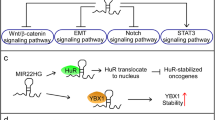
Abstract
miRNAs and lncRNAs play a central role in cancer-associated gene regulations. The dysregulated expression of lncRNAs has been reported as a hallmark of cancer progression, acting as an independent prediction marker for an individual cancer patient. The interplay of miRNA and lncRNA decides the variation of tumorigenesis that could be mediated by acting as sponges for endogenous RNAs, regulating miRNA decay, mediating intra-chromosomal interactions, and modulating epigenetic components. This paper focuses on the influence of crosstalk between lncRNA and miRNA on cancer hallmarks such as epithelial-mesenchymal transition, hijacking cell death, metastasis, and invasion. Other cellular roles of crosstalks, such as neovascularization, vascular mimicry, and angiogenesis were also discussed. Additionally, we reviewed crosstalk mechanism with specific host immune responses and targeting interplay (between lncRNA and miRNA) in cancer diagnosis and management.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Study Highlights:
-
A negative regulatory or sponging mechanism between lncRNA and miRNA has been discussed as a possible contributor to cancer aetiology.
-
This review explains how miRNA and lncRNA crosstalk affects cancer hallmarks and tumour features and how it can be used to treat carcinogenesis.
-
This review may enable the development of new immunotherapies or inhibitors targeting cell signaling or molecular effectors of miRNA-lncRNA crosstalk.
1 Introduction
Cancer is a multifactorial disease caused by the existence of driver mutations that cause proto-oncogene to be activated and inactivation of tumor suppressor genes. Such events cause a change in cell function, characterized as cancer hallmarks, and ultimately aggravates the chemoresistance to conventional therapies [1,2,3,4,5,6]. Apart from gene mutations, the expression of noncoding RNAs (ncRNAs) such as micro-RNAs (miRNAs) and long noncoding RNAs (lncRNAs) has been described [7, 8]. miRNAs are single-stranded RNAs constituting 18–25 nucleotides that play a significant role in biological processes, such as stem cell differentiation, development, and tissue identity via negatively regulating the mRNA transcripts [9, 10]. LncRNAs consisting of more than 200 nucleotides. They may interact with nucleic acids and proteins, controlling a range of cellular functions, including post-transcriptional mRNA regulation, scaffolding, post-transcriptional modification, transcription, and chromatin modification [11, 12]. In addition, miRNAs and lncRNAs play a central role in several gene regulations [13]. lncRNAs behave as molecular decoys by capturing miRNAs and preventing them from interacting with their respective messenger RNAs (mRNA) [12, 14] for their crosstalk with miRNAs to control several biological activities [15].
The dysregulated expression of lncRNAs has been reported as a hallmark of cancer progression, acting as an independent prediction marker for an individual cancer patient [16, 17]. A recent study reported a significant functional contribution of lncRNAs at transcriptional levels, targeting cis-acting regulatory sites or trans-regulatory sites. Through these mechanistic approaches, lncRNAs are expected to cause cancer progression. Therefore, they serve as potential targets to study cancer progression as their dysregulated pattern is distinctive depending on specific tumorigenic cells and tissues [18]. The interplay of miRNA and lncRNA decides the variation of tumorigenesis that could be mediated by acting as sponges for endogenous RNAs, regulating miRNA decay, mediating intra-chromosomal interactions, and modulating epigenetic components [19].
Competitive endogenous RNAs (ceRNAs) are transcripts that have been shown to compete for shared miRNA sites at the post-transcriptional level, making them ideal indicators for cancer progression [20]. The lncRNA possesses numerous miRNA response elements MREs sites, eventually leading to their interaction with more miRNAs due to the large number of MREs. The recent studies reported the remarkable effect of lncRNA-BGL3 acting as ceRNA for various miRs such as miR-17, -20a/-20b, -93, miR-106a/-106b in inhibiting repression of phosphatase and tensin homolog mRNAs causing physiological and pathological conditions. Oncocers are ceRNAs (Competing endogenous RNAs) that play essential roles in oncogenic pathways, and their crosstalk is studied by sponging miRNAs in these pathways [21].
To an extent, the role of miRNAs or lncRNAs in multiple cancers has been understood at the molecular level but requires additional studies to gain more insights into their differential cellular functions. The role of specific miRNA patterns has been studied for therapeutic intervention [22]. The role of lncRNAs in malignant cancers has recently drawn a lot of attention [23,24,25,26]. There is also a growing recognition of regulatory RNA crosstalk as a result of lncRNA-miRNA interactions, which affect many events in carcinogenesis and metastasis. A transcriptome-wide analysis of the interactions between lncRNA and miRNA resulted in the creation of a genome-wide map displaying these interactions [27]. The field of lncRNA-miRNA interaction is still nascent, and the understanding of specific regulation is still emerging. It will take time for the global research community to offer more functional data that will allow a choice of therapeutic intervention targeting lncRNA-miRNA in cancer [28].
