Abstract
Novel cycloaurated gold(III) complexes were prepared by using Schiff bases with different substituents. Effects of the synthesized gold(III) complexes and substituent groups on styrene polymerization were investigated. As a result of these investigations, it was observed that gold(III) complexes could not catalyze polymerization reaction without addition of a co-catalyst like NaBAr′4 (Ar′ = 3,5-bis(trifluoromethyl)phenyl). All ligands and complexes were characterized by using 1H nuclear magnetic resonance (NMR), 13C-NMR, LC-MS, elemental analysis and FT-IR techniques. The architecture of the polymer was determined as an atactic or sydiotactic polymer by using 13C-NMR and DSC techniques. Molecular weights of the polymers were analyzed by using gel permeation chromatogram (GPC).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclometallation reaction of gold(III) is one of the most important reactions of direct C-H bond activation. In 1989, Constable and Leese discovered first cycloauration by using 2-phenylpyridine [1]. Applications of organogold chemistry have been explored by Hashmi [2–4]. Organic derivatives of both Au(I) and (III) ions were reported in the review and in more recent papers [5–13].
Polystyrene is a hard plastic and more common in our everyday life. The stereoregular state of the polymer is important because it determines the crystallinity and physical properties of the resulting polymer. In general, transition metals are used for styrene polymerization in radicalic polymerization reaction and polystyrene obtained are generally isotactic reach atactic form [14]. But syndiotactic polystyrene (s-PD) is more useful than atactic polystyrene (a-PS) with a high melting point of ∼270°C, a glass transition temperature similar to atactic polystyrene, a fast crystallization rate, a high modulus of elasticity and an excellent resistant to heat and chemical agents [15]. Because of these properties, an immediate commercialization of syndiotactic polystyrene was successfully performed by the Idemitsu Kosan Company in a semicommerical plant of approximately 5,000 t/year in Chiba/Japan and by The Dow Chemical Company, based on a pilot plant in Midland, Michigan. The first commercial s-PS plant worldwide at a capacity of about 36,000 t/year in Schkopau, Germany opened in 1999, and continued by Idemitsu Kosan in a modernized production facility in Chiba, Japan, in 2006 [16]. In automotive systems, s-PS can be applied in power distribution centers under the hood, in electrical lighting, and electronics, s-PS can be used successfully for connectors, plugs and sockets, glass-fiber-reinforced and color-stabilized s-PS grades are materials for iron skirts and at least very promising markets of s-PS are food and water contact applications [17]. Especially half-metallocene transition metal complexes are very effective catalysts to synthesize s-PS [18, 19]. Although, gold complexes used for a wide array of transformations, there are few reports on the usage of gold complexes for polymerization reactions. In 2008, Perez and co-workers highlighted that polymerization mechanism of styrene with gold—carbene complexes is not working without using a co-catalyst like NaBAr′4. Their reaction results showed that their polymerization mechanism was cationic type [20]. But they did not explain the role of gold center.
In this work, we synthesized three cycloaurated gold(III) complexes to investigate their catalytic activity and reaction mechanism on styrene polymerization with using NaBAr′4 as a co-catalyst. As a result, when styrene is alone with NaBAr′4, reaction resulted with isotactic to reach atactic polystyrene, but when gold(III) complexes are added to the reaction, the polymer we gained was syndiotactic which is more valuable than atactic form.
Synthesis and characterization
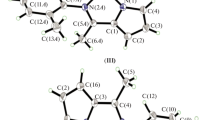
All of the synthesized ligands and complexes (Fig. 1) were characterized with a combination of elemental analysis, LC-MS, 1H and 13C nuclear magnetic resonance (NMR) techniques. All compounds elemental analysis results and mass analysis results are suitable with theoretical values.
