Abstract
Psoriatic involvement in areas of the body such as nails, palms and soles (palmoplantar), and scalp is associated with dramatically negative effects on quality of life relative to involvement elsewhere in the body. Although numerous evidence-based studies demonstrate the efficacy of biologics for overall skin clearance in moderate-to-severe plaque psoriasis (including tumor necrosis factor α [TNFα] inhibitors and interleukin [IL]-17A, IL-12/IL-23, IL-23, IL-17F, and IL-17A/F inhibitors), large, randomized, placebo-controlled clinical studies of psoriasis with nail, palmoplantar, and scalp involvement are needed to better inform decision-making in clinical practice. Moreover, biologic failure caused by drug ineffectiveness is a common occurrence in patients who do not respond, lose response, or are intolerant to treatment. Brodalumab is a fully human IL-17 receptor A antagonist that demonstrates high rates of skin clearance among the latest generation of biologic therapies for treatment of moderate-to-severe psoriasis. This review summarizes current literature on the efficacy of brodalumab and other therapies in difficult-to-treat psoriasis including psoriasis in difficult-to-treat locations (such as psoriasis with nail, palmoplantar, or scalp involvement) and psoriasis in patients whose disease did not respond to other biologics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Psoriatic lesions on the nail, palmoplantar (palms or soles), and scalp areas are recognized as difficult to treat, particularly because they tend to be unmanageable with standard therapies. |
Here, we highlight brodalumab and other psoriatic treatments and their roles in difficult-to-treat psoriasis, which is presented in two categories: psoriasis in difficult-to-treat areas of the body (nail, palmoplantar, and scalp psoriasis) and psoriasis that loses response to or never responds to prior biologic treatments. |
Systemic treatments have shown substantial improvement for psoriatic involvement in nails, palms, soles, and scalp; however, randomized clinical trials focusing on these areas have been limited. |
Patients who fail to respond or lose response to a biologic therapy require a treatment switch or an additional therapy, generally with a mechanism of action different from the therapy that did not work. |
Patients with difficult-to-treat psoriasis face distinct challenges; although more head-to-head biologic studies are warranted that focus on specialized areas of difficult-to-treat psoriasis, brodalumab may be a promising therapeutic option. |
Introduction
Psoriasis is an inflammatory skin disorder that affects up to 3% of individuals worldwide, with more than 30% of these individuals presenting with moderate-to-severe psoriasis symptoms [1, 2]. As a chronic condition, psoriasis has substantial negative effects on patients’ quality of life (QOL) and is associated with social stigma and mental health comorbidities, including depression and suicidal ideation and behavior [1, 3]. In this review, we highlight the effectiveness of brodalumab and other treatments in difficult-to-treat psoriasis, which includes psoriatic lesions located in areas of the body that are challenging to treat as well as psoriatic lesions that did not respond to previous biologic treatment.
Psoriatic lesions can occur anywhere on the body, but lesions on the nail, palmoplantar (psoriasis localized to the palms or soles), and scalp areas are recognized as difficult to treat, particularly because they tend to be recalcitrant to standard therapies and are predisposed to perpetual koebnerization (appearance of new skin lesions on previously unaffected skin secondary to trauma); therefore, additional attention may be needed to treat these areas effectively [3,4,5,6]. Typically, psoriasis in one difficult-to-treat area is associated with lesions in other difficult-to-treat areas; for instance, in patients with sole psoriasis, the risk of nail psoriasis is 91% higher [7]. Psoriatic involvement in areas of the body such as nails, palms and soles (palmoplantar), and scalp is associated with dramatically negative effects on QOL relative to involvement elsewhere in the body. Difficult-to-treat areas of psoriasis have been associated with higher physical, psychosocial, and economic burden than other types of psoriasis [3, 8].
Difficult-to-treat areas are challenging to manage with topical therapies (such as corticosteroids, retinoids, and vitamin D3 analogues) for several reasons, including inadequate penetration of active drug components of topicals, restricted treatment options for certain areas, poor treatment adherence, and location and morphological features of areas affected [4, 5, 9,10,11,12]. For instance, there are increased safety concerns of cutaneous atrophy from using corticosteroids on skin folds [3]. Intralesional corticosteroid injections into the nail may be associated with atrophy, infection, tendon rupture, and hemorrhage [3]. Although systemic therapies may overcome some of these limitations, conventional systemic therapies (such as methotrexate, cyclosporine, and acitretin) in patients with difficult-to-treat areas of psoriasis have other concerns, including hair loss, photosensitivity, peeling fingertips, and increased skin sensitivity [3].
Many studies demonstrate that several biologics—including tumor necrosis factor α (TNFα) inhibitors (adalimumab, infliximab, etanercept, and certolizumab pegol), interleukin (IL)-17A inhibitors (secukinumab and ixekizumab), IL-23 inhibitors (guselkumab, tildrakizumab, and risankizumab), the IL-17A/F inhibitor bimekizumab, and the IL-12/IL-23 inhibitor ustekinumab—have shown substantial efficacy in lesion clearance and improvements in signs and symptoms of moderate-to-severe plaque psoriasis [13, 14]. Recent evidence has highlighted the effectiveness of biologics as potential therapies for disease localized to the nails, palmoplantar regions, and scalp. However, large, randomized controlled trials (RCTs) that evaluate the efficacy and safety of psoriasis treatments within these areas are needed. Among US Food and Drug Administration (FDA)–approved biologic treatments of psoriasis, only adalimumab has evidence of treatment efficacy for nail psoriasis from an RCT in the package insert [15], whereas guselkumab and secukinumab have evidence of treatment efficacy for scalp psoriasis from RCTs in their respective package inserts [16, 17]. In a post hoc analysis, ixekizumab has shown significant improvements in genital psoriatic lesions in patients with moderate-to-severe genital psoriasis, which is another difficult-to-treat area; although reduced genital pain, resolution of genital fissures/ulcers, and improvements in sexual health were reported in a post hoc analysis, evidence of treatment efficacy and safety from large RCTs for genital psoriasis is lacking [18].
