Abstract
Background and objective
Type 2 diabetes mellitus (T2DM) is caused by insulin resistance or tissue insensitivity to insulin, as well as relative insulin insufficiency. Diabetes that is uncontrolled for an extended period of time is linked to substantial comorbidities and organ damage. The purpose of the current study is to assess the effect of coadministration of omega-3 fatty acids with glimepiride on blood glucose, lipid profile, serum irisin, and sirtuin-1 levels in T2DM patients.
Methods
This clinical trial involved 70 type 2 diabetic patients randomly assigned to glimepiride 3 mg with either omega-3 capsules contained fish oil 1000 mg, 13% of eicosapentaenoic acid (EPA) and 9% docosahexaenoic acid (DHA) (omega-3 group, n = 35) or placebo capsules contained corn oil and linoleic acid (control group, n = 35) daily for three months. Blood samples were obtained at the start of the study and 12 weeks later for biochemical examination of HbA1c%, FBG, fasting insulin, and lipid profile. In addition, the atherogenic index of plasma (AIP) was calculated. Human enzyme-linked immunosorbent assay (ELISA) kits were utilized for assessing serum irisin and sirtuin-1 levels before and after the intervention.
Results
Compared to the control group, omega-3 fatty acids decreased serum fasting blood glucose (FBG, p < 0.001), glycated hemoglobin percent (HbA1C%, p < 0.001), total cholesterol (TC, p < 0.001), triglycerides (TGs, p = 0.006), low density lipoprotein (LDL, p = 0.089), and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR, p = 0.021) after three months of intervention. However, a significant increase was reported in serum irisin and high density lipoprotein (HDL) between both groups after intervention (p = 0.026 and p = 0.007, respectively). The atherogenic index of plasma (AIP) increased in the control group but decreased in the omega-3 group, with significant differences between the two groups (p < 0.001).
Conclusion
The present study found that supplementing with omega-3 fatty acids might dramatically enhance blood irisin levels, as well as improve glycemic control and lipid profile in type 2 diabetes mellitus patients using glimepiride.
Trial Registration
This study is registered on ClinicalTrials.gov under identifier NCT03917940. (The registration date: April 17, 2019).
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic illness in which the patient’s capability to produce sufficient amounts of insulin is diminished or patients display insulin resistance; thus, the capability of insulin to accomplish its normal function becomes disrupted [1]. Physical inactivity and obesity are the foremost risk factors for type 2 diabetes mellitus [1]. The insulin resistance was attributable to an insulin signaling defect that seems to be caused by increased lipid accumulation. Previous studies have proposed a reduction in the number of insulin receptors in muscle, adipose tissue, and liver in obese subjects [2].
Inflammation is recognized as a protective immune response to infections and tissue injury causing the migration of immune cells and plasma proteins to the infection site or tissue damage. Although inflammation is a useful response to the body and may be addressed quickly, uncontrolled inflammatory reactions can result in excessive or long-term tissue damage, leading to the development of acute or chronic inflammatory illnesses [3]. Chronic inflammation, oxidative stress, and decreased mitochondrial activity in skeletal muscle or adipose tissue are all linked to the development of insulin resistance and type 2 diabetes. Consequently, oxidative stress and inflammation reduction, as well as mitochondrial function maintenance, should be therapeutic objectives for insulin resistance and T2DM [4].
Recently, Bostrom et al. [5] have identified irisin, as a bioactive molecule which is known as 'myokine' and could increase energy expenditure by enhanced thermogenesis, promote weight loss, and improve insulin resistance caused by diet.
Previous study has illustrated that inflammatory processes and oxidative stress are suppressed by situin-1 (SIRT1) [6].
Glimepiride, a second-generation sulfonylurea, stimulates insulin release and can be utilized as monotherapy or in combination with other oral hypoglycemic agents or insulin. Glimepiride has a lower risk of hypoglycemia in comparison with other sulfonylureas. It has been shown to have extra-pancreatic effects such as stimulating lipogenesis and glycogenesis [7]. Glimepiride also appears to efficiently control blood glucose levels without weight gain [8].
