Abstract
Introduction
The risk of active tuberculosis is increased in psoriasis patients receiving biologic drug therapy. The QuantiFERON-TB Gold In-Tube assay (QFT) is used for latent tuberculosis screening in these patients. This study presents a retrospective analysis on repeated QFT assays, investigating the influence of biologic drugs and isoniazid therapy on the outcome of the assay.
Methods
Serial QFTs of 58 psoriasis patients, who received biologic drug therapy, were evaluated at baseline and after 12 months of treatment. Patients were retrospectively divided in four groups according to QFT results at baseline and at follow-up: patients having a QFT reversion (from positive to negative results); patients with a conversion (from negative to positive); patients confirming the baseline results, either positive or negative.
Results
At the end of the 12-months period, 11.1% of patients with a negative QFT result at baseline presented a conversion, showing low interferon (IFN)-gamma values, whereas 6.9% of positive patients presented a QFT reversion. When the test was repeated after 2–3 months without isoniazid chemoprophylaxis, patients with QFT conversion showed negative results. No patient developed active tuberculosis.
Conclusions
In patients undergoing biologic therapy, a positive QFT assay needs to be further confirmed, as false-positive results may occur after long-term therapy. Repeating QFT tests in patients with low IFN-gamma values could reduce the incidence of false-positive latent tuberculosis infection diagnosis, thus preventing unnecessary tuberculosis chemoprophylaxis. In conclusion, a dynamic QFT response is possible in psoriasis patients undergoing biologic therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The clinical therapy for patients presenting with chronic inflammatory diseases, such as psoriasis and rheumatoid arthritis, often consists of administering biologic drugs, such as tumor necrosis factor (TNF)-alpha antagonists. However, there are some concerns on the usefulness of these drugs because they may cause side effects; notably, the possible reactivation of latent tuberculosis infection (LTBI), as the drugs act as suppressors of the immune response [1].
LTBI is a condition that is difficult to diagnose because many patients host the bacillus in a latent stage without symptoms of tuberculosis (TB) disease (after contact with Mycobacterium tuberculosis) [2, 3]. Associated with this condition is the high risk of reactivation of the infection, which can occur during a change in the immunological status of the patient, such as reduced immunity caused by immunosuppressive therapies [4]. LTBI patients are not infective, but could serve as a reservoir for future TB epidemics [5], so it is very important to avoid the evolution of this condition to active TB. It is estimated that one-third of the world’s population is infected with a LTBI [6]; therefore, it is crucial to accurately identify patients with LTBI before commencing biologic therapy for chronic inflammatory diseases, and to avoid prophylactic treatment and unnecessary exposure to toxic compounds of uninfected patients [2].
Since the early twentieth century, the diagnosis of LTBI has been based on the tuberculin skin test (TST) but, recently, the use of commercially available interferon (IFN)-gamma-releasing assays (IGRAs), named QuantiFERON®-TB Gold In-Tube (QFT; Cellestis Europe GmbH, QIAGEN, Hilden, Germany) and T-SPOT®.TB (Oxford Immunotec, Milton Park, Abingdon, UK) has led to a notable improvement [5]. IGRAs are more specific than TST and their results are not influenced by previous bacillus Calmette-Guérin (BCG) vaccination, or nontuberculous mycobacteria (NTM) infections [7].
Moreover, the sensitivity of IGRAs is higher than that of the TST, especially in patients with chronic inflammatory disease, who may be unable to produce an adequate response to the TST because of their deficient cell-mediated immune response as induced by corticosteroid and/or immunosuppressive drugs [1, 8, 9]. Nonetheless, IGRAs can be repeated over time without concerns about sensitization and boosting [8], thus allowing clinicians to monitor their patients. Since their development, IGRAs have produced promising results in the diagnosis of LTBIs [9–12] and active TB [13, 14], but their usefulness in immunosuppressed patients is still debated and limited [12, 15, 16], as discordant results between the TST and QFT have been recorded in many studies [17]. Moreover, the variability of results among QFT repeated assays in the same patient has also been noted. For these reasons, the aim of this retrospective analysis was to evaluate changes in QFT results over time, in patients affected by psoriasis vulgaris treated with biological or isoniazid therapy, and to analyze the possible causes of discordance in repeated testing.
Materials and Methods
The present study considered 58 patients affected by psoriasis vulgaris, who were eligible for continuous, long-term treatment with biologic drugs, enrolled between January 2008 and April 2012 in the Reference Center of Psoriasis at IRCCS Galeazzi Orthopedic Institute, Italy. Patient characteristics are summarized in Table 1.