Although difficult-to-treat psoriasis often refers to lesions in particular areas of the body, an added challenge in treating nonstandard psoriasis includes whether the patient had inadequate response to prior biologic treatment [19]. Psoriatic lesions generally respond to biologic treatment, but some patients present with lesions that fail to respond to biologics. An initial nonresponsiveness to treatment is called primary biologic failure, whereas an initial favorable response to treatment followed by loss of response over time is called secondary biologic failure [19]. The most common reason for biologic discontinuation is loss of efficacy [19], which is also a concern for patients with psoriasis in difficult-to-treat areas [3, 4].
Brodalumab, an anti-IL-17 receptor A antibody approved for the treatment of moderate-to-severe plaque psoriasis in adult patients with inadequate response to other systemic therapies [20], has demonstrated high rates of efficacy, including complete skin clearance [13, 21]. Studies suggest that brodalumab results in complete clearance of psoriatic lesions in patients who failed prior biologics, most likely because of its unique mechanism of action compared with other approved IL-17A monoclonal antibodies [22, 23]. Herein, we review the role of brodalumab and other biologics in difficult-to-treat psoriasis, highlighting data in psoriasis with nail, palmoplantar, and scalp involvement (and touching on psoriasis in other difficult-to-treat areas such as genital and intertriginous psoriasis), as well as in patients whose disease did not respond to prior biologics. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Unique Mechanism of Action of Brodalumab
Brodalumab is a fully human IL-17 receptor A antagonist approved for the treatment of moderate-to-severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy and have failed to respond or have lost response to other systemic therapies [20]. The clinical benefit and safety profile of brodalumab are well established in patients with moderate-to-severe psoriasis [19, 24]. In a recent systematic literature review and metaanalysis of more than 40 RCTs of psoriasis treatments, brodalumab demonstrated greater psoriasis area and severity index (PASI) response rates and had a higher likelihood of sustained PASI response rates for both short-term (16 weeks) and long-term (60 weeks) treatment of moderate-to-severe psoriasis than other biologics [13, 24].
The mechanism of action of brodalumab is distinct from approved IL-17A monoclonal antibodies (Fig. 1). Anti-IL-17A antibodies, such as secukinumab and ixekizumab, selectively inhibit IL-17A but not the other IL-17 isoforms, whereas bimekizumab targets IL-17A, IL-17F, and IL-17A/F. In contrast, brodalumab blocks the IL-17 receptor A subunit, thereby inhibiting the signaling of IL-17 family cytokines, including those involved in psoriasis (e.g., IL-17A, IL-17C, IL-17E, IL-17F, and IL-17A/F) [22, 23, 25, 26]. In addition to demonstrating clinical efficacy, brodalumab is well tolerated and exhibits low immunogenicity, comparing favorably with other biologics in RCTs [27].
Mechanism of action of brodalumab. Inflammatory response in psoriasis is mediated by TH17 and several other IL-17–producing cells. These ligands bind to the IL-17R complex, which is composed of IL-17RA and IL-17RC chains. IL-17R is expressed on various target cells in psoriatic lesions, including endothelial cells, keratinocytes, dendritic cells, and macrophages. Anti-IL-17A antibodies, such as secukinumab and ixekizumab, selectively target IL-17A but not the other isoforms; bimekizumab targets IL-17A, IL-17F, and IL-17A/F. In contrast, brodalumab blocks the IL-17RA subunit, thereby inhibiting the signaling of multiple inflammatory cytokines involved in psoriasis, including IL-17A, IL-17C, IL-17E, IL-17F, and IL-17A/F. Eventually, several downstream inflammatory factors that target gene expression are inhibited [22, 25, 26]. IL interleukin, IL-17R IL-17 receptor, IL-17RA IL-17 receptor A, IL-17RC IL-17 receptor C, TH17 helper T cell (subtype 17)
Nail Psoriasis
Nail psoriasis affects approximately 50–79% of patients with cutaneous psoriasis. Up to 90% of patients with psoriasis will develop nail psoriasis during their life [3, 12]. Multiple digits are usually affected, and although fingernails are more commonly involved than toenails, more than 50% of patients with nail psoriasis develop psoriasis in both [3]. The nail matrix, nail bed, proximal nail fold, and hyponychium may be affected [12]. Clinical manifestations include nail pitting, leukonychia, onycholysis, subungual hyperkeratosis, nail plate crumbling, and splinter hemorrhages [3]. Symptoms include intense discomfort and pain and increased risk of secondary infections [3, 28]. There is also an association between nail psoriasis and the development of psoriatic arthritis, with up to 80% of patients with psoriatic arthritis having nail involvement [28, 29]. Nail psoriasis dramatically affects patients’ QOL, leading to increased physical and emotional burden [3]. Patients with nail psoriasis report significantly more interference in aspects of daily life (such as putting on shoes and socks, household chores, school and work activities, self-esteem, and relationships) than individuals with psoriasis without nail involvement [3].
Therapies for Nail Psoriasis
Nail psoriasis is especially challenging to treat because of the nail’s anatomic structure and texture, which make it difficult for topical treatments to effectively penetrate the lesion site for inflammation resolution [3, 11]. There is a lack of published, randomized, double-blind, placebo-controlled trials on common nail psoriasis treatments, and informed decisions guiding best treatment options are limited. The nail psoriasis severity index (NAPSI) is a region-specific scoring system used to measure severity of nail matrix psoriasis (including nail plate crumbling and pitting) and nail bed psoriasis (including discoloration and hyperkeratosis) [30].
