Abstract
Objective
Rosuvastatin is a drug used for decreasing the risk of cardiovascular complications in type 2 diabetes mellitus (T2DM) patients. It is hypothesized that fetuin-A encourages lipid-induced insulin resistance and sortilin may increase the risk of atherosclerotic-related disorders. The aim of this study is to investigate the safety and efficacy of rosuvastatin co-treatment in T2DM patients and its effect on levels of sortilin and fetuin-A.
Methods
Seventy T2DM patients treated with glimepiride and metformin were randomly assigned to either co-treated with rosuvastatin 10 mg tablets (rosuvastatin group, n = 40), or placebo (placebo group, n = 30) daily for 3 months in a parallel, double-blind randomized controlled trial. Blood was collected for biochemical analysis. Serum sortilin and fetuin-A levels, glycemic and lipid profiles were measured before and 3 months after intervention.
Results
Fasting blood glucose (FBG, mg/dl) significantly decreased in placebo and rousvastatin groups from (104 ± 7.24 to 96.67 ± 7.14 vs 102.8 ± 6.43 to 93.0 ± 4.71), respectively, compared with baseline (p < 0.05). BMI and HbA1c decreased in placebo vs rosuvastatin group (29.20 ± 3.18 to 28.10 ± 3.08, p=0.08 vs 28.67 ± 3.56 to 27.66 ± 3.16, p = 0.27), and (6.59 ± 0.27 to 6.36 ± 0.27 vs 6.56 ± 0.26 to 6.29 ± 0.25), respectively, compared with baseline (p ≤ 0.001) with no significance difference between both groups (p = 0.58 and p = 0.25, respectively). Sortilin and fetuin-A levels significantly decreased in rosuvastatin vs placebo group from (1.77 ± 0.41 to 0.64 ± 0.37 vs 1.70 ± 0.36 to 1.65 ± 0.36) and from (295.33 ± 52.04 to 179.75 ± 60.22 vs 307.22 ± 50.11 to 288.94 ± 49.53), respectively, compared with baseline with significance difference between both groups (p < 0.001) compared with placebo. Significant positive correlation was found between sortilin with fetuin-A, low-density lipoprotein (LDL-C), and atherogenic index (p < 0.001). Significant positive correlation was observed between fetuin-A with FBG (p < 0.05) and atherogenic index (p < 0.001).
Conclusion
Rosuvastatin co-treatment in T2DM patients improves glycemic control and aids in decreasing the atherogenic biomarkers sortilin and fetuin-A levels, so it can be considered tolerable and efficient in improving lipid profile and atherogenic index.
Trial registration
ClinicalTrials.gov identifier (NCT number): NCT03907423, (The registration date: April 9, 2019). https://clinicaltrials.gov/ct2/show/NCT03907423.
Similar content being viewed by others
Introduction
Diabetes mellitus is a metabolic disorder identified by persistent hyperglycemia caused by defects in insulin secretion, insulin action, or both. Diabetes mellitus accounts for approximately 5% of all mortalities worldwide annually [1], as chronic hyperglycemia is linked to long-term harm, dysfunction, and deteriorating of various systems, particularly the nerves, kidneys, heart, eyes, and blood vessels owing to cardiovascular disease (CVD). Cardiovascular mortality and morbidity are significant in most diabetic patients, compared to non-diabetic individuals by a two to fourfold increase [2].
The worldwide costs of diabetes and its outcomes are enormous and will become greater by 2030 [3]. Dyslipidemia is very common in type 2 diabetes mellitus [4]. Diabetic dyslipidemia is a significant risk factor for atherosclerotic cardiovascular disease (ASCVD) [5]. Atherosclerosis is a persistent inflammatory condition of the blood vessels distinguished by intimal agglomeration with cholesterol accumulation and macrophage foam cell percolation resulting in plaque deposition at the affected vessel wall [6]. ASCVD events risk in T2DM can be assessed by LDL cholesterol level [5]. Marked evidence proposes that serum LDL-C is also a predictor of coronary artery disease (CAD) in the DM patients [7]. LDL-lowering treatment with statins is the principal remedy to decrease cardiovascular risk [8]. Rosuvastatin may be preferred in T2DM due to effective lipid profile change, reduced blood sugar variation, and their pleiotropic impacts, so current research will assess whether these rosuvastatin characteristics translated into favorable impacts on atherosclerosis and notable decreases in cardiovascular events [9]. Sortilin is related to a number of pathogenesis-related factors for cardiovascular and metabolic diseases, including atherosclerosis, lipoprotein metabolism, vascular calcification, obesity, insulin resistance, and blood sugar regulation [10]. Sortilin, one of the human vacuolar proteins, actively encourages the ingestion of low‐density lipoprotein molecules into macrophages. After that, foam cells are formed irrespective of the low‐density lipoprotein receptor, and in this way, atherosclerotic plaque constitution is prompted and continues to progress [11]. Some of the clinical findings suggest that sortilin may be a biomarker for cardiovascular risks and also discuss sortilin as an expected drug target [12]. Bourebaba et al. demonstrated that fetuin-A levels are higher in case of metabolic syndrome, and type 2 diabetes, and assumed that fetuin-A as a biomarker for atherosclerosis due to its association with hepatic steatosis and cardiovascular diseases [13]. Serum fetuin-A was also found to be associated with congestive heart diseases (CHD) [14]. Increased fetuin-A plays an important role in the occurrence of insulin resistance [15]. Some studies have expected that it might also be a prognosticate of progressing cardiovascular diseases [16]. Fetuin-A is regarded as a multifunctional protein that is involved in important biological processes, including the control of bone and calcium metabolism and the insulin signaling cascade, according to numerous studies. Additionally, it functions as an inflammatory mediator, protease inhibitor, and atherogenic and adipogenic factor [17].
