Abstract
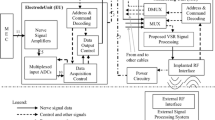
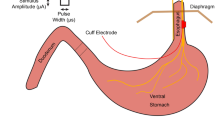
Neural interfaces have great potential to treat disease and disability by modulating the electrical signals within the nervous system. However, whilst neural stimulation is a well-established technique, current neural interfaces are limited by poor recording ability. Low signal amplitudes necessitate the use of highly invasive techniques that divide or penetrate the nerve, and as such are unsuitable for chronic implantation. In this paper, we present the first application of the velocity selective recording technique to the detection of respiration activity in the vagus nerve, which is involved with treatments for epilepsy, depression, and rheumatoid arthritis. Further, we show this using a chronically implantable interface that does not divide the nerve. We also validate our recording setup using electrical stimulation and we present an analysis of the recorded signal amplitudes. The recording interface was formed from a cuff containing ten electrodes implanted around the intact right vagus nerve of a Danish Landrace pig. Nine differential amplifiers were connected to adjacent electrodes, and the resulting signals were processed to discriminate neural activity based on conduction velocity. Despite the average single channel signal-to-noise ratio of − 5.8 dB, it was possible to observe distinct action potentials travelling in both directions along the nerve. Further, contrary to expectation given the low signal-to-noise ratio, we have shown that it was possible to identify afferent neural activity that encoded respiration. The significance of this is the demonstration of a chronically implantable method for neural recording, a result that will transform the capabilities of future neuroprostheses.





Similar content being viewed by others
References
Schuettler M, Donaldson N, Seetohul V, Taylor J. Fibre-selective recording from the peripheral nerves of frogs using a multi-electrode cuff. J Neural Eng. 2013;10:36016. https://doi.org/10.1088/1741-2560/10/3/036016.
Schuettler M, Seetohul V, Rijkhoff NJM, Moeller FV, Donaldson N, Taylor J. Fibre-selective recording from peripheral nerves using a multiple-contact cuff: report on pilot pig experiments. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:3103–6. https://doi.org/10.1109/IEMBS.2011.6090847.
Metcalfe BW, Chew DJ, Clarke CT, Donaldson NN, Taylor JT. A new method for spike extraction using velocity selective recording demonstrated with physiological ENG in rat. J Neurosci Methods. 2015;251:47–55. https://doi.org/10.1016/j.jneumeth.2015.05.003.
Connor DE, Nixon M, Nanda A, Guthikonda B. Vagal nerve stimulation for the treatment of medically refractory epilepsy: a review of the current literature. Neurosurg Focus. 2012;32:E12. https://doi.org/10.3171/2011.12.FOCUS11328.
George MS, Sackeim HA, Rush AJ, Marangell LB, Nahas Z, Husain MM, Lisanby S, Burt T, Goldman J, Ballenger JC. Vagus nerve stimulation: a new tool for brain research and therapy. Biol Psychiatry. 2000;47:287–95. https://doi.org/10.1016/S0006-3223(99)00308-X.
Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, Tracey KJ, Tak PP. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci USA. 2016;113:8284–9. https://doi.org/10.1073/pnas.1605635113.
Nielsen TN, Struijk JJ, Harreby KR, Sevcencu C. Early detection of epileptic seizures in pigs based on vagus nerve activity. In: Pons JL, Torricelli D, Pajaro M, editors. Converging clinical and engineering research on neurorehabilitation. Berlin: Springer; 2013. p. 43–7.
Sevcencu C, Nielsen TN, Kjaergaard B, Struijk JJ. A respiratory marker derived from left vagus nerve signals recorded with implantable cuff electrodes. Neuromodulation Technol Neural Interface. 2017;. https://doi.org/10.1111/ner.12630.
Haugland M. A flexible method for fabrication of nerve cuff electrodes. In: Proceedings of 18th annual international conference of the IEEE engineering in medicine and biology society. IEEE; 1996. p 359–360.
Huang Y, Miller JP. Phased-array processing for spike discrimination. J Neurophysiol. 2004;92:1944–57. https://doi.org/10.1152/jn.00088.2004.
Donaldson N, Rieger R, Schuettler M, Taylor J. Noise and selectivity of velocity-selective multi-electrode nerve cuffs. Med Biol Eng Comput. 2008;46:1005–18. https://doi.org/10.1007/s11517-008-0365-4.
Lorenzi-Filho G, Dajani HR, Leung RS, Floras JS, Bradley TD. Entrainment of blood pressure and heart rate oscillations by periodic breathing. Am J Respir Crit Care Med. 1999;159:1147–54. https://doi.org/10.1164/ajrccm.159.4.9806081.
Seidl AH. Regulation of conduction time along axons. Neuroscience. 2014;276:126–34. https://doi.org/10.1016/j.neuroscience.2013.06.047.
Taylor J, Donaldson N, Winter J. Multiple-electrode nerve cuffs for low-velocity and velocity-selective neural recording. Med Biol Eng Comput. 2004;42:634–43. https://doi.org/10.1007/BF02347545.
Grill WM, Mortimer JT. Electrical properties of implant encapsulation tissue. Ann Biomed Eng. 1994;22:23–33. https://doi.org/10.1007/BF02368219.
Struijk JJ. The extracellular potential of a myelinated nerve fiber in an unbounded medium and in nerve cuff models. Biophys J. 1997;72:2457–69. https://doi.org/10.1016/S0006-3495(97)78890-8.
Chew DJ, Zhu L, Delivopoulos E, Minev IR, Musick KM, Mosse CA, Craggs M, Donaldson N, Lacour SP, McMahon SB, Fawcett JW. A microchannel neuroprosthesis for bladder control after spinal cord injury in rat. Sci Transl Med. 2013;5:210. https://doi.org/10.1126/scitranslmed.3007186.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We wish to declare that we have no commercial affiliations, consultancies, stock or equity interests, or patent-licensing arrangements that could be considered to pose a conflict of interest regarding the submitted manuscript.
Ethical approval
All animal procedures were performed in accordance with the Danish Animal Experiments Inspectorate (approval no. 2013-15-2934-00753), as well as the care and use of laboratory animals as described by the U.S National Institutes of Health.
Rights and permissions
About this article
Cite this article
Metcalfe, B.W., Nielsen, T.N., Donaldson, N.N. et al. First demonstration of velocity selective recording from the pig vagus using a nerve cuff shows respiration afferents. Biomed. Eng. Lett. 8, 127–136 (2018). https://doi.org/10.1007/s13534-017-0054-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-017-0054-z