Patients were affected by moderate psoriasis (Psoriasis Area and Severity Index [PASI] of 12–18), and were selected for biologic therapies according to the International Consensus Conference [18]. During the screening phase before commencing therapy, all patients were evaluated for risk-factors of TB infection according to the Centers for Disease Control and Prevention (CDC) recommendations [19]. Clinically active TB or radiographic evidence of a fibrocalcified lesion in the upper lung field were exclusion criteria. A QFT test was then performed on all included patients. Determination of IFN-gamma using the QFT was performed at the baseline (T0) and after 12 months of continuous treatment with biologic drugs (T1).
Peripheral blood samples were collected into three different tubes: the first containing no antigen (negative control or Nil), the second containing TB-specific antigens (ESAT-6, CFP-10, and TB7.7[p4]) and the third containing phytohemagglutinin (positive control or Mitogen). According to the manufacturer’s instruction, samples were incubated for 18–24 h at 37 °C. Plasma was then separated by centrifugation and an enzyme-linked immunosorbent assay (ELISA) sandwich test for IFN-gamma detection was performed; absorbance readings were obtained at 450 nm, as recommended by the manufacturer’s protocol [20].
Results were considered positive, negative, or indeterminate according to the criteria established by the manufacturer [21]. Briefly, the results of the QFT assay were considered positive if the IFN-gamma level was >0.35 IU/mL in the antigen-stimulated well after subtracting the IFN-gamma value of the Nil well. The QFT was considered indeterminate if the Nil result was >8.00 IU/mL or if the Mitogen, after subtracting the Nil value, was <0.50 IU/mL. QFT conversion was defined as a baseline negative QFT assay and a value of IFN-gamma >0.35 IU/mL at T1. QFT reversion was defined as a baseline IFN >0.35 IU/mL and a negative QFT result at the follow-up.
When a positive QFT result was recorded, the patient commenced isoniazid chemoprophylaxis, except for those patients who presented a value of IFN-gamma between 0.35 and 1.0 IU/mL in the TB antigen well. These patients only started the biologic therapy and were clinically monitored for TB reactivation. After a period of 2 or 3 months, they underwent a QFT retest and, if they presented a negative result, they continued the therapy.
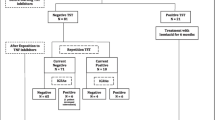
Patients were retrospectively categorized into four groups: group A presented QFT positive test at the T0 and negative at T1; group B had a negative QFT test at T0 and positive at T1; group C had both a positive QFT at T0 and T1; and group D had negative QFT results at both T0 and T1.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients included in the study.
Results
Among the 58 patients enrolled in the study, 10 (17.2%) patients had positive QFT assays at baseline and latter presented with values of IFN-gamma of >1.0 IU/mL; the QFT assay results were positive with values ranging between 0.35 and 1.0 IU/mL. No indeterminate result was recorded. Patients who were QFT positive at baseline received 300 mg/day isoniazid for 9 months. At the 1 year follow-up, 12 patients (20.7%) had a QFT positive result, six presented with a low value of IFN-gamma (ranging between 0.35 and 1.0 IU/mL).
Four patients (6.9%) presented with a positive QFT assay result at T0 and a negative result at T1 (group A), and six (11.1%) were QFT negative at T0 and QFT positive at T1 (group B); six patients (11.1%) were QFT positive at both T0 and T1 (group C), and in 42 cases (77.8%) the QFT was negative at both T0 and T1 (group D). Table 1 shows the characteristics and QFT results for each patient group. None of the 58 patients receiving biologic therapy developed active TB disease during the follow-up and no adverse events were observed.
Twelve months after the initiation of isoniazid prophylaxis and biologic therapy, there was a general decrease in IFN-gamma release in patients who were QFT positive at T0. In particular, four of them showed a reversion in QFT retesting at follow-up. All of the isoniazid chemoprophylaxis patients were treated with etanercept. The baseline and follow-up IFN-gamma levels of the LTBI cases are summarized in Table 2.
Six patients with negative QFT at baseline showed subsequent QFT conversion at the follow-up, with low IFN-gamma levels (mean ± standard deviation: 0.50 ± 0.14 IU/mL). All patients who showed QFT conversion underwent repeat QFT assays after 2–3 months, and then commenced on isoniazid chemoprophylaxis if necessary. As a consequence, they were not further considered for anti-TB prophylaxis. The other 42 patients with negative QFT at baseline did not show QFT conversion at follow-up.
