satisfaction is often highly correlated with treatment adherence and successful treatment management, it is important for physicians to evaluate patient preferences and satisfaction [1, 13]. In a 2018 systematic review [13], the authors found that treatment preferences were diverse and evolved with treatment experience among patients with PsO. Also, treatment location (e.g., home, office, hospital), probability of benefit (i.e., chance of improvement), and method of delivery (e.g., oral, topical, intravenous infusion) were considered the most important treatment attributes of those evaluated. Risk of adverse events and probability of benefit were the most important attributes associated with biologic therapy. These findings highlight the importance of the physician–patient relationship and shared decision-making necessary for successful PsO management [13, 14].
Due to the insufficient evidence available, current guidelines provide only general guidance for restarting/resuming biologic treatment in patients who have discontinued and for patients switching biologic treatments [1]. Guidelines state that when considering reinitiation of treatment after discontinuation, physicians should consider disease severity and the number of doses missed [1]. If clinically needed, all other therapies for psoriasis, including other biologics, may be switched with a different biologic agent to improve efficacy, safety, and/or tolerability [1]. It is recommended that physicians address patient preferences for all treatment modifications on a case-by-case basis [1].
In 2018, the Institute for Clinical and Economic Review (ICER) published an economic report on PsO treatments [5]. The PsO economic model constructed by ICER used a treatment algorithm wherein the patient initiated a targeted therapy (TT) such as biologics or apremilast, transitioned to a second TT upon inadequate response, and then utilized an oral NTT such as methotrexate for the remainder of their lifetime, without considering the reinitiation of TT due to relapse or drug holidays [5]. ICER’s economic model assumed that patients who transitioned to a NTT did not transition back to using any of the 12 targeted therapies available in today’s market, but this pattern does not align with common clinical practice in the treatment of PsO. Additionally, assumptions regarding treatment choices have an important bearing on the cost-effectiveness of new innovative therapies due to the cost differential between non-targeted and targeted therapies. Understanding real-world treatment patterns in patients with PsO will help to inform future economic models and optimal treatment-related decision-making.
This study evaluated US treatment patterns among patients who were initiated on TT, including biologics and apremilast. Specifically, this study examined the switch patterns among patients who initiated on TT and subsequently switched to another therapy.
Methods
Data Source
Data for this study were extracted from the IBM MarketScan insurance claims database and include individuals (and their dependents) with commercial employer-sponsored insurance and individuals with Medicare supplemental insurance. The Medicare database contains predominantly fee-for-service plan data, and only plans where both the Medicare-paid amounts and the employer-paid amounts were available and evident on the claims were selected. IBM MarketScan data represent more than 245 million patients across most geographic regions in the United States.
This database captures longitudinal, individual-level data on healthcare utilization, healthcare expenditures and plan enrollment, and contains integrated records for patient demographics, inpatient events, outpatient events, and pharmacy dispensing of drugs. Patients represented in the databases are active employees, dependents, retirees, or Consolidated Omnibus Budget Reconciliation Act (COBRA) recipients, and data are drawn from large employers, health plans, and public organizations in the United States. Records for this study were primarily drawn from the MarketScan enrollment detail, inpatient admissions, inpatient services, outpatient services, and outpatient pharmaceutical claims tables. Drug event duration was calculated from the provided days’ supply field. This retrospective study did not require review and approval by ethics committees or informed consent.
Study Design
A retrospective observational study using IBM MarketScan Commercial and Medicare Databases (January 1, 2006, to March 31, 2020) was conducted to evaluate treatment patterns in biologic- and apremilast-naive patients with moderate to severe PsO. The index date was the first prescription of apremilast or biologic during the index period (January 1, 2013, to March 31, 2020). The baseline period was any time before the index date. The follow-up period began on the index date and extended until the end of enrollment (March 31, 2020). The study schema is presented in Fig. 1.