The aim of this study is to evaluate the safety and efficacy of rosuvastatin on the atherosclerotic biomarkers sortilin and fetuin-A in type 2 diabetes mellitus patients treated with glimepiride and metformin. Our primary outcome is the change in sortilin and fetuin-A levels and to assess tolerability of rosuvastatin in T2DM patients. The secondary outcome is improvement of lipid profile and atherogenic index.
Methods and Materials
Study design
A prospective, parallel, double-blind (patients and investigator) randomized placebo-controlled study was carried on 70 type 2 diabetic patients recruited, between April 2019 through January 2021 from outpatient clinics of Damanhour National Medical Institute, Damanhour, Egypt. The study protocol adhered to the Declaration of Helsinki’s ethical guidelines and was approved by the local Ethical Committee (Faculty of Pharmacy, Damanhour University, Egypt, Code No:219PP11). Also, this study registered on ClinicalTrials.gov by its identifier number: NCT03907423. Subjects agreed to participate in this clinical study and provided informed consent at the beginning of the study. All subjects’ health status was assessed by a complete medical examination.
Patient Selection
Patients selected in the present study fulfilled the following inclusion criteria: type 2 diabetes mellitus on oral treatment, aged 21–65 years and life expectancy > 1 year. Exclusion criteria include active malignancy, chronic inflammation (such as inflammatory bowel disease, lupus, inflammatory arthritis, rheumatoid arthritis), chronic infection (such as chronic diabetic foot infection), documented CVD, planned surgical intervention, hypersensitivity to either of the study drug components, type I diabetes, insulin treatment, hepatic impairment or known hepatic failure, and pregnancy, lactation, or childbearing potential.
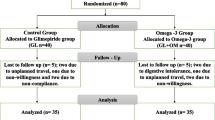
At baseline, 125 type II diabetic patients controlled on metformin-glimepiride combination who fulfilled inclusion criteria were initially evaluated (Fig. 1). According to WHO criteria, diabetes was defined as fasting glucose ≥ 126 mg/dL [18]. Based on exclusion criteria, out of 115, 77 patients were considered eligible for the present study. Eligible patients were randomly assigned using wrapped envelopes method to either receive rosuvastatin 10 mg per day (rosuvastatin group, n = 40) according to the ACC/AHA recommendation [19] or placebo (placebo group, n = 30). All patients in both groups who are included in the study were maintained on oral antidiabetic agents (OAA): glimepiride and metformin. Interventions were provided in closed, matched white containers by an independent person. Distinctive constant diet regimen is applied to all the patients to prevent weight gain or changed diet style to affect the study as confounders or effect modifiers. Clinical and biochemical measurements were done at baseline and after 3 months of interventions.
Demography and anthropometric data collection
A structured data collection sheet was constructed to record all the values studied in our study. All participants underwent physical examination, demographic data collection (age, gender, height, weight), BMI, medication history, co-morbid factors, and lifestyle habits. Body mass index (BMI = weight (kg)/height (m2)) and blood pressure (BP) were recorded. Serum urea and serum creatinine were the laboratory tests chosen for the assessment of renal functions to exclude renal disease patients. SGOT and SGPT were the diagnostic tests chosen to exclude liver problems.
Evaluation of patients’ adherence and medications tolerability
Rosuvastatin and placebo tablets were given out each month, and the subjects’ compliance was evaluated by counting the pills taken. If a patient took less than 90% of the prescribed medication during the research period, they were deemed non-adherent and were excluded from the study. Throughout the study period, participants were also followed up via phone calls and in-person meetings to gauge their adherence and report any drug-related side effects via an adverse effect questionnaire. The patients’ laboratory results and patient sheets were also used to gather the negative impacts. We tracked the start of any cardiovascular outcomes or events, as well as liver toxicity, renal decline, rhabdomyolysis, and muscle soreness.
Blood sampling and biochemical investigation
Blood samples were obtained from subjects after a 10–12-h period overnight fasting then were centrifuged at 4000 rpm for 10 min and immediately serum separated and stored at − 80 °C until assayed. Fasting blood glucose was measured using glucose oxidase method. Glycated hemoglobin (HbA1c %) was determined by ion exchange method. Total cholesterol (TC), triglycerides (TGs), and high-density lipoprotein (HDL-C) were assessed by colorimetry techniques. Low-density lipoprotein (LDL-C) was determined according to Friedewald formula as long as TG is lower than 400 mg/dl [20].