Several studies in recent years have demonstrated the importance of omega-3 supplementation including docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) when treating various diseases. For instance, in subjects with residual hypertriglyceridemia, the addition of omega-3 fatty acids to atorvastatin improved triglycerides (TG) and non-HDL-C levels in comparison with atorvastatin alone [9]. A recent study suggested that fish oil supplementation is effective in reducing inflammation and improving insulin resistance in obese and T2DM patients independent of weight loss [10]. Several studies reported that DHA and EPA could prevent cardiovascular disease through the anti-inflammatory effect [11]. Moreover, Valle Flores et al. found that supplementation with omega-3 fatty acids significantly reduced the concentrations of inflammation markers in patients with chronic kidney disease (CKD) on hemodialysis [12].
The present study aims to assess the effect of coadministration of omega-3 fatty acids and glimepiride as a hypoglycemic drug on the blood levels of irisin, sirtuin-1, glucose homeostasis, and the lipid profile of type 2 diabetic patients.
The primary outcome in this trial was the improvement of FBG, HbA1C %, lipid profile (total cholesterol (TC), triglycerides (TG), HDL-cholesterol (HDL-C), and LDL-cholesterol (LDL-C)), and atherogenic index of plasma (AIP). Secondary outcomes included the change of serum levels of the measured biomarkers irisin and sirtuin-1 after 12 weeks of intervention.
Patients and methods
Study design
Patients were randomly allocated to either the omega-3 group, managed by glimepiride 3 mg tablets plus omega 3 capsules [1000 mg fish oil, 13% of eicosapentaenoic acid (EPA) and 9% docosahexaenoic acid (DHA)] daily, or the control group, managed by glimepiride 3 mg plus placebo daily for 12 weeks, in a controlled, single-blind parallel clinical study. Placebo capsules contained corn oil, linoleic acid and were identical in shade and taste with omega 3 fish oil capsules produced by the Arab Company for Gelatin and Pharmaceutical products, El-Amreya, Alexandria, Egypt. Participants had to be willing to participate, be 30–60 years old, had T2DM for at least 2 years, have HbA1C score of more than 7% [13], and have a body mass index of 25 to 35 kg/m2. Patients were instructed to stop using any dietary supplements at least two weeks before the start of the study and for the duration of the intervention. Exclusion criteria included; using omega-3 supplements in the three months before the start of the study, having chronic renal failure, a hepatic disorder, gastrointestinal or inflammatory disease, or a thyroid disorder, being on thiazolidinediones, warfarin, fibrates, or insulin therapy or needing insulin based on a physician's recommendation, having diabetic comorbidities including micro- and macrovascular problems, being pregnant or nursing, and having a history of drug misuse. During the research period, all individuals were advised to maintain their regular levels of physical activity and nutritional intake. All participating patients provided informed consent. The study was authorized by the Research Ethics Committee of the Faculty of Pharmacy at Damanhour University (Ref. no. 1018PP5) and registered on ClinicalTrials.gov as NCT03917940. The study followed CONSORT criteria and was carried out in conformity with the ethics standards outlined in the Declaration of Helsinki in 1964 and its subsequent revisions, or similar ethics standards.
Patients and biochemical analyses
This research included 80 T2DM patients (31 men and 49 women) randomly assigned in a 1:1 ratio to one of two groups: the control group (glimepiride + placebo) or the omega-3 group (omega-3 fatty acids + glimepiride). Since the randomization was done by a study-independent individual using a computerized random number technique in Microsoft Excel, the patients would be unaware of the group to which they have been allocated. At the beginning of the study, the weight and height of all patients were taken using a measuring scale, and body mass index (BMI) was calculated using the following formula: weight (in kilograms)/(height × height) (in meters) [14].
Blood samples were collected in the morning, after an overnight fast, and centrifuged at 3,000 rpm for 10 min at room temperature for serum separation. The fasting serum samples were stored at –80 °C until analysis.
The enzymatic colorimetric approach was utilized for measuring serum triglycerides [15] and total cholesterol [16]. The precipitation technique [16] was used to determine high-density lipoprotein cholesterol (HDL-C). Low-density lipoprotein cholesterol (LDL-C) was estimated using the Friedewald formula [17]: LDL = [TC—HDL—(TG/5)] if the TG level was less than 400 mg/dL. The homeostasis model assessment (HOMA) online calculator was utilized for calculating insulin resistance (IR) by the following equation: (Fasting insulin, uIU/mL)X (Fasting glucose, mg/dL) / 405 [18]. The log (TG/HDL-C) method was used to calculate the atherogenic index of plasma (AIP) according to Dobiasova and Frohlich [19]. The enzyme-linked immunosorbent assay (ELISA) kit (Sunred Biological Technology, Shanghai, China: Catalogue No.: 201–12-5328 and 201–12-2558, respectively) was utilized for assessing irisin and SIRT1 levels according to the manufacturer’s directions.