Study Population
Adult patients (≥ 18 years of age) in the United States who newly initiated TT (biologics or apremilast) between January 1, 2013, and March 31, 2020, were included. Targeted therapies included commonly prescribed treatments (apremilast, adalimumab, etanercept, infliximab, and ustekinumab) and less commonly prescribed “other” agents (certolizumab pegol, secukinumab, brodalumab, ixekizumab, guselkumab, and tildrakizumab). The date of the first TT prescription was the index date. Patients were required to have at least 12 months of continuous medical and pharmacy enrollment prior to (baseline period) and on the index date. PsO diagnosis was confirmed by the presence of at least two International Classification of Diseases, Clinical Modification Ninth Revision (ICD-9-CM) or Tenth Revision (ICD-10-CM) diagnosis codes for PsO separated by at least 1 day prior to their index date. Patients with a diagnosis of rheumatoid arthritis (RA), ankylosing spondylitis (AS), Crohn’s disease (CD), ulcerative colitis (UC), hidradenitis suppurativa, uveitis, Behçet’s disease, or juvenile idiopathic arthritis (JIA) any time before the index date were excluded. Patients with apremilast or biologic use anytime during the baseline period were excluded. Patients with prescriptions for multiple biologics on the index date and at any time during the follow-up were also excluded. Patients with an add-on treatment to their index TT during follow-up were examined as a separate cohort. Included patients were grouped by index therapy (apremilast, adalimumab, etanercept, infliximab, ustekinumab, or other biologic), required to have ≥ 24 months of continuous enrollment post-index date, and followed for 24 months for treatment pattern analysis.
Outcomes
The main outcomes of interest were utilization of NTT by index therapy, time to first NTT by index therapy, reinitiation of TT after NTT by index therapy, and time to reinitiation of TT by index therapy in patients with moderate to severe PsO. NTT was defined as non-targeted oral systemic treatment, topical treatment, phototherapy, or no treatment. Discontinuation of the TT was identified as an absence of a new prescription for apremilast or biologic after the TT exposure episode (last prescription claim plus two times the days’ supply). Initiation of new NTT before the day of the last prescription claim plus two times the days’ supply was considered a switch.
Statistical Analysis
Descriptive characteristics were calculated for patient demographics, clinical characteristics (e.g., comorbidities, Charlson Comorbidity Index [CCI]) and NTT outcomes (utilization of NTT by index therapy, time to first NTT by index therapy, reinitiation of TT after NTT by index therapy) for the overall patient population and by each index therapy cohort. Continuous variables were summarized and reported by means and standard deviations (SDs) and/or by medians and interquartile ranges (IQRs). SDs and IQRs were reported as measures of spread for means and medians, respectively [15]. Categorical variables were summarized and reported by counts and percentages. Kaplan–Meier (KM) survival analysis was used to assess reinitiation of TT after the use of a NTT by index therapy. The entire patient population was considered for this analysis regardless of post-index enrollment (24-month continuous enrollment was not required for inclusion in the KM analysis). For the KM analysis with a log-rank test, we assumed 80% power and a 5% margin of error; the power was > 0.9. All patients were included regardless of post-index enrollment and followed until the end of continuous enrollment.
Results
A cohort of 11,256 patients with PsO were included in this analysis. Of those, 3664 initiated apremilast, 4521 adalimumab, 1651 etanercept, 100 infliximab, 1154 ustekinumab, and 436 other biologics. Mean (SD) patient age was 48.3 (12.8) years, with a balanced representation by sex (50.2% male). Of the comorbidities evaluated, the most common was cardiovascular disease, representing 58.0% of patients. The mean CCI score was 0.9 (1.4), with chronic pulmonary disease (22.1%) being the most common CCI comorbidity represented. Baseline characteristics are presented in Table 1.
On the index date, the date of the first prescription of TT, most patients initiated treatment with adalimumab (39.2%) or apremilast (31.8%), whereas the fewest initiated with infliximab (0.9%) (Table 1). Regardless of the index therapy, 69.2% of all patients switched to a NTT within 2 years of follow-up. Across the index targeted therapies, switch rates were relatively consistent. The percentage of patients who switched to NTT by index therapy is presented in Fig. 2.