The atherogenic index was determined as (LDL-C/HDL-C) and coronary risk index (CRI) also was estimated as (TC/HDL-C). They are predictor markers of the risk of coronary heart disease and atherosclerosis [21]. Serum sortilin level was determined using human sortilin 1 (SORT1) ELISA (enzyme-linked immunosorbent assay) kit (Sunred Company) and was detected at 0.08–22 ng/mL with a coefficient of variability (CV) < 10% for intra-assay precision and < 12% for inter-assay variation (sensitivity was 0.074 ng/ml). Fetuin-A was determined by using Human FETU-A ELISA kit (Sunred Company) and detected at 8–2000 mg/L with a CV < 10% for intra-assay precision and < 12% for inter-assay variation (sensitivity was 7.115 mg/L).
Calculation of sample size
Sample size was assessed using G*Power software version 3.1.0 (Institut für Experimentelle Psychologie, Heinrich Heine Universität, Dusseldorf, Germany). A total sample size of 64 patients was predicted to have a 95.2% power to detect a medium to large effect size of 0.92 in the outcome assessed.
Statistical analysis
Data were analyzed using software statistical computer package SPSS version 26.0 (SPSS Inc, Chicago, IL, USA). Categorical data were expressed as numbers and percentages and investigated using Chi-square test. Alternatively, Fisher’s exact correction test was used when more than 20% of the cells have expected count less than 5. Normality for continuous data was assessed by the Shapiro–Wilk test. Quantitative data were represented as median, range (minimum and maximum), mean, ± standard deviation. Independent Student t test was used to compare two groups for normally distributed quantitative variable. Paired t test was used for normally distributed quantitative variables, to compare between before and after treatment in the same group. The significance of the obtained results was judged at the 5% level. Pearson coefficient was used to correlate between two normally distributed quantitative variables.
The area under the ROC curve represents the diagnostic performance of the studied biomarkers. The area more than 50% gives acceptable performance and area about 100% is the best performance for the test.
Results
Figure 1 illustrates patient enrollment, randomization, and follow-up throughout the research. Seven patients were withdrawn from the study: one was in the rosuvastatin group owing to non-willingness, on the other hand, in placebo group, two patients due to non-willingness, two patients because of insufficient collected samples, and two patients because of non-compliance. Table 1 shows the baseline characteristics of the studied patients. Seventy patients who completed the study were included in the final analysis using a per-protocol approach. The mean age of placebo and rosuvastatin group was (44.63 ± 9.47 and 45.88 ± 8.76, p = 0.577) years old, respectively, with no significance difference between both groups. At baseline, both groups were comparable in respect to age, associated disease, current medications, sCr, serum urea level, and liver enzymes as illustrated in Table 1. In addition, there was non-significant difference at baseline in BMI, FBG, HbA1c%, lipid profile, CRI, AI, and biological markers (sortilin and fetuin-A) between the two studied groups as demonstrated in Table 2.
Effect on glycemic and lipid profile
The effect of co-administration of rosuvastatin with metformin-glimepiride combination for 3 months on glycemic and lipid profile is shown in Table 2. BMI and HbA1c% decreased to 28.10 ± 3.08 vs 27.66 ± 3.16 (p = 0.58) and 6.36 ± 0.27 vs 6.29 ± 0.25 (p = 0.25), respectively, in placebo vs rosuvastatin group compared with baseline with no significance difference between both groups. Also, FBG decreased to 96.67 ± 7.14 vs 93.0 ± 4.71, respectively, in placebo vs rosuvastatin group compared with baseline with a significant difference between both groups (p = 0.018). It was found that rosuvastatin group showed more decrease in FBG level compared with placebo group after 3 months of intervention as shown in Table 2.
Regarding lipid profile, rosuvastatin co-treatment for 3 months significantly decreased TG (p < 0.001), LDL-C (p = 0.002), TC (p < 0.001), CRI (p < 0.001), and atherogenic index (p < 0.001) but increased HDL-C (p < 0.001), compared with baseline. While placebo group showed significantly decreased TG (p = 0.023), a non-significant decrease in HDL-C (p = 0.19), TC (p = 0.56), and a non-significant increase in LDL-C (p = 0.45), CRI (p = 0.15), and AI (p = 0.12) after 3 months of intervention compared to the baseline. Rosuvastatin co-treatment significantly decreased TG (p < 0.001), LDL (p = 0.005), TC (p = 0.001) CRI (p < 0.001), and atherogenic index (p = 0.001) and increased HDL (p = 0.024) compared with placebo group after 3 months of intervention.
Effect on atherosclerosis biomarkers
Compared to baseline, a significant reduction was found in placebo versus rosuvastatin group regarding sortilin and fetuin-A levels (p < 0.001), respectively. However, rosuvastatin co-treatment showed a better efficacy on these biomarkers (p < 0.001) than placebo group as illustrated in Table 2.
Figure 2 shows a positive association between sortilin with fetuin-A (r = 0.595, p = 0.000). Table 3 shows correlations between measured biomarkers after treatment with rosuvastatin. Significant positive correlation was found between sortilin with LDL-C (p = 0.002) and atherogenic index (p < 0.001). Significant positive correlation was found between fetuin-A with FBG and atherogenic index (p = 0.029). Figure 3 shows the ROC-AUC of biomarkers in the studied groups. Sortilin was the most sensitive (AUC = 0.987, p < 0.001) followed by fetuin-A (AUC = 0.944, p < 0.001).