Topical therapies such as vitamin D3 analogues, retinoids, and topical steroids are commonly prescribed first-line treatments of nail psoriasis [12]. However, topical treatment of nail psoriasis is challenging because of treatment duration, poor patient adherence, and poor efficacy in improving nail bed psoriasis [3, 31]. Intralesional corticosteroids may be used but are painful [3], and their long-term use is associated with side effects, including bone atrophy (commonly known as “disappearing digit”) [12].
The consensus recommendation for nail psoriasis is systemic therapy when there is presence of severe nail disease that impacts QOL or the underlying pathology is resistant to topical therapy [12, 28, 31]. Traditional systemic treatments such as cyclosporine and methotrexate are considered to be beneficial to patients by many physicians.
Recent reviews and guidelines have highlighted the efficacy of biologics in nail psoriasis treatment [32, 33]. Biologics such as TNFα inhibitors have been recommended for severe or worsening nail lesions that are uncontrolled with topical or conventional systemic therapies, with or without concomitant psoriatic joint disease [3, 28]. Comparative studies have shown that efficacy results among different TNFα inhibitors regarding reductions in NAPSI are similar [12]. Biologics targeting the IL-17 pathway have demonstrated higher rates of efficacy versus IL-12/IL-23 or IL-23 inhibitors (brodalumab versus ustekinumab; ixekizumab versus guselkumab) and TNFα inhibitors (ixekizumab versus etanercept) in treating nail psoriasis. Certain TNFα inhibitors were shown to have similar efficacy to IL-23 inhibitors (adalimumab versus guselkumab) [32]. According to a recent network metaanalysis evaluating psoriasis with nail involvement in 17 RCTs, all active biologics were generally better than placebo in clearance of nail psoriasis. Moreover, IL-17 inhibitors were more effective in treating nail psoriasis than biologics targeting other interleukins [33]. However, these efficacy data should be interpreted with caution, as trials included in this analysis were not head-to-head studies designed to assess efficacy data for nail psoriasis as the primary endpoint, nor were they focused exclusively on patients with just nail psoriasis [33]. Current RCTs are not always optimized to include homogeneous outcomes and efficacy endpoints for nail psoriasis, limiting comparability between biologics [33, 34]. However, TRANSFIGURE, a double-blind, randomized, placebo-controlled trial evaluating the efficacy of secukinumab treatment for 16 weeks, was one of the few RCTs dedicated to patients with nail psoriasis. Patients receiving secukinumab experienced significantly greater percentage change from baseline in NAPSI compared with placebo (39–45% versus 11%, respectively; P < 0.0001) [35].
Brodalumab in the Treatment of Nail Psoriasis
A post hoc analysis of phase 3 studies evaluated patients with moderate-to-severe nail psoriasis who received brodalumab 210 mg every 2 weeks (Q2W) or ustekinumab through week 52 (AMAGINE-2/-3, ClinicalTrials.gov identifiers: NCT01708603, NCT01708629) [36]. In AMAGINE-2/-3, brodalumab was associated with significantly greater rates of complete clearance of nail psoriasis (NAPSI 0) at weeks 12, 36, and 52 (7.9%, 54.2%, and 63.8%, respectively) versus ustekinumab (2.2%, 33.7%, and 39.1%, respectively; P < 0.05; Fig. 2). Patients receiving brodalumab also had significantly lower rates of NAPSI from weeks 12 to 36 (5.3 to 1.7) compared with ustekinumab (6.7 to 2.9; P < 0.05) [36].
Percentage of patients in the AMAGINE-2/-3 trials with NAPSI 0 at weeks 12, 36, and 52 receiving either continuous brodalumab 210 mg Q2W or continuous ustekinumab [36]. Observed data analysis. Error bars are 95% CI. N1 number of patients who had a valid measurement value at the specified week, NAPSI nail psoriasis severity index, Q2W every 2 weeks. *P < 0.05 versus ustekinumab
In an open-label, single-center, unblinded study in patients with severe nail psoriasis, brodalumab 210 mg Q2W demonstrated statistically significant reductions in NAPSI for fingers and toes at weeks 12 and 24 compared with baseline (P < 0.001; Fig. 3). Furthermore, the mean (SD) dermatology life quality index (DLQI) in treated patients was significantly improved at week 24 compared with baseline (3.7 [2.2] versus 22.8 [4.4]; P < 0.001), indicating improvements in health-related QOL [37]. In a subanalysis of a phase 2, randomized trial, 12 weeks of treatment with brodalumab 210 mg Q2W versus placebo resulted in a mean (SD) NAPSI improvement of 47.6% (35.2%) versus 9.6% (86.2%), respectively [38].
Mean NAPSI of fingers and toes at baseline and weeks 12 and 24 after treatment with brodalumab (N = 30) in an open-label, unblinded study [37]. Error bars are SD. NAPSI nail psoriasis severity index. *P < 0.001 versus baseline
In a real-world case series, four patients with psoriatic nail involvement achieved significant or complete clearance with brodalumab after 12–20 weeks of treatment [23]. Improvements in QOL were also reported, with all four patients achieving PASI of ≤ 1.9 and DLQI of ≤ 1 by week 44. In two patients with psoriatic arthritis, joint pain was also resolved [23].