The patients' adherence was evaluated using a pill count and was calculated by subtracting the number of pills left in the bottle from the total number of pills supplied, during the patient-scheduled refill. The predicted number of pills consumed by participants was determined by multiplying the daily dose by the number of days since the pills were supplied. The number of pills consumed was then divided by the total number of pills at the start, and the result was multiplied by 100 to calculate the percent of pill count adherence [20]. We classified 95–100% of pills taken during the follow-up session as good adherence while less than 95% of medicine taken was considered poor adherence, hence excluded from the study. Clinical pharmacist interviewed participants weekly and asked about any specific complaints or side effects known to occur with their treatment using safety monitoring checklist.
Statistical analysis
The sample size was assessed using G*Power software version 3.1.0 (Institut fur Experimentelle Psychologie, Heinrich Heine Universitat, Dusseldorf, Germany). A total sample size of 70 patients was determined to have a power of 95% to notice a medium to large effect size of 0.88 in the measured outcomes. The obtained data were loaded into a computer and analyzed with the IBM SPSS software package version 25.0 (Armonk, NY: IBM Corp.). The Kolmogorov–Smirnov and Shapiro–Wilk tests were utilized to identify the normality of the data. Variables not having a normal distribution are presented as the median (range), and they were analyzed using the Wilcoxon signed ranks test to compare before and after the intervention, and the Mann–Whitney test was used to compare between the two groups. Continuous normal distributed variables were presented as Mean ± Standard Deviation (SD) and were assessed using paired t-test, to determine the difference between baseline and three months after treatment, and independent t-test, used to compare between groups. Spearman's rank correlation coefficient was utilized to evaluate the correlation between variables. In order to describe the predicted accuracy of different markers, a receiver operating characteristic (ROC) curve was drawn and the area under the ROC curve was determined. All analyses were conducted on an intention-to-treat (ITT) basis. P ≤ 0.05 was chosen as the significance level for the reported results.
Results
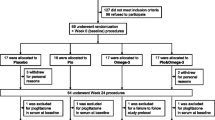
The enrollment and follow-up process of the participants are demonstrated in Fig. 1. A total of 80 patients were recruited initially, but only 70 patients completed the study successfully with no changes to their diet or the level of physical activity throughout the study duration. Ten patients withdrew from the study: two due to digestive intolerance to the supplement, one due to unplanned travel, and two due to non-willingness in omega-3 group, while in control group 5 patients was lost to follow-up; two due to unplanned travel, one due to non-willingness, and two due to non-compliance. Accordingly, 70 patients were included in the final analysis: 35 patients in each group.
Patients’ baseline data and characteristics are summarized in Table 1. The mean age of enrolled patients was 52.42 ± 7.64 years in the control group versus 50.51 ± 8.42 years in the omega-3 group, with no significant difference between both groups. The most common recorded associated diseases in the medical history of participants in the control group compared to the omega-3 group were hypertension (34.3% versus 37.1%, respectively) followed by dyslipidemia (17.1% versus 5.7%, respectively), with no significant difference between both groups as shown in Table 1.