Tolerability and adverse events
Rosuvastatin tolerability in the studied patients is shown in Table 4. There were no significant differences in tolerability and adverse events among both groups as muscle pain (weakness, tiredness, soreness, or discomfort) happened in 3 patients, (7.5%) of the rosuvastatin group and in one patient (3.3%) of the placebo group. Abdominal pain and dizziness occurred in 5% of rosuvastatin group while occurred in 6.7% and 3.3%, respectively, in placebo group. Cardiovascular events and diabetic retinopathy occurred in one patient (3.3%) of the placebo group. Urticaria occurred in 2.5% patient in rosuvastatin group and 6.7% in placebo group. All adverse events resolved within 2 weeks of treatment. No incidences of rhabdomyolysis, increased liver function enzyme larger than three times the upper limit of normal, decreased renal function, or patient death were reported during the study.
Discussion
This study reveals the effectiveness and tolerability of rosuvastatin co-treatment on biomarkers of atherosclerosis in type 2 diabetic patients. Diabetes is considered a major cause contributing to dyslipidemia [22]. The present study showed that co-administration of rosuvastatin with glimepiride and metformin treatment for 3 months significantly decreased FBG, HbA1c%, and improved lipid profile compared with placebo group, so it can prevent any CVD complication in T2DM patients.
These results were in agreement with Celik and Acbay, study on polycystic ovary syndrome (PCOS) patients [23], that concluded that combination of metformin and rosuvastatin therapy can lead to improved lipid parameters and hyperandrogenism in addition to a better reduction on atherosclerosis-related factors. Consistent with previous studies [24, 25], it is reported that rosuvastatin is effective in modulation of inflammatory biomarkers and improving lipid profile and atherogenic index.
Several previous studies have illustrated a possible effect of statins on glucose metabolism [26,27,28,29]. Consonance with Salunkhe et al. [30], it was demonstrated that rosuvastatin decreases blood glucose via improved insulin sensitivity, consequently, has a positive impact on glucose homeostasis.
Her et al. [31]’s study found that HbA1c levels increased with rosuvastatin 10 mg, and its effect was not different from atorvastatin/ezetimibe 5 mg/5 mg and atorvastatin 20 mg. Another study comparing atorvastatin and rosuvastatin stated that both regimens reduced LDL-C to a similar extent, while rosuvastatin had an HDL-C raising effect significantly greater than atorvastatin [32].
The present study found that rosuvastatin significantly decreases atherogenic index and CRI compared to placebo group after 3 months. These results were in concordant to Kazemi et al. who found a strong correlation between AI and CRI with LDL-C and TC [21].
Similar to our study, Khokhar et al. study enrolled 66 patients who were allocated consecutively for double-blind treatment with 10 mg atorvastatin (n = 33) and 10 mg rosuvastatin (n = 33) for 1 month, illustrating that rosuvastatin was significantly more effective than atorvastatin in its capability to decrease LDL-C [33]. Similarly, a study on 40 subjects illustrated that rosuvastatin should be preferred over atorvastatin in obese T2DM patients [34].
In another study, enrolled T2DM patients found that 16 weeks of rosuvastatin (10 and 20 mg) therapy safely and beneficially alter the entire spectrum of lipoproteins in Indian patients who require to control dyslipidemia [35].
Through the inhibition of HMG-CoA reductase, rosuvastatin appears to be able to lower triglyceride and apolipoprotein B (the main protein in LDL-C) serum levels and to increase high-density lipoprotein cholesterol (HDL-C), most likely through enhancing the production of apolipoprotein A (the primary HDL-C protein), preventing its breakdown, and/or by reducing the activity of the cholesteryl ester transfer protein [36, 37]. Sortilin level was significantly lowered in the current research after intervention with rosuvastatin than placebo. According to Oh et al. [38], individuals with hypercholesteremia or diabetes mellitus have higher sortilin levels than control subjects. Additionally, sortilin levels were shown by Goettsch et al. [39] to be favorably correlated with CVD and to be decreased by statin therapy.
Elevated sortilin levels are crucial risk factors of CAD in Egyptian patients [40]. Consistently a recent study stated that reducing sortilin levels, enhancing glycemic control, and improved insulin resistance in patients with T2DM [41].
Our study found a significant positive correlation between sortilin with LDL-C and AI in the recruited patients after 3 months of intervention. Along with our results, Kjolby et al. found that sortilin-knockout mice have shown decreased level of total cholesterol and LDL-cholesterol along with a decrease in atherosclerotic plaques [42] and vascular calcification [43]. These were in agreement with Simsek et al. who showed a significant positive correlation between serum sortilin with LDL-C and TC levels in subjects suffering from carotid artery disease [6].
It is hypothesized that sortilin may increase the risk of atherosclerotic-related disorders through the expression of proprotein convertase subtilisin/kexin type 9 (PCSK9) causing lower clearance of LDL-C within circulation [11]. In contrast to our findings, a study found that sortilin is associated with cardiovascular risk [44]. Likewise, macrophage sortilin-deficient animals showed a reduction in atherosclerotic lesions as a result of a reduction in LDL uptake by macrophages [45]. In contrast, when sortilin is expressed excessively in the liver, serum LDL-cholesterol levels are reduced [46].