Median time to first NTT for the overall cohort was 205 days (IQR 81–432). The longest median time to NTT was observed in patients who initiated on adalimumab with 252 days (IQR 112–483), whereas the shortest median time to NTT was observed in patients who initiated on ustekinumab with 90 days (IQR 28–343) (Fig. 3). Among patients who switched from their index TT to a NTT, 52.6% reinitiated on any TT. Patients whose index therapy was apremilast who switched to a NTT were least likely to reinitiate on any TT (45.6%) (Fig. 4). The median time to reinitiation of any TT after first NTT across index therapies was 106 days (IQR 72–200). The longest median time to reinitiation of any TT after first NTT was observed in patients who initiated other biologics [136 days (IQR 77–237)], whereas the shortest median time was observed in patients who initiated infliximab [44 days (IQR 37–69)] (Fig. 5).
Median time to NTT and reinitiating TT by index therapy. Index therapy is the first prescription of a branded systemic treatment. ITT index targeted therapy, NTT non-targeted therapy. Horizontal black lines indicate a treatment switch. Top black lines (above the pink sections) indicate a return to ITT after NTT
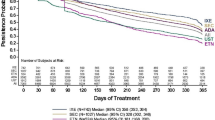
For all patients who initiated any TT and switched to a NTT, the probability of reinitiating any TT within 2 years was 60.7% (range 51.7% [apremilast] to 79.1% [ustekinumab], P < 0.0001) (Fig. 6). Treatment sequences between TT and NTT are presented in the Sankey diagram shown in Fig. 7.
For the 11,526 enrolled patients, the most frequently prescribed first targeted therapies were adalimumab (39.2%) and apremilast (31.8%). Subsequently, of the 69.2% (7972) of patients who switched to a NTT after index therapy, 79.8% either switched to a topical NTT (37.2%) or received no treatment at all (42.6%). For those who switched back to a second TT (4190), 50.6% were prescribed either apremilast (26.0%) or adalimumab (24.6%). For a complete visual description of the treatment flow, see Fig. 8.
Discussion
This real-world, retrospective claims analysis evaluated treatment patterns in patients with PsO who initiated a TT, including biologics and apremilast, and subsequently switched to another therapy. We observed frequent treatment switching among substantial proportions of patients during the study period. Our results demonstrated that most patients with PsO were treated with NTT during the follow-up period, and more than half of the patients reinitiated any TT after the first NTT. The probability of patients transitioning back to any TT after treatment with a NTT at 2 years was high.
Although slight variability was observed between treatments, our findings were consistent across all therapies studied. One variation recognized was the slightly shorter time to NTT seen for infliximab and ustekinumab. Although infliximab and ustekinumab have longer dosing schedules compared with other treatments, we found that patients experienced an immediate treatment gap following their first dose. If patients were switched to an NTT after 2 to 3 months and restarted the same TT a second time, the patient may have successfully achieved adequate or optimal skin clearance upon completion of the first dose. Subsequently, attending physicians may have suggested a therapeutic treatment pause or “drug holiday” to personalize patient care [16]. Because infliximab is primarily used in patients with more severe plaque psoriasis [17], patients in this study may have had more severe disease. Therefore, it is reasonable to consider that skin clearance may have occurred after the first dose. This is consistent with the published clinical trial results reported in the infliximab label (80%–90% clearance, as assessed by the Static Physician Global Assessment score at 10 weeks) [17]. Treatment success, the desire to use a treatment with a more favorable safety profile, prohibitive treatment costs, or other reasons may have contributed to an immediate gap after the first dose and to a switch to an NTT before returning to the index TT. Alternatively, among the infliximab and ustekinumab cohorts, large percentages of patients who initiated an NTT after a TT had no subsequent treatment. Complete treatment discontinuation may also be attributable to any of the reasons noted above. In general, results may have differed slightly from the other cohorts in part due to the small sample size of the infliximab cohort.