No significant difference was observed regarding the co-administered medications between both groups as shown in Table 1. There were no statistically significant differences observed in all the baseline characteristics between the two groups except for AIP. At the end of the study, there was a reduction in the levels of FBG [143 (110–350) to 140 (110–320) versus 145(90–214) to 118(70–158), p < 0.001], HbA1c % [8.38 ± 0.93 to 8.09 ± 1.04 versus 8.53 ± 1.19 to 6.82 ± 0.84, p < 0.001],HOMA-IR [5.3 (3–14.5) to 4.5 (2.8–13.4) versus 4.1 (1.8–11.6) to 3.8 (1.7 – 7), p = 0.021], TC [212.1 ± 31.76 to 204.7 ± 32.61 versus 198.0 ± 30.06 to 145.1 ± 25.89, p < 0.001], LDL [116.2 ± 13.05 to 111.9 ± 12.35 versus 121.2 ± 18.23 to 105.6 ± 17.50, p = 0.089] and TGs [171.5 ± 26.09 to 165.2 ± 23.63 versus 177.0 ± 23.41 to 146.8 ± 29.76, p < 0.006] in the patients of the control group in comparison with the patients receiving omega 3 plus glimepiride in omega-3 group, respectively as shown in Table 2. A significant increase in the levels of HDL [47.69 ± 8.79 to 47.57 ± 8.25 versus 43.60 ± 11.79 to 53.94 ± 10.76, p = 0.007] and irisin [3.8(0.5–14.5) to 3.9(1.5–13.1) versus 3.5(0.9–20.2) to 4.7 (1.9 – 37.6), p = 0.026] was observed in the control group in comparison with the omega-3 group, respectively (Fig. 2). Meanwhile, a non-significant difference was found in the level of sirtuin-1 when comparing both groups despite the significant increase in sirtuin-1 level in the omega-3 group after intervention in comparison with baseline (p = 0.04) as shown in Table 2 and Fig. 3. The atherogenic index of plasma (AIP) increased in the control group and decreased in the omega-3 group in comparison with baseline with significant differences between both groups (p < 0.001).
Table 3 shows the impact of baseline characteristics (Gender, age, BMI, HbA1c %, Fasting insulin and AIP) between both groups. On logistic regression analysis and adjustment of the confounders, these confounders showed no significant impact between both groups.
Figure 4 shows the validity (AUC, sensitivity, and specificity) of irisin and sirtuin-1 for predicting worsening HOMA-IR (n = 17) from improved HOMA-IR (n = 53) before treatment. Sirtuin-1 was the most sensitive (AUC = 0.613, p = 0.54) followed by irisin (AUC = 0.536, p = 0.16).
Regarding tolerability of the used medications during the presented study, there were no reported significant adverse events or complaints among participants in both groups.
Discussion
The present study aims to assess the effect of coadministration of glimepiride as a hypoglycemic drug and omega-3 fatty acids on the blood levels of irisin, sirtuin-1, glucose homeostasis, and lipid profile of type 2 diabetic patients.
Our results revealed no significant difference in the case of body mass index between both studied groups after 3 months of intervention, which seems in accordance with some previous findings [21].
In our study, we found that FPG, HOMA-IR, and HbA1C levels were reduced in the omega-3 group when compared to the control group, suggesting enhanced insulin sensitivity in the studied diabetic patients. This suggests that the consumption of omega-3 fatty acids supplements could improve glycemic control, FPG, HbA1c %, and HOMA-IR, in T2DM patients, which seems in accordance with some previous findings [21, 22]. Induction of Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) by omega-3 fatty acids has been linked to increased glucose transport and insulin sensitivity through glucose transporter 4 (GLUT4) [23]. Omega-3 fatty acids can regulate metabolism and other cell and tissue responses, including adipocyte differentiation and inflammation through activating peroxisome proliferator-activated receptor (PPAR) and inhibiting NFκB. This mechanism might explain the ability of omega-3 fatty acids to increase insulin sensitivity and reduce inflammation [24].
The current study revealed no change in plasma insulin in response to omega 3. Our results agree with those of Mostad et al., [25] who could not find better fasting insulin concentrations after omega-3 supplementation. Furthermore, our results are in line with those reported by Sirtori et al. [26], who found no significant changes in serum insulin levels between the treatment and control groups [26]. On the contrary, Mori et al. reported that both EPA and DHA significantly increased fasting insulin in mildly hyperlipidemic men [27].
The present study indicated a significant reduction in total cholesterol (TC) after omega-3 fatty acids supplementation, which seems in accordance with some previous findings [21].