Palmoplantar Psoriasis
Psoriasis of the palms and soles, which manifests as plaques or pustular lesions, is considered to be the most disabling form of psoriasis and occurs in 12–16% of psoriasis cases [39,40,41]. Beyond palm and sole involvement, palmoplantar psoriasis can also involve the fingers and toes. Compared with moderate-to-severe plaque psoriasis, palmoplantar psoriasis is associated with a greater effect on QOL. Painful fissures, tissue hardening, and hyperkeratosis affect locations that are crucial for daily functional activities; patients typically experience impaired mobility, self-care, and activities of daily living [41,42,43]. The severity of skin disease and response to treatment are monitored using the palmoplantar PASI [44] and palmoplantar psoriasis physician’s global assessment (PPPGA) [45,46,47].
Therapies for Palmoplantar Psoriasis
Patients with palmoplantar psoriasis demonstrate increased dependence on topical treatments, with almost 80% using topical corticosteroids as first-line treatment, followed by second-line mainstay systemic treatment with acitretin [42]. Palmoplantar psoriasis is resistant to treatment and has high rates of recurrence. Treatment with conventional drugs has limited efficacy [43], and more than 70% of patients with palmoplantar psoriasis require systemic treatment [44]. Challenges in managing palmoplantar psoriasis have led to emerging new treatments, including biologics [40, 42]. The efficacy of secukinumab was investigated in GESTURE, a large, double-blind, randomized, placebo-controlled, phase 3b study of 205 patients with chronic moderate-to-severe palmoplantar psoriasis. Among those receiving secukinumab, one-third of patients achieved complete or almost complete clearance of palms and soles, with approximately 35–50% improvement in palmoplantar disease at week 16 [35]. Similar large, randomized, placebo-controlled studies using other biologics in patients with palmoplantar psoriasis are warranted to identify the most effective treatment options for this disease [42].
Brodalumab in the Treatment of Palmoplantar Psoriasis
Although these findings are exploratory, a case series of 16 patients suggests that brodalumab also shows efficacy in nonplaque psoriasis. Politou et al. assessed treatment initiation with brodalumab in patients who failed prior treatment with secukinumab and revealed that 100% (4/4) of patients with palmoplantar pustulosis achieved PPPGA of 0 (complete skin clearance) at week 16 [46].
Scalp Psoriasis
The scalp is one of the most commonly affected body regions in psoriasis, with 45–90% of patients with psoriasis having scalp psoriasis. In adolescents with psoriasis, the scalp is often the only affected area [3, 48, 49]. Scalp psoriasis generally presents as erythematous plaques and flaky silvery white scales associated with dry skin, cracking, bleeding, and itch. Patients are often predisposed to recurrence of the same psoriatic lesions due to scratching and routine hair care [3]. Diagnosis of scalp psoriasis may be delayed because of resemblance to other conditions including seborrheic dermatitis [8]. The psoriasis scalp severity index (PSSI) is a region-specific scoring system that measures erythema, induration, and desquamation of disease affecting the scalp [50]. Like nail and palmoplantar psoriasis, scalp psoriasis is associated with poor QOL and substantial negative psychosocial impairment (e.g., self-consciousness, embarrassment, avoidance of certain clothing or hairstyles) due to physical symptoms [3, 8].
Therapies for Scalp Psoriasis
Topical corticosteroids are typically the first-line treatment of scalp psoriasis and are generally used in various formulations, including foam, gel, solutions, shampoo, and spray; they can also be used in combination with vitamin D analogues [48]. Scalp psoriasis is, however, difficult to treat with topical agents because of the presence of hair, poor accessibility to lesions, unappealing cosmetic effects (e.g., greasiness, unpleasant odors), difficulties in application, and increased frequency of applications required, thus leading to poor patient adherence and dissatisfaction with treatments. Use of systemic or biologic therapies in such cases may therefore be warranted [3, 8, 48].
Systemic therapies (e.g., methotrexate, cyclosporine) may be used as second-line therapy when topicals or phototherapy have failed [48]. The efficacy of conventional systemic agents for psoriasis is well documented [51, 52]. Although effective in select patients, systemic therapies can cause notable complications. For instance, methotrexate can cause exacerbated hair loss, photosensitivity, oral ulcerations, and burning sensations, and cyclosporine can cause skin burning/tingling, renal toxicity, and arthritic pain [3]. Additionally, many individuals with severe scalp psoriasis may not have psoriatic involvement elsewhere and therefore may not receive systemic therapy because of prescriber hesitation or payer restrictions [1, 8, 48].
Because few studies have specifically assessed psoriasis with scalp involvement, lack of robust efficacy data remains an unmet need in the psoriasis treatment landscape [8, 48]. A review of RCTs and observational studies that evaluated the effects of biologics and small molecules suggests that, on average, guselkumab, infliximab, ixekizumab, and brodalumab demonstrate the highest efficacy for treatment of scalp psoriasis [48]. This review also suggests that, in individuals with scalp plaques and concomitant whole-body psoriasis, biologics are suitable first-line options [48]. Guselkumab and secukinumab have efficacy evidence for scalp psoriasis on the FDA label [16, 17]. Efficacy and head-to-head clinical trial data comparing biologics among patients with disease isolated to the scalp are necessary to determine the most effective treatments [48].
Brodalumab in the Treatment of Scalp Psoriasis
In a post hoc analysis of the phase 3 AMAGINE-1 study (ClinicalTrials.gov identifier: NCT01708590), patients with moderate-to-severe scalp psoriasis received either brodalumab 210 mg Q2W or placebo through 12 weeks. Significant improvement rates from baseline in mean PSSI were seen as early as 2 weeks in patients receiving brodalumab versus placebo (67.6% versus 6.7%; P < 0.001). Brodalumab continued to demonstrate significantly greater improvements from baseline PSSI versus placebo through week 12 (92.8% versus 14.4%; P < 0.001). At week 12, 89.0% and 63.4% of patients receiving brodalumab, compared with 9.5% and 3.2% of patients receiving placebo, achieved PSSI 75% and PSSI 100% improvement from baseline, respectively [36].