Discussion
Accurate diagnosis of LTBI has become mandatory before starting a treatment based on biologic drug administration [9], in an attempt to avoid TB reactivation caused by the immunosuppressive effect of the therapy. The diagnosis is usually based on the TST and/or IGRAs assays; the latter measure the IFN-gamma production from effector lymphocytes, which are activated by contact with specific TB antigens.
Since the introduction of IGRAs, some meta-analysis has reported variable sensitivity and specificity values for LTBI diagnosis, with only a relative advantage over TST [22, 23]. Although most of the current guidelines advocate the use of the TST as the main screening tool for LTBI in psoriasis patients receiving treatment with biologic drugs, the European S3-guidelines present a more flexible approach, suggesting the use of the TST and/or QFT [24]. However, discordance between the TST and QFT result is often recorded [12], because the TST may be influenced by the deficient cell-mediated immunity and its specificity is limited by the cross-reactivity of the TST with BCG vaccination [9].
The QFT test for LTBI diagnosis may also have some limitations, as a number of situations, such as a low immune response, extrapulmonary TB [25], and high intra-assay variability [20], can affect QFT results.
In the present study, the QFT test was positive in 27.6% of the study population. However, in 17.2% of cases, the QFT test was positive only at baseline, whereas the rest of QFT positivity was recorded at follow-up. These results are consistent with those obtained in previous retrospective studies that reported a LTBI prevalence rate in psoriasis patients of 20% and 11%, respectively, prior to and after treatment with biologic drugs [26, 27].
Corticosteroids and immunosuppressive therapy administered to patients with chronic inflammatory disease, such as psoriasis, has been proven to be a cause of QFT indeterminate results [28]. Indeterminate QFT results have not been recorded in this study population, either at the baseline, before starting the therapy, or after 1 year of treatment. Discordances between QFT results at baseline and follow-up pose a major clinical problem.
Dynamic QFT responses were evident during active biologic drug administration and after isoniazid chemoprophylaxis. Without a reference standard for LTBI diagnosis, the clinical significance of results from different tests is unknown. If a patient is recognized as a false-positive LTBI case, interruption of biologic therapy and unnecessary chemoprophylaxis with the risk of drug toxicity would be avoided. In the present study, patients with positive QFT at baseline showed IFN-gamma concentrations ranging between 7.92 and 30.0 IU/mL.
Patients who were QFT positive at baseline received isoniazid chemotherapy, and showed a lower value of IFN-gamma when retested after 1 year; noticeably four of these patients had negative QFT results. Interestingly, these latter patients had baseline IFN-gamma values below 10.0 IU/mL, whereas the IFN-gamma levels in patients who maintained positive results were >10.0 IU/mL (Table 2). These results are in agreement with those obtained by Kazue et al. [29], who observed that IFN-gamma responses significantly decrease after therapy. It was also observed that patients with higher values of IFN-gamma had a generalized decrease of their QFT value of approximately 11.0 IU/mL after 1 year of treatment. Interestingly, all patients in whom a reversion of QFT was observed at follow-up had received etanercept therapy.
Concerning those patients with a QFT conversion after 1 year, but having a negative result after a further 2–3 months, the low IFN-gamma values observed at 1 year follow-up (ranging between 0.35–1.0 IU/mL), suggest that they could probably represent false-positive results. These patients, in fact, presented a QFT reversion after a short period of time without receiving isoniazid chemotherapy. The causes of these transiently positive results remained unclear as these patients did not apparently differ from the others regarding age, therapy, or clinical conditions.
To reduce QFT variability and decrease the number of false-positive test results, the authors observed that it could be useful to consider a “grey zone,” ranging from 0.35 to 1.0 IU/mL before giving a definitive positive result. Although more prospective data are needed, the authors have adopted a strategy of retesting positive QFTs with a low IFN-gamma level in patients with psoriasis vulgaris. Therefore, these patients are categorized in the “grey zone,” introducing a period of accurate clinical observation, with evaluation of the TST and radiography results, before starting isoniazid prophylaxis. Indeed, causes of variability in serial IGRA testing could also be intrinsic to the assay or due to variability in the immune response. Potential causes of intra-assay variability include improper collection, storage, incubation, and processing of blood tubes, and variation of the IFN-gamma ELISA measurement, which is performed in 96-well plates [20]. Potential sources for variable immune responses include medications, stress, and infections.