This review may interest global research community to study cancer by focusing on lncRNA-miRNA interactions rather than only miRNA or lncRNA (Fig. 1). We have also covered the potential mechanism of action and influence of miRNA and lncRNA interactions on cancer hallmarks.
2 Mechanism of the interplay between miRNA and lncRNA
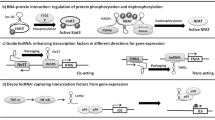
LncRNAs regulate gene expression at multiple levels (DNA, RNA, and proteins) by modulating chromatin structure, post-translational modification, RNA modification, and stability. We have focused on explaining the two functioning processes that are responsible for the interaction between lncRNA and miRNA (Fig. 2).
2.1 Sponge effect of lncRNAs on miRNAs
LncRNAs and miRNAs play essential roles in genetic control. Unfortunately, many of these transcripts’ roles are yet unknown [29]. Substantial crosstalk between miRNAs and lncRNAs results in binding competition between the miRNA and lncRNA via targets for regulating multiple proteins and transcription factors [30]. In the case of ceRNAs, a lncRNA performs its job by sequestering miRNAs, hence decreasing their levels. As a result, miRNAs are less likely to bind to their mRNA targets, a process known as the sponge effect. Predicting miRNA targets in mRNAs is possible using a variety of techniques. Sponge ceRNAs are lncRNAs, yet few methods exist for predicting miRNA response elements (MREs) in these RNAs [31,32,33]. Hence, the sponge effect of MRE-lncRNA crosstalk irreplaceably impacts diverse cancer progressions. Crosstalk and communication between lncRNAs with several common MREs play a significant role in biochemical events at the cellular and molecular level, causing distinct physiological and pathological states. The positionally selective phenomenon of lncRNA BC032469 targeting miR-1207-5p rather than its corresponding miR-1266 seed region inducing gene downregulation was recently reported. This shows that lncRNAs target their core miRNA sites while leaving others unaffected. Sponging effects mediate lncRNA and miRNA biochemical regulation in cancer progression [34].
The above-mentioned sponging activity could be described by explaining the two kinds of crosstalk between lncRNAs and miRNAs, which involve either complete or partial complementary modes [34]. MREs contain conserved target sites and mediate the mechanism by which miRNA typically attaches to a partially complementary target gene sequence. lncRNAs regulate miRNA MREs and play an important role in post-transcriptional regulation [35]. The sponging impact of miRNA and lncRNA involves lncRNA, which is adversely controlled by miRNA. The mature lncRNA incomplete complementation has a hat-like structure with a poly5-A tail at the end, a 5’UTR, a 3’UTR and multiple MREs, connecting with miRNAs. The more lncRNAs share multiple MREs, the crosstalk will proceed successfully [21].
Some lncRNAs without MRE use the sponging effect with the reciprocal miRNA to express a superior selection when there are several miRNAs present. Similarly, in the instance of lncRNA BC032469, which possessed elements complementary to miR-1207-5p and miR-1266 in the seed regions but no MRE, weakening the down-regulation of miR-1207-5p-dependent target genes [36]. MREs in lncRNAs express a position preference in mid-region and at the 3’ends of the lncRNAs when it comes to the AGO binding sites, regulatory elements harbor a particular pattern crosswise to the transcripts [37]. Furthermore, the sponging effect’s influence is dependent on a specific spatial-temporal distribution [38]. The lncRNA UCA1 is one such case where it interacts with miR-184 via a sponging mechanism for causing cellular proliferation for prostate cancer [39, 40]. In summary, the lncRNA may compete for miRNA MREs, reducing miRNA repression of target genes, so indirectly affecting the expression levels of target genes and ultimately aiding in cancer regulation. miRNAs and lncRNAs constitute a negative feedback loop.
2.2 2.2. Negative feedback loop:
The capacity to produce a negative feedback regulation pathway for the crosstalk of lncRNA and miRNA is an additional mechanism critical for a functional miRNA and lncRNAs interaction. Both miRNAs and lncRNAs play roles in regulating gene expression. The expression of lncRNAs is indirectly influenced by miRNAs. In a manner analogous to an enhancer function, miRNA and lncRNA interactions generate regulatory network transcriptomes that affect the expression of nearby genes [41]. By inhibiting DNA methylase, the DLK1-MEG3 imprinted domain, which contains the tumor suppressor factor MEG3 lncRNA, promotes cancer growth [42]. MiRNAs degrade lncRNAs in an AGO-dependent way by binding to target lncRNA 3’UTR inside the RISC, causing complete mRNA destruction or ribosomal machinery blockage for gene silencing in tumors [34, 43].