In a subanalysis of a phase 2, randomized, placebo-controlled, Japanese trial, efficacy and safety of brodalumab were evaluated in patients with moderate-to-severe scalp psoriasis. After 12 weeks of receiving brodalumab 210 mg Q2W versus placebo, patients achieved a mean (SD) PSSI improvement of 94.5% (14.8%) versus 12.6% (63.0%), respectively (P < 0.001) [38].
Brodalumab Treatment in Psoriasis Not Responsive to Previous Biologics
Although difficult-to-treat psoriasis often refers to lesions in particular areas of the body, biologic failure is another challenge in psoriasis treatment [19]. Biologic failure caused by drug ineffectiveness occurs when patients do not initially respond, lose response over time, or are intolerant to treatment; in such situations, therapy switch or adjuvant treatment is often required, which in practice is usually with a biologic that has a different mechanism of action [19, 53]. The most common reason for biologic discontinuation is loss of efficacy [19], which is also a concern for patients with psoriasis in difficult-to-treat areas [3, 4].
In a subgroup analysis of AMAGINE-2/-3 trials of brodalumab, patients in the ustekinumab group with inadequate response were eligible to switch to brodalumab or continue ustekinumab; those who switched showed an increase in PASI 75% improvement from baseline (PASI 75), PASI 90, and PASI 100 response rates from week 12 to week 52 (24.2–72.6%, 4.8–58.1%, and 0–36.3%, respectively) [54]. Patients who had been rescued with brodalumab at week 16 after experiencing inadequate response to ustekinumab had greater improvements in health-related QOL measures than patients who continued ustekinumab following an inadequate response after week 16 (increased DLQI responder rate of 3.6% versus decreased DLQI responder rate of 16.2%, respectively, from weeks 12 to 52) [54].
Evidence of efficacy for switching to brodalumab after loss or failure of response to IL-17A inhibitors was demonstrated in a retrospective study in patients with moderate-to-severe psoriasis [53]. Among ten individuals, 57% (4/7) and 67% (2/3) of patients who switched from secukinumab or ixekizumab, respectively, achieved PASI 75 after 12 weeks of treatment with brodalumab [53].
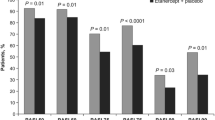
In other studies of patients with inadequate or loss of response to secukinumab and ixekizumab, patients achieved partial or complete skin clearance after switching to brodalumab. In a retrospective study of 47 patients treated with brodalumab after discontinuing secukinumab or ixekizumab, 62%, 47%, and 43% of patients achieved PASI 75, PASI 90, and PASI 100, respectively, at week 16 (Fig. 4a) [55]. In another multicenter study, among 39 patients treated with brodalumab after treatment failure (defined as treatment with either secukinumab or ixekizumab for ≥ 3 months without achieving PASI 75 response or with a 50% loss of original improvement), 76%, 50%, and 32% achieved PASI 75, PASI 90, and PASI 100, respectively, at week 16 (Fig. 4b) [56].
Proportion of PASI 75, PASI 90, and PASI 100 responders at week 16 after rescue with brodalumab (a) in a study of 47 individuals who switched treatment from secukinumab, ixekizumab, or both [55] and (b) in a separate study of 39 patients who failed treatment with secukinumab or ixekizumab [56]. PASI 75, 90, and 100 psoriasis area and severity index 75%, 90%, and 100% improvement
Data show that there are increased rates of patients switching to brodalumab after stopping previous biologics. For instance, among 16 patients with moderate-to-severe psoriasis who had prior biologic use, 88%, 81%, and 69% achieved PASI 75, PASI 90, and PASI 100, respectively, with brodalumab [57]. Furthermore, real-world data from the CORRONA Psoriasis Registry show that, among 202 patients initiating brodalumab, many had previously received TNFα inhibitors (74%), IL-17A inhibitors (72%), or IL-12/IL-23 inhibitors (50%). Although the reasons for switching biologics were not provided (e.g., biologic failure, adverse events from other biologics, cost-effectiveness), the percentage of patients with a history of ≥ 4 previous biologics was 41% for those initiating brodalumab [58].
Discussion
Difficult-to-treat psoriasis, including psoriatic lesions located in areas of the body that are challenging to treat as well as psoriatic lesions that did not respond to previous biologic treatment, results in significant physical impairments and substantial impairment in QOL, including elevated emotional distress, reduced work productivity, and challenges in relationships [3]. Consensus on the best therapeutic options for psoriasis with nail, palmoplantar, or scalp involvement is generally limited, and unmet needs remain for safe and efficacious treatments [33, 34, 42, 48]. Challenges associated with managing difficult-to-treat areas include poor accessibility to lesions, reduced efficacy from greater treatment resistance and sensitivity to strong topicals, and decreased adherence to treatment, resulting in more frequent use of systemic drugs [3, 39]. Because of their mechanism of action, biologic therapies may result in faster and greater clearance of skin lesions compared with traditional systemic agents [13, 39]. For individuals with scalp plaques and whole-body psoriasis, biologics may be suggested as suitable first-line options. Although head-to-head studies are lacking, a recent review suggests that guselkumab, infliximab, ixekizumab, and brodalumab achieve the highest clearance rates in psoriasis with scalp involvement [48]. Moreover, the consensus is that biologic therapies will generally improve skin psoriasis with coexisting nail involvement without significant adverse events [34]. Additionally, IL-17 inhibitors may be more effective in treating nail psoriasis compared with biologics targeting other interleukins [33]. The role of IL-17 inhibitors in treating palmoplantar psoriasis is evolving, suggesting utility of these biologics beyond plaque psoriasis [35]. Brodalumab has shown complete clearance of skin, nail, palmoplantar, and scalp lesions in greater proportions of patients compared with placebo and ustekinumab, or patients who failed prior treatment with previous biologics [19, 36, 46]. Patient-related QOL is also improved with brodalumab treatment in areas challenging to treat [23, 37].
