Abstract
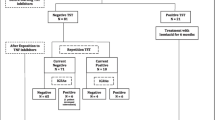
Screening for active tuberculosis (TB) and latent TB infection (LTBI) is mandatory to the initiation of biological therapy in patients with rheumatic diseases. To determine the prevalence of LTBI in patients with rheumatoid arthritis before treatment with biological therapy (anti-TNF, abatacept, and tocilizumab) and the rate of TB conversion during treatment in rheumatoid arthritis (RA) patients, we evaluated the file of 275 patients with RA treated with biological agents. We considered patients with negative baseline TB screening (tuberculin skin test (TST); quantiferon TB gold in tube (QFT-GIT); chest x-ray) and with rescreening for a TB assay every year. Twenty-six patients (10.6%) resulted positive to TB screening at baseline. Two hundred and forty-nine patients (mean age 55.3 ± 11.9; median 55.8 years, range 16–81.9; 210 female) with TB screening negative at baseline were enrolled. One hundred and sixty-eight (67.5%) patients were treated with anti-TNF, 37 (14.9%) patients with abatacept, and 44 (17.7%) patients with tocilizumab. After a period of 12–120 months (median 24), 34 (13.6%) patients displayed conversion of at least one screening assay. Out of the 34 patients with conversion, 6 (16.2%) were treated with abatacept, 7 (15.9%) with tocilizumab, and 21 (12.5%) with anti-TNF. During the follow-up period, no patients developed active TB. Our study shows that a proportion of patients (13.6%) converts at least one TB screening assay during biological therapy. This study underscores the American College of Rheumatology advice for annual screening in some or all biologically treated patients.
Similar content being viewed by others
References
Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO (2004) Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis 38(9):1261–1265
Gómez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD, BIOBADASER Group (2003) Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum 48(8):2122–2127
Carmona L, Gómez-Reino JJ, Rodríguez-Valverde V, BIOBADASER Group et al (2005) Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum 52(6):1766–1772
World Health Organization. Global tubercolosis report (2013) URL: http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf
Souto A, Maneiro JR, Salgado E, Carmona L, Gomez-Reino JJ (2014) Risk of tuberculosis in patients with chronic immune-mediated inflammatory diseases treated with biologics and tofacitinib: a systematic review and meta-analysis of randomized controlled trials and long-term extension studies. Rheumatology (Oxford) 53(10):1872–1885. doi:10.1093/rheumatology/keu172
Singh JA, Furst DE, Bharat A et al (2012) 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 64(5):625–639. doi:10.1002/acr.21641
Vassilopoulos D, Tsikrika S, Hatzara C et al (2011) Comparison of two gamma interferon release assays and tuberculin skin testing for tuberculosis screening in a cohort of patients with rheumatic diseases starting anti-tumor necrosis factor therapy. Clin Vaccine Immunol 18(12):2102–2108. doi:10.1128/CVI.05299-11
Keystone EC, Papp KA, Wobeser W (2011) Challenges in diagnosing latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. J Rheumatol 38(7):1234–1243. doi:10.3899/jrheum.100623
Park JH, Seo GY, Lee JS, Kim TH, Yoo DH (2009) Positive conversion of tuberculin skin test and performance of interferon release assay to detect hidden tuberculosis infection during anti-tumor necrosis factor agent trial. J Rheumatol 36(10):2158–2163. doi:10.3899/jrheum.090150
Sanduzzi A, Bocchino M, Atteno M et al (2012) Screening and monitoring of latent tubercular infection in patients taking tumor necrosis factor-α blockers for psoriatic arthritis. J Rheumatol Suppl 89:82–85. doi:10.3899/jrheum.120252
Hatzara C, Hadziyannis E, Kandili A et al (2015) Frequent conversion of tuberculosis screening tests during anti-tumour necrosis factor therapy in patients with rheumatic diseases. Ann Rheum Dis 74(10):1848–1853. doi:10.1136/annrheumdis-2014-205376
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd (2010) Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62:2569–2581
American Thoracic Society (2000) Targeted tuberculin testing and treatment of latent tubercolosis infection. Am J Respir Crit Care Med 161:5221–5247
Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K, IGRA Expert Committee; Centers for Disease Control and Prevention (CDC) (2010) Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep 59(RR-5):1–25
Nobre CA, Callado MR, Lima JR, Gomes KW, Martiniano GV, Vieira WP (2012) Tuberculosis infection in rheumatic patients with infliximab therapy: experience with 157 patients. Rheumatol Int 32(9):2769–2775. doi:10.1007/s00296-011-2017-5
Marques CDL, Duarte ALBP, Lorena VMB et al (2009) Resposta atenuada ao PPD no diagnostico de infection tuberculosa latente em pacientes com artrite reumatoide. Rev Bras Reumatol 49(2):121–131
Westhovens R, Yocum D, Han J, START Study Group et al (2006) The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo-controlled trial. Arthritis Rheum 54(4):1075–1086
Chen DY, Shen GH, Chen YM, Chen HH, Hsieh CW, Lan JL (2012) Biphasic emergence of active tuberculosis in rheumatoid arthritis patients receiving TNFα inhibitors: the utility of IFNγ assay. Ann Rheum Dis 71(2):231–237. doi:10.1136/annrheumdis-2011-200489
Garcovich S, Ruggeri A, D'Agostino M et al (2012) Clinical applicability of quantiferon-TB-gold testing in psoriasis patients during long-term anti-TNF-alpha treatment: a prospective, observational study. J Eur Acad Dermatol Venereol 26(12):1572–1576. doi:10.1111/j.1468-3083.2011.04220.x
Chen DY, Shen GH, Hsieh TY, Hsieh CW, Lan JL (2008) Effectiveness of the combination of a whole-blood interferon-gamma assay and the tuberculin skin test in detecting latent tuberculosis infection in rheumatoid arthritis patients receiving adalimumab therapy. Arthritis Rheum 59(6):800–806. doi:10.1002/art.23705
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Informed consent
“Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.”
Funding
This study was not funded.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Key messages
1. Screening for active tuberculosis and latent TB infection is mandatory to the initiation of biological therapy.
2. Rate of TB conversion during treatment in rheumatoid arthritis patients.
3. Dual screening and rescreening strategy (TST and QFT-GIT) in RA patients
An erratum to this article is available at http://dx.doi.org/10.1007/s10067-017-3614-9.
Rights and permissions
About this article
Cite this article
Cuomo, G., D’Abrsca, V., Iacono, D. et al. The conversion rate of tuberculosis screening tests during biological therapies in patients with rheumatoid arthritis. Clin Rheumatol 36, 457–461 (2017). https://doi.org/10.1007/s10067-016-3462-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-016-3462-z