Contrary, Demir et al. found a negative correlation between sortilin levels with TC, LDL-C, and TG while finding a positive correlation between sortilin levels and HDL-C in patients who had just been diagnosed with T2DM, indicating that sortilin may play a role in dyslipidemia in T2DM patients [47].
Our finding revealed that rosuvastatin treatment showed a greater efficacy in the reduction of fetuin-A level than placebo group. Also, we observed a significant positive correlation was found between fetuin-A with FBG, and atherogenic index. To our knowledge, this is the first study evaluating the effect of rosuvastatin on fetuin-A level in type 2 diabetic patients. Though, fetuin-A might be considered as a novel biological marker for the diagnosis of dyslipidemia and other related metabolic disorders [48].
ROC-AUC of biomarkers in our studied patients showed that sortilin was the most sensitive followed by fetuin-A. Moreover, a significant positive correlation was found between fetuin-A and sortilin in the recruited subjects after the 3 months period of treatment with rosuvastatin. In the same context, Liu et al. reported a significant positive correlation between fetuin-A with each of BMI, HOMAIR, TG, TC, and LDL-C [49].
Preclinical and clinical evidence have demonstrated that a high fetuin-A is considered an outstanding early predictor for the diagnosis of various liver-related metabolic disorders like obesity, T2DM, nonalcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and insulin resistance (IR) [48].
Moreover, a previous study by Demirbaş et al. considered fetuin-A as a new risk factor for cardiovascular diseases and also proposed the association between high level of fetuin-A and atherosclerosis. These studies justified its proatherogenic effect by increasing insulin resistance [50].
Fetuin-A is an early predictive biomarker for the detection of IR-associated metabolic diseases by inhibiting the glucose transporter type 4 (GLUT-4) and the insulin receptor substrate (IRS) [51, 52]. Similarly, a recent clinical study showed that elevated serum fetuin-A levels were linked to the development of NAFLD and T2DM because nuclear factor kappa B cells were activated, inhibiting IRS [53].
Consistence with our results, Trepanowski et al. found a clear correlation between high hemoglobin A1c levels and high serum fetuin-A levels, which are linked to high serum glucose levels and the risk of diabetes-related liver disorders [54]. According to Priya et al., T2DM patients who have diabetic retinopathy have greater blood level of fetuin-A than T2DM patients who do not have this complication [55].
Neves et al. study illustrated that elevated serum fetuin-A levels activate toll-like receptor 4 (TLR-4) and Nox1/4 to induce vascular dysfunction, which is a risk factor for metabolic diseases [56].
According to Kadoglou et al., [57] simvastatin treatment for 6 months significantly decreased serum fetuin-A, in patients with CAD compared with healthy controls. Our study outcomes can be explained as rosuvastatin works by inhibiting the enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase leading to slow down the formation of mevalonic acid, which is the rate-limiting step in the synthesis of cholesterol. In addition, this increases the low-density lipoprotein receptors on hepatocyte membranes and stimulates the breakdown of low-density lipoprotein.
Fetuin-A interacts with a wide range of receptors, such as insulin, growth hormones, TGF-β, and toll-like receptors (TLR), to produce these varied effects. Fetuin-A can be a target for the diagnosis and treatment of clinical disorders linked to it as well as a biomarker when taken together [17].
By acting as an endogenous ligand of toll-like receptor 4, fetuin-A encourages lipid-induced insulin resistance. Recently, it is revealed that dysfunctional beta cells release fetuin-A in response to palmitate stimulation [58].
The clinical relevance of this may require further investigation to confirm their findings.
Study limitations
Compared to earlier trials, our study was short duration and had a smaller patient cohort with no healthy control. Thus, larger sample size studies with longer duration are needed.
Conclusions
In summary, our study showed that the co-administration of rosuvastatin with glimepiride and metformin treatment for 3 months in T2DM patients improves lipid profile, atherogenic index (LDL-C/HDL-C), and coronary risk index (TC/HDL-C) which may be attributed due to its synergistic ability to decrease serum sortilin and fetuin-A levels, in addition to its non-cholesterol-lowering effects (anti-inflammatory, antioxidant, and antithrombotic properties). Also, our result could elucidate association between sortilin and fetuin-A with atherogenic and coronary index which may highlight new treatment target in type 2 diabetic patients. Rosuvastatin may be considered as an essential aid for both primary and secondary CV prevention in type 2 diabetic patients. Further larger and longer studies are warranted.
Data Availability
The data are available from the corresponding author on reasonable request.
References
GBD. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2016;390(10100):1345–422. https://doi.org/10.1016/S0140-6736(17)32366-8. (Erratum in: Lancet. 2017; 390(10104):1736. Erratum in: Lancet. 2017; 390(10106): e38).
Hernández C, Candell-Riera J, Ciudin A, Francisco G, Aguadé-Bruix S, Simó R. Prevalence and risk factors accounting for true silent myocardial ischemia: a pilot case-control study comparing type 2 diabetic with non-diabetic control subjects. Cardiovasc Diabetol. 2011;10:9. https://doi.org/10.1186/1475-2840-10-9.
Bommer C, Sagalova V, Heesemann E, et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care. 2018;41(5):963–70. https://doi.org/10.2337/dc17-1962.