Immunosuppressive drug treatment in autoimmune disease patients are also known to cause a decrease in the production of IFN-gamma, which, in turn, could reduce the accuracy of QFT testing [30]. Hypoproteinemia and hypoalbuminemia are also risk factors for secondary immunodeficiency, which results in decreased T cell production and functional activity [31].
Several studies using the previous generation of the QFT assay reported that age and extrapulmonary TB may also cause false-negative QFT results [25, 32].
The 42 patients with negative QFT at baseline in the present study remained negative at follow-up. As in vitro T cell responses to QFT antigens are diminished in the presence of biologic drugs [33], biological therapy could represent a risk factor for false-negative QFT. Therefore, careful interpretation of negative QFT results is necessary and an annual chest radiograph for at-risk patients on biologic therapy should be considered [34].
In conclusion, the present study showed dynamic QFT responses in psoriasis vulgaris patients undergoing long-term biologic therapy. More prospective data are needed to better define the predictive value of positive and transiently positive QFT assays, but the authors’ direct experience suggests that a QFT-positive assay with a low IFN-gamma level (0.35–1.0 IU/mL) should be reconsidered before assuming it is a definitive positive QFT result and commencing isoniazid chemoprophylaxis. The value of 1.0 IU/mL could be considered as a threshold value, an indication to further evaluate questionable positive results, but it should not be considered as a cut-off value. This study does not validate a new reference interval, but instead presents the authors’ experience regarding multiple QFT testing.
Definitively, it was observed that positive values of QFT <1.0 IU/mL were not confirmed as positive when retested after a short time period without chemoprophylaxis, and it was also observed that all patients with a QFT reversion were treated with etanercept. However, this hypothesis needs further investigation in future studies with an enlarged study population.
References
Carmona L, Gómez-Reino JJ, Rodríguez-Valverde V, et al. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients with tumor necrosis factor antagonists. Arthritis Rheum. 2005;52:1766–72.
González-Salazar F, Vargas-Villarreal J, Rivera G, et al. Snapshot of Quantiferon TB gold testing in Northern Mexico. Tuberculosis (Edinb). 2011;91:S34–7.
Leung EC, Leung CC, Leung WW, et al. Role of whole-blood interferon-gamma release assay in the diagnosis of smear negative tuberculosis. Int J Tuberc Lung Dis. 2010;14:1564–70.
Chiappini E, Fossi F, Bonsignori F, Sollai S, Galli L, de Martino M. Utility of interferon-gamma release assay results to monitor anti-tubercular treatment in adults and children. Clin Ther. 2012;34:1041–8.
Bautista J, Banaei N. Sensitivity of QuantiFERON-TB GOLD In-Tube for diagnosis of recent versus remote M. tuberculosis infection. Diagn Microbiol Infect Dis. 2012;73:257–9.
Sotgiu G. Diagnosis and treatment of latent tubercular infections. G Ital Med Lav Ergon. 2010;32:264–8.
Mazurek GH, LoBue PA, Daley CL, et al. Comparison of a whole blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA. 2001;286:1740–7.
Panés J, Gomollón F, Taxonera C, Hinojosa J, Clofent J, Nos P. Crohn’s disease: a review of current treatment with focus on biologics. Drugs. 2007;67:2511–37.
Mínguez S, Latorre I, Mateo L, et al. Interferon-gamma release assays in the detection of latent tuberculosis infection in patients with inflammatory arthritis scheduled for anti-tumor necrosis factor treatment. Clin Rheumatol. 2012;31:785–94.
Lalvani A, Nagvenkar P, Udwadia Z, et al. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J Infect Dis. 2001;183:469–77.
Connell TG, Curtis N, Ranganathan SC, Buttery JP. Performance of a whole blood interferon gamma assay for detecting latent infection with Mycobacterium tuberculosis in children. Thorax. 2006;61:616–20.
Domínguez J, Latorre I, Altet N, et al. Interferon-gamma-release assays to diagnose TB infection in immunocompromised individual. Expert Rev Respir Med. 2009;3:309–27.
Ravn P, Munk ME, Andersen AB, et al. Prospective evaluation of a whole-blood test using Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10 for diagnosis of active tuberculosis. Clin Diagn Lab Immunol. 2005;12:491–6.
Ruhwald M, Dominguez J, Latorre I, et al. A multicentre evaluation of the accuracy and performance of IP-10 for the diagnosis of infection with M. tuberculosis. Tuberculosis (Edinb). 2011;91:260–7.