Emerging evidence demonstrates long-term resolution of psoriasis with brodalumab in patients who have responded poorly to prior biologics, including etanercept, adalimumab, ixekizumab, and secukinumab [23]. Furthermore, brodalumab has successfully rescued individuals who failed treatment with IL-17A antagonists [55]. In a retrospective, multicenter study of 78 patients initiating brodalumab treatment, including some whose disease did not respond to treatment with IL-17A inhibitors, complete or almost complete clearance was achieved in 89% (16/18) of patients with genital psoriasis. Patients in this real-world setting reported statistically significant improvements in QOL and satisfaction with brodalumab relative to baseline [59]. Although it remains unclear why such switching has proven beneficial to some patients, multiple cytokines beyond IL-17A (e.g., IL-A/F, IL-17C, IL-17E, IL-17F) underlie inflammatory pathways in psoriasis [22]. Clinical benefit may be derived from using an agent that blocks multiple cytokines implicated in psoriasis over inhibition of IL-17A alone, and patients whose disease does not respond to an anti-IL-17A agent may respond to an anti-IL-17 receptor A agent [46].
The unique mechanism of blocking multiple IL-17 family cytokines may account for the effectiveness of brodalumab in achieving skin clearance in patients with inadequate response to other biologics [23]. Brodalumab also has faster onset compared with other commonly used biologics, which may help alleviate the QOL deficits associated with psoriasis with nail, palmoplantar, or scalp involvement [36]. Although not the focus of this review, exploratory evidence suggests that brodalumab also shows efficacy in other difficult-to-treat areas, such as psoriasis with genital involvement [59]. Brodalumab is well tolerated, and its safety profile in moderate-to-severe psoriasis is comparable to that of IL-17 antagonists [60]. Therefore, brodalumab may be an effective treatment option for psoriasis with nail, palmoplantar, or scalp involvement (or other difficult-to-treat areas) and addresses unmet needs in patients with inadequate or loss of response to other biologic therapies.
Conclusion
Psoriasis with nail, palmoplantar, or scalp involvement substantially impairs patients’ physical function, QOL, and well-being. Additionally, despite recent advances in biologic therapies, some patients with psoriasis do not respond to biologic treatment. Switching among biologics when efficacy (e.g., PASI 75) is not achieved commonly occurs in clinical settings [53]. Brodalumab may be a promising option for treatment of nail, palmoplantar, or scalp psoriasis, as well as for patients whose disease did not respond to previous biologics [36], including IL-17A inhibitors [23, 37]. Further investigations into the efficacy and safety of brodalumab in the treatment of other difficult-to-treat psoriasis types, such as intertriginous and genital psoriasis, are warranted.
References
Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9:504–13.
Naik GS, Ming WK, Magodoro IM, et al. Th17 inhibitors in active psoriatic arthritis: a systematic review and meta-analysis of randomized controlled clinical trials. Dermatology. 2017;233:366–77.
Aldredge LM, Higham RC. Manifestations and management of difficult-to-treat psoriasis. J Dermatol Nurces Assoc. 2018;10:189–97.
Kivelevitch D, Frieder J, Watson I, Paek SY, Menter MA. Pharmacotherapeutic approaches for treating psoriasis in difficult-to-treat areas. Expert Opin Pharmacother. 2018;19:561–75.
Callis Duffin K, Mason MA, Gordon K, et al. Characterization of patients with psoriasis in challenging-to-treat body areas in the Corrona Psoriasis Registry. Dermatology. 2021;237:46–55.
Sanchez DP, Sonthalia S. Koebner phenomenon. Treasure Island: StatPearls; 2022.
Egeberg A, See K, Garrelts A, Burge R. Epidemiology of psoriasis in hard-to-treat body locations: data from the Danish skin cohort. BMC Dermatol. 2020;20:3.
Merola JF, Qureshi A, Husni ME. Underdiagnosed and undertreated psoriasis: nuances of treating psoriasis affecting the scalp, face, intertriginous areas, genitals, hands, feet, and nails. Dermatol Ther. 2018;31: e12589.
Hjuler KF, Iversen L, Rasmussen MK, Kofoed K, Skov L, Zachariae C. Localization of treatment-resistant areas in patients with psoriasis on biologics. Br J Dermatol. 2019;181:332–7.
Rich P, Gooderham M, Bachelez H, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with difficult-to-treat nail and scalp psoriasis: results of 2 phase III randomized, controlled trials (ESTEEM 1 and ESTEEM 2). J Am Acad Dermatol. 2016;74:134–42.
Wasel N, Thaci D, French LE, et al. Ixekizumab and ustekinumab efficacy in nail psoriasis in patients with moderate-to-severe psoriasis: 52-week results from a phase 3, head-to-head study (IXORA-S). Dermatol Ther (Heidelb). 2020;10:663–70.
Bardazzi F, Starace M, Bruni F, Magnano M, Piraccini BM, Alessandrini A. Nail psoriasis: an updated review and expert opinion on available treatments, including biologics. Acta Derm Venereol. 2019;99:516–23.
Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156:258–69.
Papp KA, Merola JF, Gottlieb AB, et al. Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol. 2018;79:277-86.e210.