Kaze AD, Santhanam P, Musani SK, Ahima R, Echouffo-Tcheugui JB. Metabolic dyslipidemia and cardiovascular outcomes in type 2 diabetes mellitus: findings from the look AHEAD study. J Am Heart Assoc. 2021;10(7): e016947. https://doi.org/10.1161/JAHA.120.016947. (Erratum in: J Am Heart Assoc. 2021;10(14):e020749).
Aguilar-Ballester M, Hurtado-Genovés G, Taberner-Cortés A, Herrero-Cervera A, Martínez-Hervás S, González-Navarro H. Therapies for the treatment of cardiovascular disease associated with type 2 diabetes and dyslipidemia. Int J Mol Sci. 2021;22(2):660. https://doi.org/10.3390/ijms22020660.
Simsek Z, Alizade E, Güner A, Zehir R. Correlation between serum sortilin levels and severity of extracranial carotid artery stenosis. Int J Clin Pract. 2021;75(11): e14733. https://doi.org/10.1111/ijcp.14733.
Shi R, Gao Y, Shen LL, et al. The effect of LDL-C status on the association between increased coronary artery calcium score and compositional plaque volume progression in statins-treated diabetic patients: evaluated using serial coronary CTAs. Cardiovasc Diabetol. 2022;21(1):121. https://doi.org/10.1186/s12933-022-01556-y.
Bahiru E, Hsiao R, Phillipson D, Watson KE. Mechanisms and treatment of dyslipidemia in diabetes. Curr Cardiol Rep. 2021;23(4):26. https://doi.org/10.1007/s11886-021-01455-w.
Tuomilehto J, Leiter LA, Kallend D. A review of the efficacy of rosuvastatin in patients with type 2 diabetes. Int J Clin Pract Suppl. 2004;143:30–40. https://doi.org/10.1111/j.1368-504x.2004.00390.x.
Mitok KA, Keller MP, Attie AD. Sorting through the extensive and confusing roles of sortilin in metabolic disease. J Lipid Res. 2022;63(8): 100243. https://doi.org/10.1016/j.jlr.2022.100243.
Su X, Chen L, Chen X, Dai C, Wang B. Emerging roles of sortilin in affecting the metabolism of glucose and lipid profiles. Bosn J Basic Med Sci. 2022;22(3):340–52. https://doi.org/10.17305/bjbms.2021.6601.
Goettsch C, Kjolby M, Aikawa E. Sortilin and its multiple roles in cardiovascular and metabolic diseases. Arterioscler Thromb Vasc Biol. 2018;38(1):19–25. https://doi.org/10.1161/ATVBAHA.117.310292.
Bourebaba L, Marycz K. Pathophysiological implication of fetuin-A glycoprotein in the development of metabolic disorders: a concise review. J Clin Med. 2019;8(12):2033. https://doi.org/10.3390/jcm8122033.
Zheng J, Huang M, Huang Q, Chen Q, Chen Z. The relationship between fetuin-A and coronary atherosclerotic heart disease (CHD) and CHD-related risk factors: a retrospective study. Medicine (Baltimore). 2021;100(43): e27481. https://doi.org/10.1097/MD.0000000000027481.
Icer MA, Yıldıran H. Effects of fetuin-A with diverse functions and multiple mechanisms on human health. Clin Biochem. 2021;88:1–10. https://doi.org/10.1016/j.clinbiochem.2020.11.004.
Karadeniz H, Güler AA, Koca G, et al. Serum levels of fetuin-A as a novel biomarker for disease activity in patients with Takayasu arteritis and granulomatous polyangiitis. Clin Rheumatol. 2022;41(4):1169–76. https://doi.org/10.1007/s10067-021-06020-y.
Chekol Abebe E, Tilahun Muche Z, Behaile T/Mariam TA, et al. The structure, biosynthesis, and biological roles of fetuin-A: a review. Front Cell Dev Biol. 2022;10:945287. https://doi.org/10.3389/fcell.2022.945287.
Gillett MJ. International Expert Committee report on the role of the A1c assay in the diagnosis of diabetes: Diabetes Care 2009; 32(7): 1327–1334. Clin Biochem Rev. 2009; 30(4):197–200
Kim KJ, Yoon J, Won KH, Lim SW, Chae IH, Lee SY, Kim SW, Kim HS. Assessment of the efficacy of lowering LDL cholesterol with rosuvastatin 10 mg in Four Korean Statin Benefit Groups as per ACC/AHA Guidelines (NewStaR4G). J Clin Med. 2020;9(4):916. https://doi.org/10.3390/jcm9040916.PMID:32230818;PMCID:PMC7230727.
Knopfholz J, Disserol CC, Pierin AJ, et al. Validation of the Friedewald formula in patients with metabolic syndrome. Cholesterol. 2014;2014: 261878. https://doi.org/10.1155/2014/261878.
Kazemi T, Hajihosseini M, Moossavi M, Hemmati M, Ziaee M. Cardiovascular risk factors and atherogenic indices in an Iranian population: Birjand East of Iran. Clin Med Insights Cardiol. 2018;20(12):1179546818759286. https://doi.org/10.1177/1179546818759286.
Thambiah SC, Lai LC. Diabetic dyslipidaemia. Pract. Lab Med. 2021;26:e00248. https://doi.org/10.1016/j.plabm.2021.e00248.