Another negative feedback loop mechanism is the lncRNA-mediated downregulation of miRNA expression. Although, there are variations in sequence between lncRNAs and miRNAs, the lncRNAs could be used as miRNA precursors to alter miRNA regulation [30]. Although, H19 has a G as its major base and miR675 has either a G or a C, their interaction is typical of the ncRNA family, which includes members with widely varying degrees of nucleotide conservation and mutation rates. Inverted regions in the mature miRNAs ensure the stability of the stem-loop structure in pre-miRNAs. The integrity of the stem-loop structure of pre-miRNA is ensured by its structure. Due to the nucleotide makeup, different lengths are required for optimal functioning. Additionally, lncRNAs compete with miRNA targets (particular mRNAs), and there is negative regulation of miRNA for miRNA targets by lncRNAs, which compete with the target 3’UTR of the mRNA [32, 44]. In addition, nanog silencing in breast cancer is caused by the interaction of lncRNA FEZF1-AS1, which blocks miRNA30a, competitively [45]. In addition, lncRNA H19 binds to proteins in the miRNA production regulatory complex, PCAF/hnRNPU/Pol RNA II, to boost histone acetylation upstream of miR200 [46].
Overexpression of lncRNAs such as miR100HG, miR100, and miR125b is associated with resistance to the anti-cancer drug cetuximab in several cancer types. Coordination between miR100 and miR125b inhibits five Wnt-beta catenin negative regulators for mitochondrial genome preservation via a negative feedback loop involving MIR100HG and TF GATA binding protein 6 (GATA6). Using miR125b as a target, GATA6 suppresses MIR100HG, which in turn suppresses Wnt signaling through miR125b suppression and restores cetuximab sensitivity in cells that have been resistant to the drug [47]. Furthermore, lncRNA UCA1 interacts with miR-204 via a negative impacting mechanism, resulting in glioma progression by glioma cell migration and proliferation via downregulation of anti-miR-182 protein [48].
UCA1, a lncRNA involved in cellular proliferation and migration, interacts with miR-28-5p to upregulate homeobox B3 and cause angiogenesis in colon cancer [49]. Additionally, the lncRNA UCA1 promotes cellular proliferation and migration in a bromodomain 4, c-Myc, and growth factor-binding protein 5-dependent way [50,51,52] after interacting with miRNA 135 in a competitive method. Similar research also discovered that EZH2 cooperates with miR-26a-2 and miR-101 to suppress c-Myc and HIF-1a/1b. It has been found that EZH2 collaborates with miR-26a-2 and miR-101 to negatively regulate c-Myc and HIF-1a/1b [53, 54]. Moreover, a negative feedback loop involving miRNA-101, miR-26a, and EZH2 inhibits cell cycle regulatory factors [53,54,55].
By further chromatin changes, the lncRNAs affect miRNA expression [56]. This process underlies the crosstalk between microRNAs and lncRNAs in hepatocellular carcinoma, where overexpression of histone deacetylase-4 (HDAC4) inhibits miRNA-200a and vice versa [57].
3 Ultra-conserved regions and crosstalk of miRNA and lncRNA
A significant proportion of transcripts produced by these UCRs (Ultraconserved regions) have secondary RNA structures and are found in clusters with other functional noncoding elements [58]. B-cell lymphocytes have the greatest number of transcribed UCRs, and various UCR signatures have been associated to human carcinomas and leukemia. The expression of nineteen UCRs, eight raised and eleven decreased, differed in CLL versus normal hematological tissues [58, 59]. The cancer-associated genomic region (CAGR) comprises a cluster of 7 UCRs (uc. 347 to uc. 353), two of which are transcribed UCRs (T-UCRs) expressed differentially between malignant and normal B-CLL CD5 + cells [58]. Additionally, there is a relationship between the expression of ZAP-70, a well-established prognostic marker for CLL, and a signature of five T-UCRs, including uc. 348 A(P) (N), uc. 346 A(P), uc. 215(N), uc. 160(N), and 269 A. (N). The levels of expression of the five T-UCRs mentioned above were discovered to be negatively linked with the expression profile of CLL-specific miRNAs [59].