Infliximab had the shortest time to reinitiation of a TT. As noted previously, infliximab patients may have had more severe disease, given its label indication [17]. Because patients restarted in less than 2 months, early relapse may be a reason for restarting treatment more quickly. The published median time to relapse since last injection for infliximab is slightly longer than what we observed in this study at approximately 15 weeks (3 months); however, they are similar [18]. Previous infliximab efficacy may also be a factor affecting rapid retreatment. Compared with ustekinumab, adalimumab, and apremilast, the estimated number needed to treat (a measure of efficacy) relative to that of placebo for short-term 75% and 90% improvement from baseline in the Psoriasis Area and Severity Index (PASI) 75 [19] and PASI 90 [20]) scores at 10–16 weeks and at 12 weeks, respectively, was highest for infliximab, followed by ustekinumab, adalimumab, and apremilast (reported for PASI 75 only). Therefore, the short-term efficacy for infliximab is supported and provides a rationale for retreatment with infliximab. Another consideration for retreatment with infliximab or other index TTs is that it may be because of recapture rates of clinical efficacy after treatment discontinuation. The highest average recapture of clinical efficacy as measured by PASI 75 was for patients treated with ustekinumab [21], and was comparable for infliximab [22] and adalimumab [23]. This may also explain the short time to retreatment with ustekinumab after discontinuation of index ustekinumab treatment and the short time to initiation of an NTT. Among patients who transitioned to an NTT, the apremilast cohort had the longest time to reinitiation of TT and the highest percentage of patients who had topical treatment. This may result from the patient’s preference for continuing topical treatment because of cost, dissatisfaction with efficacy, an unfavorable safety profile, or a lack of evidence supporting recapture of clinical efficacy after treatment discontinuation. Recapture rates for apremilast were not identified in the literature. Lower efficacy rates and the lack of published recapture rates for apremilast compared with other treatments may also explain why retreatment was lower with apremilast. Higher recapture rates likely translate into higher confidence levels of health care professionals that the initial extent of skin clearance will be regained by restarting the same treatment. Alternatively, lower proportions of patients who reinitiated on TT may indicate that the patients initiating treatment with apremilast in this study had lower disease severity. More research is needed to further explain the reasons for differences in retreatment observed across index TTs.
These findings provide new evidence regarding real-world treatment patterns in patients with PsO. A PsO economic model recently published by ICER [5] used a treatment algorithm wherein patients initiated a TT such as biologics or apremilast, transitioned to a second TT upon inadequate response, and then utilized a NTT such as methotrexate for the remainder of their lifetime, without considering a restart of TT due to relapse or drug holidays. As evidenced by the results of this retrospective cohort study, the treatment algorithm used by ICER may not align with treatment patterns in patients with PsO as identified using real-world data. Furthermore, an inaccurate economic model could lead to inaccurate assessment of the cost-effectiveness of treatments for patients with PsO.
Several published studies report treatment patterns in patients with PsO [10,11,12, 24, 25]; however, few report treatment reinitiation [24, 25] or switching [10, 12, 24]. Higa et al. [24] reported treatment patterns, including persistence, switching, and restarting, for all systemic therapies available at the time of this study. Regardless of treatment type, > 50% of patients lacked persistence (i.e., discontinued) by 12 months. Of those who lost persistence, 60.2% and 79.9% restarted their index oral and biologic treatment, respectively. In a retrospective matched cohort study of treatment-naive patients initiating apremilast or biologics, the primary reason for nonpersistence was due to treatment switching and was similar between treatment groups. Treatment switching was associated with significantly higher per patient per month associated costs compared with those who were persistent on the index therapy (P < 0.0001) [12]. With the aim to measure overall treatment patterns and undertreatment over a 5-year period, Armstrong et al. [25] found that 59% of patients from the US National Health and Wellness Survey with moderate to severe PsO were not treated for their condition in the last year, and among patients who received treatment the previous year, approximately 50% lapsed treatment. Total numbers reported in the previous year (prior to September 2012) reflected that 438,000 patients stopped their PsO treatment. In addition, 384,000 restarted PsO treatment and 807,000 had a lapse in treatment. Each of these studies provides important information about treatment patterns; however, most focused on systemic therapies only [10,11,12, 24]. Our study provides unique insights into switch patterns among patients who initiated on TT and subsequently switched to non-targeted therapies or took drug holidays. Consideration must be given to these real-world treatment patterns for treatment optimization and for the development of future economic models.