HUMIRA [package insert]. North Chicago: AbbVie Inc; 2021.
COSENTYX [package insert]. East Hanover: Novartis Pharmaceuticals Corporation; 2020.
TREMFYA [package insert]. Horsham: Janssen Biotech, Inc; 2020.
Merola JF, Ghislain PD, Dauendorffer JN, et al. Ixekizumab improves secondary lesional signs, pain and sexual health in patients with moderate-to-severe genital psoriasis. J Eur Acad Dermatol Venereol. 2020;34:1257–62.
Menter A, Armstrong A, Van Voorhees A, Liu C, Jacobson A. Brodalumab to the rescue: efficacy and safety of brodalumab in patients with psoriasis and prior exposure or inadequate response to biologics. Dermatol Ther (Heidelb). 2020;10:615–21.
SILIQ [package insert]. Bridgewater: Bausch Health US, LLC; 2017.
Sawyer L, Fotheringham I, Wright E, Yasmeen N, Gibbons C, Holmen MA. The comparative efficacy of brodalumab in patients with moderate-to-severe psoriasis: a systematic literature review and network meta-analysis. J Dermatolog Treat. 2018;29:557–68.
Green L, Weinberg JM, Menter A, Soung J, Lain E, Jacobson A. Clinical and molecular effects of interleukin-17 pathway blockade in psoriasis. J Drugs Dermatol. 2020;19:138–43.
Pinter A, Bonnekoh B, Hadshiew IM, Zimmer S. Brodalumab for the treatment of moderate-to-severe psoriasis: case series and literature review. Clin Cosmet Investig Dermatol. 2019;12:509–17.
Sawyer LM, Cornic L, Levin LÅ, Gibbons C, Møller AH, Jemec GB. Long-term efficacy of novel therapies in moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. J Eur Acad Dermatol Venereol. 2019;33:355–66.
Boniface K, Moynet D, Mossalayi MD. Role of Th17 cells in the pathogenesis of rheumatoid arthritis. World J Rheumatol. 2013;3:25–31.
Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–76.
Bagel J, Lebwohl M, Israel RJ, Jacobson A. Immunogenicity and skin clearance recapture in clinical studies of brodalumab. J Am Acad Dermatol. 2020;82:344–51.
Crowley JJ, Weinberg JM, Wu JJ, Robertson AD, Van Voorhees AS, National Psoriasis Foundation. Treatment of nail psoriasis: best practice recommendations from the Medical Board of the National Psoriasis Foundation. JAMA Dermatol. 2015;151:87–94.
Sobolewski P, Walecka I, Dopytalska K. Nail involvement in psoriatic arthritis. Reumatologia. 2017;55:131–5.
Rich P, Scher RK. Nail Psoriasis Severity Index: a useful tool for evaluation of nail psoriasis. J Am Acad Dermatol. 2003;49:206–12.
Rigopoulos D, Stathopoulou A, Gregoriou S. Small molecules and biologics in the treatment of nail psoriasis. Skin Appendage Disord. 2020;6:134–41.
Hadeler E, Mosca M, Hong J, Brownstone N, Bhutani T, Liao W. Nail psoriasis: a review of effective therapies and recommendations for management. Dermatol Ther (Heidelb). 2021;11:799–831.
Szebényi J, Gede N, Hegyi P, et al. Efficacy of biologics targeting tumour necrosis factor-alpha, interleukin-17 -12/23, -23 and small molecules targeting JAK and PDE4 in the treatment of nail psoriasis: a network meta-analysis. Acta Derm Venereol. 2020;100:adv00318.
Thomas L, Azad J, Takwale A. Management of nail psoriasis. Clin Exp Dermatol. 2021;46:3–8.
Armstrong AW, Vender R, Kircik L. Secukinumab in the treatment of palmoplantar, nail, scalp, and pustular psoriasis. J Clin Aesthet Dermatol. 2016;9(6 suppl 1):S12–6.
Elewski B, Rich P, Lain E, Soung J, Lewitt GM, Jacobson A. Efficacy of brodalumab in the treatment of scalp and nail psoriasis: results from three phase 3 trials. J Dermatolog Treat. 2022;33:261–5.
Gregoriou S, Tsiogka A, Tsimpidakis A, Nicolaidou E, Kontochristopoulos G, Rigopoulos D. Treatment of nail psoriasis with brodalumab: an open-label unblinded study. J Eur Acad Dermatol Venereol. 2021;35:e299–301.
Nakagawa H, Niiro H, Ootaki K, Japanese Brodalumab Study Group. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81:44–52.
Dopytalska K, Sobolewski P, Błaszczak A, Szymańska E, Walecka I. Psoriasis in special localizations. Reumatologia. 2018;56:392–8.
Misiak-Galazka M, Zozula J, Rudnicka L. Palmoplantar pustulosis: recent advances in etiopathogenesis and emerging treatments. Am J Clin Dermatol. 2020;21:355–70.
Chung J, Callis Duffin K, Takeshita J, et al. Palmoplantar psoriasis is associated with greater impairment of health-related quality of life compared with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2014;71:623–32.
Engin B, Aşkın Ö, Tüzün Y. Palmoplantar psoriasis. Clin Dermatol. 2017;35:19–27.
Murakami M, Terui T. Palmoplantar pustulosis: current understanding of disease definition and pathomechanism. J Dermatol Sci. 2020;98:13–9.
Miceli A, Schmieder GJ. Palmoplantar psoriasis. Treasure Island: StatPearls; 2021.
Bissonnette R, Haydey R, Rosoph LA, et al. Apremilast for the treatment of moderate-to-severe palmoplantar psoriasis: results from a double-blind, placebo-controlled, randomized study. J Eur Acad Dermatol Venereol. 2018;32:403–10.