Celik O, Acbay O. Effects of metformin plus rosuvastatin on hyperandrogenism in polycystic ovary syndrome patients with hyperlipidemia and impaired glucose tolerance. J Endocrinol Invest. 2012;35(10):905–10. https://doi.org/10.3275/8371.
Werida R, Khairat I, Khedr NF. Effect of atorvastatin versus rosuvastatin on inflammatory biomarkers and LV function in type 2 diabetic patients with dyslipidemia. Biomed Pharmacother. 2021;135: 111179. https://doi.org/10.1016/j.biopha.2020.111179.
Adams SP, Sekhon SS, Wright JM. Lipid-lowering efficacy of rosuvastatin. Cochrane Database Syst Rev. 2014; 21;2014(11):CD010254. https://doi.org/10.1002/14651858.CD010254.pub2
Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207. https://doi.org/10.1056/NEJMoa0807646.
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. https://doi.org/10.1016/S0140-6736(02)09327-3.
Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentration, in the anglo-Scandinavian cardiac outcomes trial. Lipid lowering arm (ASCOT-LLA): a multicenter randomized controlled trial. Lancet. 2003;361(9364):1149–58. https://doi.org/10.1016/S0140-6736(03)12948-0.
Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495–504. https://doi.org/10.1056/NEJMoa040583.
Salunkhe VA, Mollet IG, Ofori JK, et al. Dual effect of rosuvastatin on glucose homeostasis through improved insulin sensitivity and reduced insulin secretion. EBioMedicine. 2016;10:185–94. https://doi.org/10.1016/j.ebiom.2016.07.007.
Her AY, Kim JY, Kang SM, et al. Effects of atorvastatin 20 mg, rosuvastatin 10 mg, and atorvastatin/ezetimibe 5 mg/5 mg on lipoproteins and glucose metabolism. J Cardiovasc Pharmacol Ther. 2010;15(2):167–74. https://doi.org/10.1177/1074248409357922.
Milionis HJ, Rizos E, Kostapanos M, et al. Treating to target patients with primary hyperlipidemia: comparison of the effects of atorvastatin and rosuvastatin (the ATOROS study). Curr Med Res Opin. 2006;22(6):1123–31. https://doi.org/10.1185/030079906X112462.
Khokhar SA, Farooq Ur Rehman RM, Masood S. Comparison of efficiency between rosuvastatin and atorvastatin in reducing low-density lipoprotein (LDL-C) in patients with diabetes mellitus. J Pak Med Assoc. 2022;72(11):2288–90. https://doi.org/10.47391/JPMA.4823.
Sindhu S, Singh HK, Salman MT, Fatima J, Verma VK. Effects of atorvastatin and rosuvastatin on high-sensitivity C-reactive protein and lipid profile in obese type 2 diabetes mellitus patients. J Pharmacol Pharmacother. 2011;2(4):261–5. https://doi.org/10.4103/0976-500X.85954.
Shah SN, Arneja J. Efficacy of rosuvastatin in achieving target HDL, LDL, triglycerides and total cholesterol levels in type 2 diabetes mellitus (T2DM) with newly diagnosed dyslipidaemia: an open label, nonrandomised, non-interventional and observational study in India. J Assoc Physicians India. 2013;61(10):721-6,732.
Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol. 2003;92(2):152–60. https://doi.org/10.1016/s0002-9149(03)00530-7.
Long SB, Blaha MJ, Blumenthal RS, Michos ED. Clinical utility of rosuvastatin and other statins for cardiovascular risk reduction among the elderly. Clin Interv Aging. 2011;6:27–35. https://doi.org/10.2147/CIA.S8101.
Oh TJ, Ahn CH, Kim BR, et al. Circulating sortilin level as a potential biomarker for coronary atherosclerosis and diabetes mellitus. Cardiovasc Diabetol. 2017;16(1):92. https://doi.org/10.1186/s12933-017-0568-9.
Goettsch C, Iwata H, Hutcheson JD, et al. Serum sortilin associates with aortic calcification and cardiovascular risk in men. Arterioscler Thromb Vasc Biol. 2017;37(5):1005–11. https://doi.org/10.1161/ATVBAHA.116.308932.
Werida RH, Omran A, El-Khodary NM. Sortilin and homocysteine as potential biomarkers for coronary artery diseases. Int J Gen Med. 2021;27(14):6167–76. https://doi.org/10.2147/IJGM.S324889.
El-Khodary NM, Dabees H, Werida RH. Folic acid effect on homocysteine, sortilin levels and glycemic control in type 2 diabetes mellitus patients. Nutr Diabetes. 2022;12(1):33. https://doi.org/10.1038/s41387-022-00210-6.
Kjolby M, Andersen OM, Breiderhoff T, et al. Sort1, encoded by the cardiovascular risk locus 1p13.3, is a regulator of hepatic lipoprotein export. Cell Metab. 2010;12(3):213–23. https://doi.org/10.1016/j.cmet.2010.08.006.
Goettsch C, Hutcheson JD, Aikawa M, et al. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J Clin Invest. 2016;126(4):1323–36. https://doi.org/10.1172/JCI80851.
Ogawa K, Ueno T, Iwasaki T, et al. Soluble sortilin is released by activated platelets and its circulating levels are associated with cardiovascular risk factors. Atherosclerosis. 2016;249:110–5. https://doi.org/10.1016/j.atherosclerosis.2016.03.041.