FT-IR spectra of the complexes gave us important information about complexation. A comparison was made between ligands and their complex forms. Shifting frequency of C=N double bond of ligands with complexation, ranging from ∼1,600 to ∼1,700 cm−1 displays out the coordination of ligand to the metal. 1H-NMR gave us more important evidences about complexation than FT-IR. After complexation, H(4) proton shifts to downfield because H(4) proton is located near to the halogens. In the spectrums of L1 and AuL1, R=CH3 peak was seen at 2.1 ppm. In AuL2's spectrum, the effect of strongly electronegative CF3 group was seen clearly. H(1) proton, which is patient from CF3 group, split up into two shifts from 7.89 to 7.72 ppm. This shifting implies that after coordination to metal center, electronegativity effect of CF3 decreases. In the spectrum of L3 and AuL3, R=H peak is seen at 8.22 ppm in the ligand and 9.12 ppm in the complex. Shifting of proton to downfield displays the coordination of the ligand to the central metal atom.
Catalytic effects of complexes on styrene polymerization
In the first series of experiments, L1Au–L3Au complexes were employed in catalytic amounts in bulk polymerization reactions involving styrene. Under these reaction conditions, no polymerization was observed. When 1 equiv of NaBAr'4 was added to neat styrene solution as a halide scavenger, sudden polymerization was observed. To determine the effect of NaBAr′4, blank experiments were carried out. No reaction was observed when L1–L3 ligands were used as catalyst without metal center. Interestingly, NaBAr′4 alone with styrene gave polystyrene with high yield a-PS (Table 1; run nos., 1–5). When gold complexes added to the reaction media s-PS was formed (Fig. 2; Table 1; run nos., 6–20)
After these results were evaluated, series of experiments were conducted at room temperature with different solvents and results. Bulk polymerization was carried out at different catalyst concentrations but conversion did not pass 25% yield. Polymerization reactions were traced for 48 h and it was observed that polymerization reactions finalized at the end of 24th hour. Therefore we carried out several experiments in the presence of co-solvent. When toluene and chloroform was used as a co-solvent, conversion occurred in 24 h. When toluene was used, PDI value of polymers were recorded under 2.0 but yields of polymerization reactions were pretty less (ranging from 2% to 5% within 5 h; Table 1, run nos., 10, 15, 20). Maximum yields of polymerization reactions were ranging from 35% to 50% after 24 h. When chloroform was used as a co-solvent, conversion increased to 100%, although the polydispersity was also higher than both bulk and toluene containing experiments. Conversion was inhibited completely after 24 h when THF or acetonitrile was added to the polymerization media. All complexes yielded syndiotactic polystyrene.
Characterization of polymers
The number and weight-average molecular weights (M n and M w) were determined by GPC in CHCl3 as calibrated with polystyrene standards. The triad tacticities of polymers were determined by 13C-NMR spectra in deuterated chloroform.
1H-NMR is very useful to identify polystyrene. Chemical shifts between 6.39 and 7.42 ppm are aromatic hydrogen signals. The 1.72–2.43 ppm are methyne hydrogen signals and chemical shifts between 0.97 and 1.76 ppm are methylene hydrogen signals in polystyrene [21].
Figure 3 shows the comparison 13C-NMR spectrums of polystyrene in CDCl3 solution with catalyst and without catalyst. All spectrums were analyzed in terms of triads. Three main peaks left to right in Fig. 3 were assigned to isotactic triad (mm), heterotactic triad (mr), and syndiotactic triad (rr), respectively [22]. When we did not use a catalyst, the isotactic peak (mm) seen as a major peak and the polymer obtained as isotactic reach atactic. On the other hand, when we used Au catalyst, the stereoregularity of polymer changed and syndiotactic (rr) peak seen as a major peak.