This study has several strengths and a few limitations. A key strength of this study is the large patient population and its generalizability, which is increased by the longitudinal nature of this study. Additionally, this is the first study to examine reinitiation of TT after NTT or a drug holiday in a population of patients with PsO in the United States. Retrospective analyses provide a better understanding of the insured population in clinical practice as compared with the controlled conditions of a clinical trial. They also allow for observations of patients who are often underrepresented in clinical trials, such as patients with comorbidities and the elderly. In addition, patient histories taken prior to treatment can be well observed in claims data, as can posttreatment experience. A better picture of the true efficacy and safety of treatments may also be observed. Claims data are generated for the purpose of provider reimbursement and not for analysis; therefore, information derived from medical billing codes may be subject to omissions, errors, or other differences in billing and reimbursement practices of clinicians and individual insurance plans, and are not connected to medical records. The data captured are limited and not reflective of medical records. There may be missing and uninterpretable data, which is expected to be minimal. There is an inherent risk of information bias resulting from misclassification. Claims databases only include information on patients if patients are covered by the health plan. This study represents real-world practice patterns for patients with PsO but is limited to individuals with employer-sponsored health coverage and/or Medicare supplemental coverage and may not be representative of the entire US population.
Conclusion
Patients with PsO frequently experience drug holidays or changes in treatment, and most transition back to any TT after using NTT. It is important that future PsO economic models include these treatment patterns to remain consistent with trends observed during the real-world usage of these therapies and that real-world treatment patterns be subsequently incorporated into their designs. These models may help to generate more insightful findings on the clinical effectiveness and value of current and future treatments for PsO within these targeted to NTT transitions.
References
Menter A, Strober BE, Kaplan DH, Kivelevitch D, Prater EF, Stoff B, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–72.
Armstrong AW, Mehta MD, Schupp CW, Gondo GC, Bell SJ, Griffiths CEM. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157(8):940–6.
Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–60.
Elmets CA, Leonardi CL, Davis DMR, Gelfand JM, Lichten J, Mehta NN, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80(4):1073–113.
Institute for Clinical and Economic Review. Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: effectiveness and value. Condition update (final evidence report). Boston: Institute for Clinical and Economic Review; 2018.
Bhutani T, Wong JW, Bebo BF, Armstrong AW. Access to health care in patients with psoriasis and psoriatic arthritis: data from National Psoriasis Foundation survey panels. JAMA Dermatol. 2013;149(6):717–21.
WHO. Global report on psoriasis. Geneva: World Health Organization; 2016.
Armstrong AW, Edson-Heredia E, Naegeli AN, Burge R, Poon JL, Anatchkova M, et al. Development of the Psoriasis Symptoms Scale (PSS) in patients with moderate-to-severe psoriasis: qualitative and quantitative analyses. J Dermatolog Treat. 2020;31(5):452–9.
Yeung H, Wan J, Van Voorhees AS, Callis Duffin K, Krueger GG, Kalb RE, et al. Patient-reported reasons for the discontinuation of commonly used treatments for moderate to severe psoriasis. J Am Acad Dermatol. 2013;68(1):64–72.
Doshi JA, Takeshita J, Pinto L, Li P, Yu X, Rao P, et al. Biologic therapy adherence, discontinuation, switching, and restarting among patients with psoriasis in the US Medicare population. J Am Acad Dermatol. 2016;74(6):1057-65.e4.
Dommasch ED, Lee MP, Joyce CJ, Garry EM, Gagne JJ. Drug utilization patterns and adherence in patients on systemic medications for the treatment of psoriasis: a retrospective, comparative cohort study. J Am Acad Dermatol. 2018;79(6):1061-8.e1.
Wu JJ, Pelletier C, Ung B, Tian M. Real-world treatment patterns and healthcare costs among biologic-naive patients initiating apremilast or biologics for the treatment of psoriasis. J Med Econ. 2019;22(4):365–71.
Florek AG, Wang CJ, Armstrong AW. Treatment preferences and treatment satisfaction among psoriasis patients: a systematic review. Arch Dermatol Res. 2018;310(4):271–319.
Larsen MH, Hagen KB, Krogstad AL, Wahl AK. Shared decision making in psoriasis: a systematic review of quantitative and qualitative studies. Am J Clin Dermatol. 2019;20(1):13–29.
The Pennsylvania State University. Measures of spread or variation: State College: The Pennsylvania State University; 2022.
Rubin DT. Restarting biologic agents after a drug holiday. Gastroenterol Hepatol (N Y). 2019;15(11):612–5.