As a result, lncRNAs and small RNA biology are intricately intertwined; many lncRNAs appear to function as precursors for small RNA species, particularly miRNAs. The possibility of complex regulation processes involving long noncoding RNAs and short RNAs, meaning that the two types of noncoding RNAs may interact. Given the increasing importance of lncRNAs in biomolecular regulatory interactions within cells and their role in the etiology of human disease and cancer, a thorough examination of their functions is required to shed light on the specific pathways and regulatory circuits in which they are involved [59]. Notwithstanding the fact that certain research suggests a link between UCRs and small RNA biology. A slew more investigations are needed to establish a clear link between UCRs and the crosstalk of lncRNAs and miRNAs in cancer.
4 Impact of the interplay of miRNA and lncRNA
Several hallmarks, tumor features, and the tumor microenvironment (TME) are modulated by the crosstalk of miRNA and lncRNA, ultimately leading to progression. The tumor growth of several cancer pathways relies heavily on the regulatory network of the interplay between lncRNA and miRNA. This review explains how miRNA and lncRNA crosstalk can lead to immunomodulation, which can cause cancer development via angiogenesis, neovascularization, Epithelial-Mesenchymal Transition (EMT), Vascular Mimicry (VM), suppression of apoptosis, invasion, and metastasis.
4.1 Angiogenesis and interplay of miRNA and lncRNA
The process by which pre-existing blood vessels are used to form capillary networks because of the requirement for nutrients in specific tissue regions is called angiogenesis [60]. It is one of the most critical aspects of all cancer processes, and it is closely regulated by a network of angiogenesis inhibitors (such as VASH2) and activators (such as VEGF) [61]. Tumor cells need resources, therefore, they manipulate angiogenesis to their benefit, causing the delicate activator-inhibitor balance to shift [62]. Therapeutic strategies targeting the crosstalk between miRNA and lncRNA, or the development of inhibitors against the downstream effector molecule that causes angiogenesis upon crosstalk, require a thorough understanding of the regulatory network and role of angiogenesis. Figure 3 illustrates the impact of crosstalk between miRNA and lncRNA via angiogenesis and is summarized in Table 1.
MiRNA-299 promotes VEGF-A-dependent angiogenesis in glioblastoma through interacting with lncRNA TUG1. Regression of tumors was induced by therapeutic knockdown of the lncRNA TUG1 [63]. TUG1 can potentially stimulate angiogenesis by interacting with miRNA-34a-5p through a sponging mechanism. Knockdown of the lncRNA TUG1 induces tumor regression [66], and crosstalk regulates VEGF-A to promote invasion and cellular proliferation. Oral squamous cells benefit from increased cell proliferation via angiogenesis when LINC00668, another lncRNA, works in concert with miR-297 to negatively regulate the miRNA in a VEGF-A-dependent way. Tumor regression was observed after LINC00668 was knocked down therapeutically [64].
VEGF-A-dependent upregulation of differentiation, migration, and angiogenesis in osteoclastogenesis was also shown in a similar study involving the lncRNA AK131850, which interacts with miR-93-5p via a sponging mechanism. The TGF-β pathway is activated by VEGF-A, which then promotes tumor growth by encouraging angiogenesis [65]. The lncRNA Snhg1 also interacts with miR-199a to trigger VEGF-A-dependent angiogenesis. This feedback loop stimulates the proliferation of microvascular endothelial cells in bone marrow, resulting in angiogenesis [67].
In a VEGF-A-dependent way, lncRNA H19 interacts with miR-29a to promote glioma progression via angiogenesis (cell proliferation and invasion). Regression of gliomas and suppression of angiogenesis can be achieved by knocking down the lncRNA H19 [68]. Similar research identified WTAPP1, a lncRNA that mediates angiogenesis through invasion, cellular differentiation, and proliferation by interacting with miR-3120-5P via a molecular decoy method. Tumor growth in endothelial progenitor cells occurs via the PI3K/Akt/mTOR pathway, and this interaction is reliant on MMP-1 in the autophagy route [69].
To prevent the signaling pathway for tumor progression caused by miRNA and lncRNA crosstalk, MMP − 1 based inhibitors should be utilized. Using immunomodulation of EFGR and TGF-alpha signaling, MMP-1 1-based inhibitor has been used earlier in a breast cancer xenograft model to suppress invasion and metastasis [70]. VASH2 is abundantly expressed in ovarian cell carcinoma, where it stimulates tumor angiogenesis and its knockdown causes tumor regression. In this way, it can serve as a therapeutic molecular target [71].