Previous studies seem to be compatible with our current result, revealing that triglyceride levels and AIP decreased effectively among patients receiving n–3 fatty acids [28, 29]. Some studies reported that triglyceride levels were markedly reduced among patients receiving omega-3 fatty acids in comparison to those receiving a placebo [30]. The present results demonstrated a significant reduction in TG levels and an increase in HDL-C levels (p = 0.006 and p = 0.007, respectively). Consistent with our results, Kesavulu et al. [31] reported that TG levels significantly decreased, and HDL-C levels significantly increased with the combined treatment with omega-3 fatty acids in diabetic patients. The reported mechanisms by which omega-3 fatty acids reduce TG levels are through the stimulation of fatty acid oxidation, resulting in the reduction of fatty acids substrate for triglyceride synthesis [11]. Furthermore, omega-3 fatty acids can increase plasma lipolytic activity and enhance clearance of plasma TG [32].
Although both groups showed a significant reduction in LDL after 12 weeks, changes in LDL did not differ significantly between groups. Consistent with our results, Eftekhari et al. found that omega-3 fatty acids did not have any significant effect on serum LDL [33]. On the contrary, Agh et al. [34] demonstrated that serum LDL decreased in participants with omega-3 fatty acids supplementation.
The current study indicated that omega-3 supplementation resulted in an increased serum irisin level of type 2 diabetic patients after the intervention between both groups. The results of a study on human rhabdomyosarcoma cells have shown that treatment with omega-3 fatty acids for 24 and 48 h leads to increased expression of the irisin level [23]. Consistent with our results, Agh et al. found that serum irisin levels increased significantly after omega-3 fatty acids supplementation in male patients with coronary artery disease (CAD) [34]. In addition, Ansari et al. [1] revealed that omega-3 supplementation could significantly increase serum irisin levels in type 2 diabetic patients. Treatment with omega-3 fatty acids has been shown to increase the expression of peroxisome proliferator-activated receptor co-activator 1 alpha (PGC-1α) in white adipocytes. PGC-1α increases fatty acid oxidation through the induction of peroxisome proliferator-activated receptor alpha (PPARα). Irisin is induced by PGC-1α expression and increases metabolic rate through activating uncoupling protein 1 (UCP1) in mitochondria [23]. This process increases energy expenditure and improves metabolic parameters including insulin sensitivity [35]. According to a recent study, individuals with chronic renal failure who are receiving hemodialysis may benefit clinically from omega 3 by having higher levels of the protective vascular calcification inhibitors: fetuin-A and osteoprotegerin [36]. Accordingly, physical activity stimulates the transcriptional regulator peroxisome proliferator-activated receptor-coactivator 1 (PGC-1), promoting the expression and proteolytic cleavage of fibronectin type III domain-containing protein 5 (FNDC5) with the release of the irisin in the blood flow. This in turn promotes a browning of white adipose tissue by increasing the expression of mitochondrial uncoupling protein 1 (UCP1) [5].
Another study stated that irisin promotes cell proliferation, as well as insulin production and secretion. Increased circulating irisin has been linked to better glucose tolerance and weight loss [37, 38]. These findings seem to agree with a previous study which displayed that irisin enhanced β cell generation and improved insulin activity in mice [39].
Although serum sirtuin-1 level increased significantly in the omega-3 group, the changes in serum sirtuin-1 level did not differ significantly between groups in the present study. In line with our study, the addition of omega-3 fatty acids in a study on male Sprague–Dawley rats conducted by Son et al. resulted in a relative increase in SIRT1 expression, but this difference was not statistically significant compared to placebo group [40]. Another study found that after six weeks of therapy, ligarglutide dramatically boosted SIRT-1 expression, and this effect persisted for an additional six weeks beyond the end of the regimen. Additionally, they observed a further decline in total cholesterol, ceruloplasmin, fasting glucose, BMI, and HbA1c [41]. Situin-1 (SIRT1) inhibits oxidative stress and inflammatory processes by interfering with nuclear factor kappa-B (NF-κB) signaling by deacetylation of the NF-κB p65 subunit and regulation of its transcriptional activity [6]. SIRT1 has the ability to control PGC-1α deacetylation, which activates peroxisome proliferator-activated receptor-α (PPAR-α), leading to enhanced insulin resistance and increased fatty acid oxidation [4]. Given that SIRT-1 is implicated in the epigenetic regulation of pathogenic pathways in several disorders, including type 2 diabetes [42, 43]. Consistently, given the bidirectional relationship between kidney and cardiovascular events in diabetic patients, antidiabetic therapies with a positive impact on renal and cardiovascular outcomes will play a critical role in reshaping the way diabetes complications are managed [44, 45].