Janssen Biotech, Inc. Remicade (package insert). Horsham, PA: Janssen Biotech, Inc.; 2021.
Masson Regnault M, Shourick J, Jendoubi F, Tauber M, Paul C. Time to relapse after discontinuing systemic treatment for psoriasis: a systematic review. Am J Clin Dermatol. 2022;1–15.
Armstrong AW, Puig L, Joshi A, Skup M, Williams D, Li J, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156(3):258–69.
Leonardi CL, See K, Burge R, Sun Z, Zhang Y, Mallbris L, et al. Number needed to treat network meta-analysis to compare biologic drugs for moderate-to-severe psoriasis. Adv Ther. 2022;39(5):2256–69.
Griffiths CEM, Gordon K, Szaspary PO, Li S, Fakharzadeh S, Papp K. Response to retreatment with ustekinumab after withdrawal from therapy in patients with moderate to severe psoriasis: results from the PHOENIX 1 and ACCEPT phase 3 clinical trials (abstract P-55). Br J Dermatol. 2011;165(suppl 1):43.
Menter A, Feldman SR, Weinstein GD, Papp K, Evans R, Guzzo C, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56(1):31.e1–15.
Gordon KB, Gottlieb AB, Langely RG, van de Kerkhof P, Belasco KT, Sundaram M, et al. Adalimumab retreatment successfully restores clinical response and health-related quality of life in patients with moderate to severe psoriasis who undergo therapy interruption. J Eur Acad Dermatol Venereol. 2015;29(4):767–76.
Higa S, Devine B, Patel V, Baradaran S, Wang D, Bansal A. Psoriasis treatment patterns: a retrospective claims study. Curr Med Res Opin. 2019;35(10):1727–33.
Armstrong AW, Koning JW, Rowse S, Tan H, Mamolo C, Kaur M. Under-treatment of patients with moderate to severe psoriasis in the United States: analysis of medication usage with health plan data. Dermatol Ther (Heidelb). 2017;7(1):97–109.
Acknowledgements
Funding
This study was sponsored by Bristol Myers Squibb, Princeton, NJ, USA, which also provided funding for the journal’s Rapid Service Fees.
Medical Writing Assistance
Professional medical writing from LeeAnn Braun, MPH, MEd, and editorial assistance were provided by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, USA, and were funded by Bristol Myers Squibb. The authors acknowledge Chitra Krishnamurthi for her contributions to research analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
April Armstrong, Qian Xia, Vardhaman Patel, and Lauren Seigel contributed to the study design and served as study investigators. Qian Xia and Anand Rojer John participated in the collection and assembly of data and conducted data analysis. All authors contributed to data interpretation and to the drafting, critical review, and revision of the manuscript. All authors granted approval of the final manuscript for submission.
Prior Presentation
Data from this study were initially presented at the European Academy of Dermatology and Venereology 30th Congress (Virtual), September 29–October 2, 2021.
Disclosures
April Armstrong has received grants and personal fees from AbbVie, Bristol Myers Squibb, Eli Lilly, Janssen, Leo Pharma, and Novartis; personal fees from Boehringer Ingelheim/Parexel, Celgene, Dermavant, Genentech, GlaxoSmithKline, Menlo Therapeutics, Merck, Modernizing Medicine, Ortho Dermatologics, Pfizer, Regeneron, Sanofi Genzyme, Science 37, Sun Pharma, and Valeant; and research grants from Dermira, Kyowa Hakko Kirin, and UCB outside the submitted work. Qian Xia, Vardhaman Patel, and Lauren Seigel are employees and shareholders of Bristol Myers Squibb. Anand Rojer John is an employee of Mu Sigma, which has received consulting fees from Bristol Myers Squibb.
Compliance with Ethics Guidelines
This retrospective study did not include human or animal participants; therefore, review and approval by ethics committees or informed consent were not required.
Data Availability
The Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Armstrong, A., Xia, Q., John, A.R. et al. Treatment Patterns for Targeted Therapies, Non-Targeted Therapies, and Drug Holidays in Patients with Psoriasis. Dermatol Ther (Heidelb) 12, 2087–2103 (2022). https://doi.org/10.1007/s13555-022-00775-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00775-1