Figure 4 shows the DSC thermogram of PS obtained by using L2Au as catalyst (Table 1, entry 13) and using without catalyst (Table 1, entry 3). Experiment without catalyst, the glass transition temperature (T g = 105°C) was obtained from the curve. No sharp melting peak above 200°C was observed. This indicates that the resulting polymer is amorphous. Also the melting points at 270 and 240°C were observed in the syndiotactic and isotactic polystyrene, respectively. No melting point was observed for polystyrene suggesting that the obtained polymer was atactic. Moreover, polystyrene obtained with using catalysts, a melting point at 266°C was observed which indicates that the synthesized polystyrene is in crystalline and syndiotactic form.
Experimental
General
All reactions were performed under dry, oxygen-free, nitrogen atmosphere. 1H and 13C-NMR spectra were recorded at room temperature in CDCl3 on a Bruker/BioSpin NMR 300 MHz with SiMe4 (0.0 ppm) as internal reference. Elemental analysis was performed on a LECO CHNS-932. Melting points were measured on a Buchi Melting Point B-540 apparatus and they are uncorrected. FT-IR spectra were recorded on a Jasco 300E spectrometer with KBr palletes in the 4,000–400 cm−1 range. Atmospheric pressure chemical ionization mass spectra were recorded with an AGILENT 1100 MS LC-MS mass spectrometer. Molecular weights and molecular weight distributions were determined with a Knauer model gel permeation chromatograph (GPC) with CHCl3 as eluent and calibration was conducted with polystyrene standards. Differential scanning calorimetry (DSC) was obtained on Perkin Elmer Diamond DSC instrument under a nitrogen atmosphere with a heating/cooling rate of 20°C/min of NaBAr′4 was synthesized according to the reference [23].
Preparation of ligands, general procedure
One millimole of acetylpyridine, pyridinecarboxyaldehyde, or acetophenone was dissolved in 2 ml ethanol. Two millimoles aniline and 30 ml of toluene were added dropwise to the above solution. In order to remove water from the solution, a Dean–Stark apparatus was used and resulting solution was refluxed for 2 h. Precipitation was accomplished by cooling the mixture to room temperature. The precipitate (L1–L3) was isolated by filtration and then it was washed with cold methanol and finally it was dried under vacuum.
(E)-N-(1-Phenylethylidene)benzenamine (L1)
Yield 79%. M.p. 122°C. Anal. Calcd for C14H13N: C, 86.43; H, 6.51; N, 7.06. Found, C, 86.12. H, 6.71. N, 7.17. 1H-NMR (DMSO-d6): δ 2.11 (s, 3H), 6.90 (m, 2H), 7.12 (m, 2H), 7.25 (m, 2H), 7.39 (m, 2H), 7.48 (m, 2H) ppm. 13C-NMR (DMSO-d6): δ 17.531, 119.44, 124.64, 123.21, 127.25, 128.35, 128.65, 130.45, 139.55, 151.83, 165.36 ppm. FT-IR(KBr, ν, cm−1) 1630 (C=N). LC-MS m/z 218 (+Na+).
(Z)-N-(2,2,2-Trifluoro-1-phenylethylidene)aniline (L2)
Yield 84%. M.p. 138°C. Anal. Calcd for C14H10F3N: C, 67.11; H, 4.41; N, 5.61. Found, C, 67.47. H, 4.04. N, 5.62. 1H-NMR (DMSO-d6): δ 6.75 (m, 2H), 6.86 (m, 2H), 6.95 (m, 1H), 7.24 (m, 1H), 7.58 (m, 2H), 7.89 (m, 2H) ppm. 13C-NMR (DMSO-d6): δ 120.25, 120.94, 125.18, 128.51, 129.88, 130.53, 131.25, 132.04, 151.51, 158.04 ppm. FT-IR (KBr, ν, cm−1) 1598 (C = N). LC-MS m/z 272 (+Na+).