Conclusion
The current study illustrated that omega-3 fatty acids supplementation could significantly increase serum irisin levels and showed better glycemic control and improved lipid profile and insulin sensitivity in type 2 diabetec patients.
Study limitations
The current study has some limitations. The main limitation is its relatively small sample size. Other limitations include the intervention duration which was short to understand the real effects of omega-3 fatty acids supplementation and being single blind, controlled study. Hence, further double-blind controlled studies should be carried out on a large scale and for a longer duration to confirm our research findings.
Availability of data and materials
The data are available from the corresponding author upon reasonable request.
References
Ansari S, Djalali M, Mohammadzadeh Honarvar N, et al. The effect of n-3 polyunsaturated fatty acids supplementation on serum irisin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Int J Endocrinol Metab. 2017;15(1):e40614. https://doi.org/10.5812/ijem.40614.
Ighbariya A, Weiss R. Insulin resistance, prediabetes, metabolic syndrome: what should every pediatrician know? J Clin Res Pediatr Endocrinol. 2017;9(Suppl 2):49–57. https://doi.org/10.4274/jcrpe.2017.S005.
Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. https://doi.org/10.1038/sigtrans.2017.23.
Kitada M, Ogura Y, Monno I, Koya D. Sirtuins and type 2 diabetes: role in inflammation, oxidative stress, and mitochondrial function. Front Endocrinol (Lausanne). 2019;27(10):187. https://doi.org/10.3389/fendo.2019.00187.
Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–8. https://doi.org/10.1038/nature10777.
Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23(12):2369–80. https://doi.org/10.1038/sj.emboj.7600244.
Basit A, Riaz M, Fawwad A. Glimepiride: evidence-based facts, trends, and observations (GIFTS). [corrected]. Vasc Health Risk Manag. 2012;8:463–72. https://doi.org/10.2147/HIV.S33194.
Davis SN. The role of glimepiride in the effective management of type 2 diabetes. J Diabetes Complications. 2004;18(6):367–76. https://doi.org/10.1016/j.jdiacomp.2004.07.001.
Jun JE, Jeong IK, Yu JM, et al. Efficacy and safety of omega-3 fatty acids in patients treated with statins for residual hypertriglyceridemia: a randomized, double-blind, Placebo-Controlled Clinical Trial. Diabetes Metab J. 2020;44(1):78–90. https://doi.org/10.4093/dmj.2018.0265.
Souza DR, Pieri BLDS, Comim VH, et al. Fish oil reduces subclinical inflammation, insulin resistance, and atherogenic factors in overweight/obese type 2 diabetes mellitus patients: a pre-post pilot study. J Diabetes Complications. 2020;34(5):107553. https://doi.org/10.1016/j.jdiacomp.2020.107553.
Kromhout D, Yasuda S, Geleijnse JM, Shimokawa H. Fish oil and omega-3 fatty acids in cardiovascular disease: do they really work? Eur Heart J. 2012;33(4):436–43. https://doi.org/10.1093/eurheartj/ehr362.
Valle Flores JA, Fariño Cortéz JE, Mayner Tresol GA, Perozo Romero J, Blasco Carlos M, Nestares T. Oral supplementation with omega-3 fatty acids and inflammation markers in patients with chronic kidney disease in hemodialysis. Appl Physiol Nutr Metab. 2020;45(8):805–11. https://doi.org/10.1139/apnm-2019-0729.
International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–34. https://doi.org/10.2337/dc09-9033.
Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. Int J Epidemiol. 2014;43(3):655–65. https://doi.org/10.1093/ije/dyu058.
Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19(5):476–82.
Allian CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20(4):470–5.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. https://doi.org/10.1007/BF00280883. PMID: 3899825.
Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin Biochem. 2001;34(7):583–8. https://doi.org/10.1016/s0009-9120(01)00263-6. PMID: 11738396.
Sangeda RZ, Mosha F, Prosperi M, et al. Pharmacy refill adherence outperforms self-reported methods in predicting HIV therapy outcome in resource-limited settings. BMC Public Health. 2014;14:1035. https://doi.org/10.1186/1471-2458-14-1035.
