Abstract
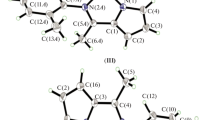
In recent years, a variety of novel late transition metal catalysts have been used for polymerization of methyl methacrylate (MMA); in order to study the effects of fine-tuning of catalyst structure on polymerization of MMA, we synthesized a series of mono(imine)pyrrole ligands (L1–L5) with different substituents in the same position using microwave reactions. Then, the ligands were directly coordinated with NiCl2·6H2O through liquid-phase reaction method to prepare the Ni(II) complexes (1–4). All the compounds were characterized by spectroscopic methods, and compounds L1, L3, L5 and 2 were characterized by X-ray crystallography. The results showed that the structures of the three ligands were mono(imine)pyrrole Schiff base structure. Complex 2 contains two molecular ligands’ structure and a Ni(II) atom to form a standard square plane geometry. The total molecular energy and other data for each Ni(II) complex were obtained by Gaussian 09 calculation program. The negative total molecular energy indicated the structures of complexes were relatively stable. Additionally, the bond parameters were in good agreement with X-ray single-crystal diffraction data. With the aid of azodiisobutyronitrile (AIBN), the synthesized Ni(II) complexes were used in the polymerization of MMA. By comparing the Ni(II) catalysts with different structures, we found catalytic activities were in a sequence of 4 > 1 > 2 > 3, which indicated the stronger electron-withdrawing ability of the substituent, the better catalytic activity of catalyst for MMA. We also studied the effects of polymerization temperature and time on catalytic performance, the optimum activity (11.71 × 104 g mol−1·h−1) was obtained at 100℃ and 10 h, and the polymer with relatively narrow molecular weight distribution (PDI = 1.91) and large molecular weight (Mn = 36.10 × 104 g mol−1) was obtained under the same condition.









Similar content being viewed by others
References
Chen CY, Wang SN, Hu Y (2021) Chem Ind 49:1–2
Chen XL, Zhu FM, Lin SA (2007) Acta Polom 11:1064–1068
Li JC, Gu CH, Shan YH (2007) J Chem Mater 8:40–42
Long RJ, Gibson VC, Williams DJ (2006) Inorg Chem 45:511–513
Chan HF, He LJ, Ren ST (2012) Polym Bull 8:13–23
Hu YJ, Jiang HL, Wang HH (2007) Ind Catal 15:23–27
Lv JH, Zhang DF, Chen Q (2011) Chem Sci Eng 5:19–25
Zhang DF, Li S, Yu W (2014) Chem J Chinese U 7:1559–1564
Su BY, Li XT, Wang XD (2015) Heterocycles 91:1955–1963
Su BY, Wang XD, Wang JX (2015) J Coord Chem 68:4212–4223
Su BY, Li YN, Pan DD (2019) Curr Org Synth 16:444–448
Su BY, Yan TY, Li XT (2020) Chin J Struc Chem 39:1093–1102
Sun L, Liu HX, Zhou HL (2015) Chinese J Inorg Chem 31:1207–1214
Barbas R, Kumar V, Vallcorba O (2020) Curr Comput-Aided Drug Des 10:1126–1135
Datta P, Mukhopadhyay AP, Manna P (2011) J Inorg Biochem 10:577–588
Carabineiro SA, Bellabarba RM, Gomes PT (2008) Inorg Chem 47:8896–8911
Hou YF, Study on the Catalysis of New Type Salicylaldehyde-Pyrroleimine Complexes for Ethylene Polymerization. Xi'an Shiyou University 2019
Wang JX, Liu X, Su BY (2013) Fine Chem 30:1389–1393
Zhang SL, Zhao B, Qin DB (2019) Ind Catal 27:62–68
Su BY, Pan DD, Yan TY (2020) Russ J Coord Chem 46:355–364
Yaman M, Aydemir E, Dege N (2018) Acta Cryst 74:1528–1634
Mao PD, Yan LL, Wu WN (2016) Chinese J Inorg Chem 32:879–883
Yuan ZL, Synthesis, Characterization and Spectroscopic Properties of a New Type of Macrocyclic Schiff Base. Guizhou University 2006
Zhang W, Synthesis and fluorescence properties of novel pyridine derivatives and their Eu and Tb complexes. Central South University 2009
Hao L, Design, Synthesis, Structure and Fluorescence Properties of D-A Conjugated Schiff Base Complex. Beijing Institute of Technology 2017
Li YN, Development and QSAR study of five-membered heterocyclic imine transition metal polyolefin catalyst. Xi'an Shiyou University 2018
Lan TY, Zhang N, Chen LD (2021) ACS Omega 6:3354–3362
Pang RL, Li JX, Cui ZZ (2019) Dalton T 48:7242–7248
Altomare A, Burla MC, Camalli M (1999) J Appl Crystallogr 32:339–340
Burla MC, Camalli M, Carrozzini B (1999) Acta Cryst 55:991–999
Cheng ZZ, Gong K, Wang Y (2014) J Wuhan Univ Technol 29:1294–1301
Kim S, Kim D, Lee HJ (2014) J Mol Struct 1063:70–76
Fondo M, Ocampo N, García-Deibe AM (2009) Inorg Chem 48:9861–9873
Ahmad S, Nadeem S, Anwer A (2017) J Mol Struct 1141:204–212
Adejumo TT, Tzouras NV, Zorba LP (2020) Molecules 25:4043–4061
Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (51674200), the Young Scientific Research and Innovation Team Program of Xi'an Shiyou University (2019QNKYCXTD16), Xi'an Science and Technology Innovation Project (2020KJRC0099) and the Innovation and Practical Ability Training Project for Postgraduates of Xi'an Shiyou University (YCS21211010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Su, B., Liu, Y., Yan, T. et al. Nickel(II) complexes with mono(imino)pyrrole ligands: preparation, structure, DFT calculation and catalytic behavior. Transit Met Chem 46, 601–611 (2021). https://doi.org/10.1007/s11243-021-00478-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-021-00478-0