(E)-N-Benzylideneaniline (L3)
Yield 79%. M.p. 114°C. Anal. Calcd for C13H12N: C, 85.31; H, 6.88; N, 7.81. Found, C, 85.68. H, 6.64. N, 7.69. 1H-NMR (DMSO-d6): δ 7.18 (m, 6H), 7.37 (m, 2H), 7.48 (m, 2H), 8.22 (s, 1H) ppm. 13C-NMR (DMSO-d6): δ 126.62, 129.42, 129.51, 132.53, 137.18, 153.47, 161.11 ppm. FT-IR(KBr, ν, cm−1) 1645 (C=N). LC-MS m/z 204 (+Na+).
Preperation of complexes, general procedure
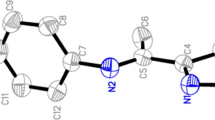
A solution of the ligand (L1–L3; 1.00 mmol) in CH3CN (5 ml) was added to a solution of HAuCl4 (1.00 mmol) in H2O (20 ml), forming a yellow suspension (Fig. 1). The suspension was stirred at room temperature for 2 h and a yellow solid was collected by filtration. After the precipitate was washed with H2O and a small amount of diethyl ether, it was suspended in mixed CH3CN/H2O (50 ml; 1/5, v/v). The suspension was stirred at room temperature for 10 min, and then it was heated at reflux for an hour. The reaction mixture was cooled to room temperature. After the filtration of this mixture, obtained precipitate was washed with H2O. The precipitate was extracted with hot acetone or CH2Cl2, and the extract was filtered through filter paper. The solvent was removed by evaporation under vacuum and the residual solid (AuL1–AuL3) was soaked with a small amount of pre-cooled acetone and then hexanes.
[AuL1], [AuCl2(L1)], yield 58%. M.p. 233°C (decomposition). Anal. Calcd for C14H12AuCl2N: C, 36.39; H, 2.62; N, 3.03. Found, C, 37.14. H, 2.33. N, 3.41. 1H-NMR (DMSO-d6): δ 2.11 (s, 3H), 7.07 (m, 2H), 7.33 (m, 2H), 7.49 (m, 2H), 7.63 (m, 2H), 7.91 (d, 1H) ppm. 13C-NMR (DMSO-d6): δ 18.51, 121.24, 124.64, 128.31, 129.89, 129.30, 130.93, 135.63, 141.16, 152.09, 166.42 ppm. FT-IR(KBr, ν, cm−1) 1702 (C=N). LC-MS m/z 484 (+Na+).
[AuL2], [AuCl2(L2)], yield 69%. M.p. 212–214°C (decomposition). Anal Calcd for C14H9AuCl2F3N: C, 32.58; H, 1.76; N, 2.71. Found, C, 32.82. H, 1.54. N, 2.44. 1H-NMR (DMSO-d6): δ 6,89 (m, 1H), 6.97 (m, 2H), 7.12 (t, 1H), 7.59 (m, 1H), 7.83 (d, 2H), 8.07 (d, 2H) ppm. 13C-NMR (DMSO-d6): δ 160.31, 153.74, 134.26, 133.22, 132.92, 131.25, 130.97, 130.32, 126.27, 122.93, 122.14 ppm. FT-IR(KBr, ν, cm−1) 1716 (C=N). LC-MS m/z 514 (+Na+).
[AuL3], [AuCl2(L3)], yield 77%. M.p. 247°C (decomposition). Anal Calcd for C13H10AuCl2N: C, 34.84; H, 2.25; N, 3.13. Found, C, 35.02; H, 1.99; N, 3.38. 1H-NMR(DMSO-d6): δ 9.12 (s, 1H), 7.92(d, 1H), 7.76 (d, 1H), 7,57 (m, 4H), 7.38 (m, 3H) ppm. 13C-NMR (DMSO-d6): δ 163.72, 154.21, 138.10, 135.61, 133.70, 131.21, 130.88, 130.11, 127.22 ppm. FT-IR(KBr, ν, cm−1) 1708 (C=N). LC-MS m/z 446 (+Na+).