Khalili L, Valdes-Ramos R, Harbige LS. Effect of n-3 (Omega-3) polyunsaturated fatty acid supplementation on metabolic and inflammatory biomarkers and body weight in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of RCTs. Metabolites. 2021;11(11):742. https://doi.org/10.3390/metabo11110742.
Sarbolouki S, Javanbakht MH, Derakhshanian H, et al. Eicosapentaenoic acid improves insulin sensitivity and blood sugar in overweight type 2 diabetes mellitus patients: a double-blind randomised clinical trial. Singapore Med J. 2013;54(7):387–90. https://doi.org/10.11622/smedj.2013139.
Vaughan RA, Garcia-Smith R, Bisoffi M, Conn CA, Trujillo KA. Conjugated linoleic acid or omega 3 fatty acids increase mitochondrial biosynthesis and metabolism in skeletal muscle cells. Lipids Health Dis. 2012;11:142. https://doi.org/10.1186/1476-511X-11-142.
Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142(3):592S-599S. https://doi.org/10.3945/jn.111.155259.
Mostad IL, Bjerve KS, Bjorgaas MR, Lydersen S, Grill V. Effects of n-3 fatty acids in subjects with type 2 diabetes: reduction of insulin sensitivity and time-dependent alteration from carbohydrate to fat oxidation. Am J Clin Nutr. 2006;84(3):540–50. https://doi.org/10.1093/ajcn/84.3.540.
Sirtori CR, Crepaldi G, Manzato E, et al. One-year treatment with ethyl esters of n-3 fatty acids in patients with hypertriglyceridemia and glucose intolerance: reduced triglyceridemia, total cholesterol and increased HDL-C without glycemic alterations. Atherosclerosis. 1998;137(2):419–27. https://doi.org/10.1016/s0021-9150(97)00298-0. PMID: 9622285.
Mori TA, Burke V, Puddey IB, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000;71(5):1085–94. https://doi.org/10.1093/ajcn/71.5.1085.
Kabir M, Skurnik G, Naour N, et al. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr. 2007;86(6):1670–9. https://doi.org/10.1093/ajcn/86.5.1670.
Thota RN, Acharya SH, Garg ML. Curcumin and/or omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance and blood lipids in individuals with high risk of type 2 diabetes: a randomised controlled trial. Lipids Health Dis. 2019;18(1):31. https://doi.org/10.1186/s12944-019-0967-x.
Bosch J, Gerstein HC, Dagenais GR, et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367(4):309–18. https://doi.org/10.1056/NEJMoa1203859.
Kesavulu MM, Kameswararao B, Apparao Ch, Kumar EG, Harinarayan CV. Effect of omega-3 fatty acids on lipid peroxidation and antioxidant enzyme status in type 2 diabetic patients. Diabetes Metab. 2002;28(1):20–6.
Jacobson TA. Role of n-3 fatty acids in the treatment of hypertriglyceridemia and cardiovascular disease. Am J Clin Nutr. 2008;87(6):1981S-S1990. https://doi.org/10.1093/ajcn/87.6.1981S. PMID: 18541599.
Eftekhari MH, Aliasghari F, Beigi MA, Hasanzadeh J. The effect of conjugated linoleic acids and omega-3 fatty acids supplementation on lipid profile in atherosclerosis. Adv Biomed Res. 2014;3:15. https://doi.org/10.4103/2277-9175.124644. PMID:24600599;PMCID:PMC3929013.
Agh F, Mohammadzadeh Honarvar N, Djalali M, et al. Omega-3 fatty acid could increase one of myokines in male patients with coronary artery disease: a randomized, double-blind, placebo-controlled trial. Arch Iran Med. 2017;20(1):28–33 PMID: 28112528.
Novelle MG, Contreras C, Romero-Picó A, López M, Diéguez C. Irisin, two years later. Int J Endocrinol. 2013;2013:746281. https://doi.org/10.1155/2013/746281. PMID:24298283;PMCID:PMC3835481.
Werida RH, Abou-Madawy S, Abdelsalam M, Helmy MW. Omega 3 fatty acids effect on the vascular calcification biomarkers fetuin A and osteoprotegerin in hemodialysis patients. Clin Exp Med. 2022;22(2):301–10. https://doi.org/10.1007/s10238-021-00740-w. PMID: 34286397.