Lalvani A, Millington KA. Screening for tuberculosis infection prior to initiation of anti-TNF therapy. Autoimmun Rev. 2008;8:147–52.
Domínguez J, Latorre I. Role of the T-cell interferon-gamma release assays in preventing reactivation of latent tuberculosis infection in immunosuppressed patients in treatment with anti-TNF agents. J Crohns Colitis. 2008;2:250–4.
Mancuso JD, Mazurek GH, Tribble D, et al. Discordance among commercially available diagnostics for latent tuberculosis infection. Am J Respir Crit Care Med. 2012;185:427–34.
Centers for Disease Control and Prevention. Controlling tuberculosis in the United States: recommendations from the American Thoracic Society, CDC, and the Infectious Disease Society of America. MMWR. 2005;54:1–81.
Mori T, Sakatani M, Yamagishi F, et al. Specific detection of tuberculosis infection: an interferon-gamma based assay using new antigens. Am J Resp Crit Care Med. 2004;170:59–64.
QuantiFERON-TB Gold In-Tube assay (package insert). Valencia: Cellestis Inc.; 2009.
Sterry W, Barker J, Boheneke WH, et al. Biological therapies in the systemic management of psoriasis: International Consensus Conference. Br J Dermatol. 2004;151:3–17.
Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177–84.
Diel R, Goletti D, Ferrara G, et al. Interferon-gamma release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur Respir J. 2011;37:88–99.
Pathirana D, Nast A, Ormerod AD, et al. On the development of the European S3 guidelines on the systemic treatment of psoriasis vulgaris: structure and challenges. J Eur Acad Dermatol Venereol. 2010;24:1458–67.
Kobashi Y, Mouri K, Yagi S, et al. Clinical utility of QuantiFERON TB-2G test for elderly patients with active tuberculosis. Chest. 2008;133:1196–202.
Laffitte E, Janssens JP, Roux-Lombard P, et al. Tuberculosis screening in patients with psoriasis before antitumour necrosis factor therapy: comparison of an interferon-gamma release assay vs. tuberculin skin test. Br J Dermatol. 2009;161:797–800.
Chiu H, Hsueh P, Tsai T. Clinical experience of QuantiFERON(®)-TB Gold testing in patients with psoriasis treated with tumour necrosis factor blockers in Taiwan. Br J Dermatol. 2011;164:553–9.
Helwig U, Müller M, Hedderich J, Schreiber S. Corticosteroids and immunosuppressive therapy influence the result of QuantiFERON TB Gold testing in inflammatory bowel disease patients. J Crohns Colitis. 2012;6:419–24.
Kazue H, Nobuyuki H, Toru M. Interferon-gamma responses after isoniazid chemotherapy for latent tuberculosis. Respirology. 2008;13:468–72.
Ashwell JD, Lu FW, Vacchio MS. Glucocorticoids in T cell development and function. Annu Rev Immunol. 2000;18:309–45.
Chinen J, Shearer WT. Secondary immunodeficiency, including HIV infection. J Allergy Clin Immunol. 2010;125:S195–203.
Dewan PK, Grinsdale J, Kawamura LM. Low sensitivity of a whole blood interferon-gamma release assay for detection of active tuberculosis. Clin Infect Dis. 2007;44:69–73.
Hamdi H, Mariette X, Godot V, et al. Inhibition of anti-tuberculosis T-lymphocyte function with tumour necrosis factor antagonists. Arthritis Res Ther. 2006;8:R114.
Sivamani RK, Goodarzi H, Garcia MS, et al. Biologic therapies in the treatment of psoriasis: a comprehensive evidence-based basic science and clinical review and a practical guide to tuberculosis monitoring. Clinic Rev Allerg Immunol. 2012 [Epub ahead of print].
Acknowledgments
The page charges were paid for by the IRCCS Galeazzi Orthopaedic Institute, via Galeazzi 4, 20161 Milan. Prof. Drago is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Conflict of interest
Prof. Drago declares he has no conflict of interest. Dr. Nicola declares she has no conflict of interest. Dr. Signori declares she has no conflict of interest. Dr. Palazzi declares she has no conflict of interest. Dr. Garutti declares she has no conflict of interest. Dr. Spadino declares she has no conflict of interest. Dr. Altomare declares he has no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Drago, L., Nicola, L., Signori, V. et al. Dynamic QuantiFERON Response in Psoriasis Patients Taking Long-Term Biologic Therapy. Dermatol Ther (Heidelb) 3, 73–81 (2013). https://doi.org/10.1007/s13555-013-0020-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-013-0020-3