Patel KM, Strong A, Tohyama J, et al. Macrophage sortilin promotes LDL uptake, foam cell formation, and atherosclerosis. Circ Res. 2015;116(5):789–96. https://doi.org/10.1161/CIRCRESAHA.116.305811.
Musunuru K, Strong A, Frank-Kamenetsky M, et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466(7307):714–9. https://doi.org/10.1038/nature09266.
Demir İ, Yildirim Akan O, Guler A, Bozkaya G, Aslanipour B, Calan M. Relation of decreased circulating sortilin levels with unfavorable metabolic profiles in subjects with newly diagnosed type 2 diabetes mellitus. Am J Med Sci. 2020;359(1):8–16. https://doi.org/10.1016/j.amjms.2019.10.003.
Sardana O, Goyal R, Bedi O. Molecular and pathobiological involvement of fetuin-A in the pathogenesis of NAFLD. Inflammopharmacology. 2021;29(4):1061–74. https://doi.org/10.1007/s10787-021-00837-4.
Liu S, Hu W, He Y, et al. Serum fetuin-A levels are increased and associated with insulin resistance in women with polycystic ovary syndrome. BMC Endocr Disord. 2020;20(1):67. https://doi.org/10.1186/s12902-020-0538-1.
Demirbaş A, Kurtipek GS, Tunçez A, Akyürek F, Demirbaş GU. The role of cystatin-C and fetuin-A in the determination of early atherosclerotic risk in psoriasis patients. Dermatol Ther. 2020;33(6): e13898. https://doi.org/10.1111/dth.13898.
Shim YS, Kang MJ, Oh YJ, Baek JW, Yang S, Hwang IT. Fetuin-A as an alternative marker for insulin resistance and cardiovascular risk in prepubertal children. J Atheroscler Thromb. 2017;24(10):1031–8. https://doi.org/10.5551/jat.38323.
Jirak P, Stechemesser L, Moré E, et al. Clinical implications of fetuin-A. Adv Clin Chem. 2019;89:79–130. https://doi.org/10.1016/bs.acc.2018.12.003.
Filardi T, Panimolle F, Tiberti C, et al. Circulating levels of fetuin-A are associated with moderate-severe hepatic steatosis in young adults. J Endocrinol Invest. 2021;44(1):105–10. https://doi.org/10.1007/s40618-020-01274-w.
Trepanowski JF, Mey J, Varady KA. Fetuin-A: a novel link between obesity and related complications. Int J Obes (Lond). 2015;39(5):734–41. https://doi.org/10.1038/ijo.2014.203.
Priya E, Jayashree K, Senthilkumar GP, Yasir M, Babu KR, Devi TD. Role of fetuin-A and vascular endothelial growth factor in type 2 diabetes mellitus patients without and with retinopathy. Diabetes Metab Syndr. 2019;13(4):2699–703. https://doi.org/10.1016/j.dsx.2019.07.026.
Neves KB, Lopes RA, Strembitska A, et al. Fetuin-a induces vascular dysfunction through toll-like receptor 4 and Nox1/4 activation. Hypertension. 2020;76:AP083. https://doi.org/10.1161/hyp.76.suppl_1.P083.
Kadoglou NP, Kottas G, Lampropoulos S, Vitta I, Liapis CD. Serum levels of fetuin-A, osteoprotegerin and osteopontin in patients with coronary artery disease: effects of statin (HMGCoA-reductase inhibitor) therapy. Clin Drug Investig. 2014;34(3):165–71. https://doi.org/10.1007/s40261-013-0157-y.
Mukhuty A, Fouzder C, Kundu R. Fetuin-A excess expression amplifies lipid induced apoptosis and β-cell damage. J Cell Physiol. 2022;237(1):532–50. https://doi.org/10.1002/jcp.30499.
Acknowledgments
Authors thank medical staff members of Endocrinology and Diabetes Departments, Damanhour National Medical Institute, Egypt, for their help in collecting patient’s data and samples for analysis. Authors also thank Faculty of Pharmacy, Damanhour University for their technical support of our research.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors shared in literature reviewing, study design construction, wrote and revised the manuscript. Eligibility assessment and enrolment of participants were performed by R.A.M.A. Collection of clinical data, following up participants, and laboratory investigations of the collected samples were performed by R.H.W. and O.M.E. All authors read and approved the final manuscript.
The data are available from the corresponding author on reasonable request.
Corresponding author
Ethics declarations
Ethics approval and consent to participate.
The study protocol followed the ethical guidelines of the Declaration of Helsinki and was approved by the local Ethical Committee (Faculty of Pharmacy, Damanhour University, Egypt with Code No:219PP11). Also, this study was registered on ClinicalTrials.gov by its identifier number: NCT03907423, https://clinicaltrials.gov/ct2/show/NCT03907423, (The registration date: April 9, 2019). Subjects agreed to participate in this clinical study and provided informed consent.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Werida, R.H., Elattar, O.M., Abdelghafour, R.A. et al. Effect of rosuvastatin on sortilin and fetuin-A in type 2 diabetic patients: a randomized controlled trial. Int J Diabetes Dev Ctries (2024). https://doi.org/10.1007/s13410-024-01324-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13410-024-01324-6