Mostafa TM, El-Gharbawy NM, Werida RH. Circulating IRAPe, irisin, and IL-34 in relation to insulin resistance in patients with type 2 diabetes. Clin Ther. 2021;43(7):e230–40. https://doi.org/10.1016/j.clinthera.2021.05.003. PMID: 34103176.
Gizaw M, Anandakumar P, Debela T. A Review on the role of irisin in insulin resistance and type 2 diabetes mellitus. J Pharmacopuncture. 2017;20(4):235–42. https://doi.org/10.3831/KPI.2017.20.029. Retraction in: JPharmacopuncture.2020;23(1):42-43.PMID:30151293;PMCID:PMC6104716.
Liu S, Du F, Li X, Wang M, Duan R, Zhang J, Wu Y, Zhang Q. Effects and underlying mechanisms of irisin on the proliferation and apoptosis of pancreatic β cells. PLoS ONE. 2017;12(4):e0175498. https://doi.org/10.1371/journal.pone.0175498. PMID:28394923;PMCID:PMC5386279.
Son SH, Lee SM, Lee MH, Son YK, Kim SE, An WS. Omega-3 fatty acids upregulate SIRT1/3, activate PGC-1α via deacetylation, and induce Nrf1 production in 5/6 nephrectomy rat model. Mar Drugs. 2021;19(4):182. https://doi.org/10.3390/md19040182. PMID:33810216;PMCID:PMC8066610.
Savchenko LG, Digtiar NI, Selikhova LG, Kaidasheva EI, Shlykova OA, Vesnina LE, Kaidashev IP. Liraglutide exerts an anti-inflammatory action in obese patients with type 2 diabetes. Rom J Intern Med. 2019;57(3):233–40. https://doi.org/10.2478/rjim-2019-0003. PMID: 30901315.
Ling C, Groop L. Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes. 2009;58(12):2718–25. https://doi.org/10.2337/db09-1003. PMID:19940235;PMCID:PMC2780862.
Strycharz J, Rygielska Z, Swiderska E, Drzewoski J, Szemraj J, Szmigiero L, Sliwinska A. SIRT1 as a therapeutic target in diabetic complications. Curr Med Chem. 2018;25(9):1002–35. https://doi.org/10.2174/0929867324666171107103114. PMID: 29110598.
Elnaem MH, Mansour NO, Nahas AF, Baraka MA, Elkalmi R, Cheema E. Renal outcomes associated with the use of non-insulin antidiabetic pharmacotherapy: a review of current evidence and recommendations. Int J Gen Med. 2020;13:1395–409. https://doi.org/10.2147/IJGM.S285191. PMID:33324086;PMCID:PMC7733337.
Berbari AE. Links between chronic kidney disease and cardiovascular disease: a bidirectional relationship In: Cardiorenal Syndrome: Mechanisms, Risk and Treatment. Springer Milan; 2010:3–14. https://doi.org/10.1007/978-88-470-1463-3_1
Acknowledgements
The authors are so thankful to all participants and to the physicians in the Endocrinology Clinics at Damanhour Medical National Institute for their help and recommendations.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The study did not receive any type of grant from funding groups in commercial, public, or non-profit organizations.
Author information
Authors and Affiliations
Contributions
R.H.W., A.R. and M.W.H reviewed the literatures, and constructed the study design. Eligibility assessment and enrolment of participants were performed by Y.N.E. Collection of clinical data and laboratory investigations of the collected samples and data analysis were performed by R.H.W., and A.R. All authors wrote, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki. The study was approved by the Research Ethics Committee of the Faculty of Pharmacy, Damanhour University, Egypt (Ref. no. 1018PP5) before the beginning of the study. This study was registered on ClinicalTrial.gov with code no.: NCT03917940, https://clinicaltrials.gov/ct2/show/NCT03917940 (The registration date: April 17, 2019). All participants have agreed to be included in this clinical study and provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Werida, R.H., Ramzy, A., Ebrahim, Y.N. et al. Effect of coadministration of omega-3 fatty acids with glimepiride on glycemic control, lipid profile, irisin, and sirtuin-1 in type 2 diabetes mellitus patients: a randomized controlled trial. BMC Endocr Disord 23, 259 (2023). https://doi.org/10.1186/s12902-023-01511-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01511-2