Polymerization reactions
In a 50 ml ampoule, equipped with a magnetic stir bar, 1 × 10−3 mmol of L1Au–L3Au were introduced along with 1 equivalent of NaBAr′4. The co-solvent and styrene were added via syringe and the mixture was stirred at room temperature. The initial brownish color mixture readily converted into a colorless mixture. At the end of the reaction, addition of 20 ml of methanol into this colorless mixture induced the precipitation of the polymer. The polymer was separated by filtration and washed with two portions of MeOH. Finally, the polymer was dried first under vacuum and later in the oven at 100°C for 12 h before isolated yield was calculated.
Conclusions
It's already known that, gold is very effective for both homogeneous and heterogeneous catalysis. But effects of gold on polymerization reactions are not explored. In conclusion, we have found that styrene can be polymerized in the presence of gold(III) complexes and NaBAr′4. Collected results seem very interesting because gold catalysts did not affect the yield of reaction. But significantly they affect to the stereoregularity of the polymer. The problem is high polydispersity values and low yields of the resulting polymers. This problem can be achieved by using different ligand systems. Results are very promising to use gold catalysts in polymerization reactions.
References
Constable EC, Leese TA (1989) J Organomet Chem 363:419
Hashmi ASK (2003) Gold Bull 36:3–9
Hashmi ASK (2004) Gold Bull 37:51–65
Hashmi ASK, Hutchings GJ (2006) Angew Chem Int Ed 45:7896
Grohmann A, Riede J, Schmidbaur H (1992) Z Naturforsch B 47:1255–1260
M. A. Cinellu, A. Zucca, S. Stoccoro, G. Minghetti, M. Manassero, M. Sensoni, J. Chem. Soc. Dalton Trans,1993, 4217
Soloshomok VA, Hayashi T (1994) Tetrahedron Asymetry 5(6):191
M. A. Cinellu, A. Zucca, S. Stoccoro, G. Minghetti, M. Manassero, M. Sansoni, J. Chem. Soc. Dalton Trans,1995, 2865
H. Q. Liu, T. C. Chaung, S. M. Peng, C. M. Che, J. Chem. Soc. Chem. Commun, 1995, 1787
Parish RV (1998) Gold Bull 31(1):14
Vicente J, Chicote MT, Lozano MI, Huertas S (1999) Organometallics 18:753
Kronje S, Raubenheimer HG, Spies HSC, Esterhuysen C, Schmidbaur H, Schier A, Kruger GJ (2003) Dalton Trans 14:2859–2866
Hashmi ASK, Weyrauch JP, Rudolph M, Kurpejovic E (2004) Angew Chem Int Ed 43:5645–6547
Baundry-Barber D, Camus E, Dormund A, Visseaux M (1999) Appl Organomet Chem 13:813–817
Zang H, Chen Q, Qian Y, Huary J (2005) Appl Organomet Chem 19:68–75
Malang M, Newman TH (2002) Encyclopedia of Polymer Science and Technology. Wiley, New York, online version
Schellenberg J, Leder JJ (2006) T. Adv Polym Sci 25:141–151
Schellenberg J, Tomotsu N (2002) Prog Polym Sci 9:1925–1982
Schellenberg J (2009) Prog Polym Sci 8:688–718
J. Urbano, A. J. Hormigo, P. de Fremont, S. P. Nolan, M. M. Diaz-Requejo, P. J. Perez, Chem. Commun, 2008, 759
Li Y, Gao M, Wu Q (2008) Appl Organomet Chem 22:659
Istihara N, Seimiya T, Kuramoto M, Uoi M (1986) Macromolecules 19:2464
Brookhart M, Grant B, Volpe AF (1992) Organometallics 11:3920
Acknowledgments
We gratefully acknowledge financial support for this work from TUBITAK, 108T240, Turkey.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Emre Hanhan, M. Novel cycloaurated gold(III) complexes and their effects on styrene polymerization. Gold Bull 44, 43–47 (2011). https://doi.org/10.1007/s13404-011-0006-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13404-011-0006-9