Politou M, Pompou M, Afroditi KI, Giannoukos A, Nikolaos F. Twenty patients with moderate to severe psoriasis successfully treated with brodalumab after a failed treatment with secukinumab. J Am Acad Dermatol. 2020;83(6 suppl):AB214.
Brunasso AM, Puntoni M, Aberer W, Delfino C, Fancelli L, Massone C. Clinical and epidemiological comparison of patients affected by palmoplantar plaque psoriasis and palmoplantar pustulosis: a case series study. Br J Dermatol. 2013;168:1243–51.
Mosca M, Hong J, Hadeler E, Brownstone N, Bhutani T, Liao W. Scalp psoriasis: a literature review of effective therapies and updated recommendations for practical management. Dermatol Ther (Heidelb). 2021;11(3):769–97.
Mahé E. Optimal management of plaque psoriasis in adolescents: current perspectives. Psoriasis (Auckl). 2020;10:45–56.
Blakely K, Gooderham M. Management of scalp psoriasis: current perspectives. Psoriasis (Auckl). 2016;6:33–40.
Schmitt J, Rosumeck S, Thomaschewski G, Sporbeck B, Haufe E, Nast A. Efficacy and safety of systemic treatments for moderate-to-severe psoriasis: meta-analysis of randomized controlled trials. Br J Dermatol. 2014;170:274–303.
Menter A, Korman NJ, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011;65:137–74.
Gasslitter I, Kirsten N, Augustin M, et al. Successful intra-class switching among IL-17 antagonists: a multicentre, multinational, retrospective study. Arch Dermatol Res. 2019;311:421–4.
Langley RG, Armstrong AW, Lebwohl MG, et al. Efficacy and safety of brodalumab in patients with psoriasis who had inadequate responses to ustekinumab: subgroup analysis of two randomized phase III trials. Br J Dermatol. 2019;180:306–14.
Yeung J, Vender R, Turchin I, et al. Brodalumab success in patients with moderate-to-severe psoriasis who failed previous interleukin-17A inhibitors. J Am Acad Dermatol. 2021;84:1169–71.
Kimmel G, Chima M, Kim HJ, et al. Brodalumab in the treatment of moderate to severe psoriasis in patients when previous anti-interleukin 17A therapies have failed. J Am Acad Dermatol. 2019;81:857–9.
Papp K, Menter A, Strober B, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2015;72(3):436-9.e1.
Armstrong A, Strober B, Drew S, Cronin A, Jacobson A. Real-world characteristics of patients with psoriasis initiating brodalumab: findings from the Corrona Psoriasis Registry. J Am Acad Dermatol. 2020;83(6 suppl):AB181.
Fargnoli MC, Esposito M, Dapavo P, et al. Brodalumab for the treatment of moderate-to-severe plaque-type psoriasis: a real-life, retrospective 24-week experience. J Eur Acad Dermatol Venereol. 2021;35:693–700.
Rusta-Sallehy S, Gooderham M, Papp K. Brodalumab: a review of safety. Skin Therapy Lett. 2018;23:1–3.
Acknowledgements
Funding
This review and the journal’s Rapid Service Fee were sponsored by Ortho Dermatologics. Ortho Dermatologics is a division of Bausch Health US, LLC.
Medical Writing and Editorial Assistance
Medical writing and editorial support were provided under the direction of the authors by Prithvi Shah, PhD, and Jenna Lewis, MA, ELS, of MedThink SciCom, with support from Ortho Dermatologics. Ortho Dermatologics is a division of Bausch Health US, LLC.
Author Contributions
Alan Menter, Tina Bhutani, Benjamin Ehst, Boni Elewski, and Abby Jacobson contributed to conception, planning, drafting, critical revision, and final approval of the manuscript.
Disclosures
Alan Menter has received compensation from or served as an investigator, consultant, advisory board member, or speaker for AbbVie, Abbott Labs, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly & Co, Janssen Biotech Inc., Leo Pharma, Mindera, Novartis, Pfizer, SunPharma, and UCB. Tina Bhutani is currently an investigator for studies sponsored by AbbVie, Clementia, Galderma, Mindera, Pfizer, and Regeneron; and has served as an advisor for Arcutis, Boehringer Ingelheim, Bristol Myers Squibb, Clarify, Janssen, Leo, Lilly, Novartis, Sun, and UCB. Benjamin Ehst has received compensation from or served as an investigator, advisory board member, consultant, or speaker for AbbVie, Allergan, Amgen, Arcutis, Bristol Myers Squibb, Dermavant Sciences, Eli Lilly, Evelo Biosciences, Janssen Biotech, Novartis, Ortho Dermatologics, Sun Pharma, and UCB. Boni Elewski has served as a consultant for Arcutis, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Leo, Lilly, Menlo, Novartis, Pfizer, Sun Pharma, UCB, Valeant (Ortho Dermatologics), and Verrica; and has received clinical research support from AbbVie, AnaptysBio, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Incyte, Leo, Lilly, Merck, Menlo, Novartis, Pfizer, Regeneron, Sun, Valeant (Ortho Dermatologics), and Vanda. Abby Jacobson is an employee of Ortho Dermatologics (a division of Bausch Health US, LLC).
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Availability of Data and Material
Articles used in this review are available in the public domain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Menter, A., Bhutani, T., Ehst, B. et al. Narrative Review of the Emerging Therapeutic Role of Brodalumab in Difficult-to-Treat Psoriasis. Dermatol Ther (Heidelb) 12, 1289–1302 (2022). https://doi.org/10.1007/s13555-022-00746-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00746-6