4.2 Neovascularization and interplay of miRNA and lncRNA
Neovascularization is the process of forming new blood vessels from existing ones. Angiogenesis inhibitors and inductors coordinate the procedure after endothelial cell proliferation and migration [72]. In order to receive the nutrients and oxygen they need to grow, tumors need to have close proximity to blood capillaries. Due to oxygen’s long diffusion distance (100-200 m), tumors larger than 12 mm necessitate the development of new blood vessels [73]. In this hypoxic setting, HIF-1 promotes hypervascularization by increasing the production of several growth factors including vascular endothelial growth factor and hepatocyte growth factor [74, 75]. Herein as shown in Fig. 4, and summarized in Table 2, the pre-clinical importance of crosstalk between miRNA and lncRNA impacting neovascularization is being clarified.
Through influencing downstream effectors including c-myc and HMGA, which in turn causes neovascularization via cellular proliferation and invasion in hepatocellular carcinoma, the CCAT1 lncRNA promotes tumor growth through a sponging mechanism. Tumors shrank after CCAT1 lncRNA was inhibited [76]. TUG1 is another lncRNA that interacts with miR-34a-5p to promote glioma progression in in vitro and in vivo studies. It’s VEGF-A dependent, therefore it produces neovascularization through invasion and metastasis [77].
The neovascularization of gliomas is facilitated by an interaction between the lncRNA H19 and microRNA-140. Cancer cells shrank after H19 lncRNA was knocked out and p53’s apoptosis-inhibiting protein was targeted therapeutically [78]. Similarly, PVT1, a lncRNA, was found to interact with miR-186 in glioma cells via a negative feedback loop that promotes cell proliferation, motility, and angiogenesis. The upregulation of PVTI and miR-186 may offer new therapeutic targets for anti-angiogenic therapy of gliomas [79].
4.3 EMT and interplay of lncRNA and miRNA
Studies over the past decade have demonstrated the reciprocal role that miRNAs and lncRNAs play in regulating cancer progression via epithelial-mesenchymal plasticity [28, 80, 81]. Because of the interplay between lncRNA and miRNA, EMT is a cancer characteristic that controls the cellular and molecular level processes of malignancies. Whenever lncRNAs interact with miRNAs, their stability is compromised. LncRNAs (ceRNAs) can antagonize microRNAs (miRNAs) by binding to them and preventing them from reaching their targets, as well as binding to mRNAs to compete with miRNAs. In addition, certain lncRNAs produce miRNAs, leading to the silencing of specific mRNAs. Further study of the patterns of interaction between lncRNAs and miRNAs in the biological process of cancer is warranted, as these patterns influence cancer progression [82]. It is essential to regulate the prognosis of early-stage carcinomas by targeting the bidirectional mechanism of epithelial-mesenchymal plasticity [82]. Yet, when cancer cells have spread, inhibiting EMT could be counterproductive because of the positive effects it has on MET [83]. Therefore, it is essential for diagnosis to learn the specific process of secondary site colonization. Here, the impact of miRNA and lncRNA crosstalk on EMT in pre-clinical studies is explained and illustrated (Fig. 5) and summarized (Table 3).
Tumor suppressor miR-217 blocks EMT by preventing EZH2-mediated H3K27me3 through suppression of the lncRNA MALAT-1 via an Ago2-mediated pathway. The therapeutic intervention of downregulating vimentin and N-cadherin and upregulating E-cadherin in HBE cells generated by cigarette smoke extract (CSE) [96] would be consistent with this hypothesis. Moreover, MALAT-1 promotes EMT in triple-negative breast cancer by up-regulating Slug expression via suppression of miR-1 expression via negative feedback loop regulation [85]. This is accomplished by forming complementary base pairs between MALAT-1 and miR-1.
Similarly, lncRNA H19 promotes EMT in CRC by downregulating endogenous target vimentin ZEB1 and ZEB2 through negative regulation of miR-138 and miR-200a [84]. In addition, H19 functions as a sponge interacting with miRlet-7 to promote EMT in HCC [91].
Also, the lncRNA DLX6-AS1 interacts with miR181-b in pancreatic cells, inducing EMT enhancement via cell proliferation, migration, and MMP2 dependence. Knockdown of the lncRNADLX6-AS1 increased E-cadherin and decreased MMP2, preventing malignant cells from undergoing EMT [92]. In addition, EMT in breast cancer is caused by DLX6-AS1 interacting with miR-223 via a sponging mechanism via the Wnt β-catenin signaling pathway in an oncogene HSP90B1-dependent manner [94]. According to the aforementioned research, silencing DLX6-AS1 also causes an increase in apoptosis and a reduction in ESC migration, proliferation, invasion, and EMT.
A related study found that miRNA-lncRNA interactions promoted the development of cancer. Using a sponging mechanism, LncARSR interacts with miR-129-5p, leading to bladder cancer due to SOX4 overexpression during EMT and metastasis. Cancer progression and EMT can be slowed by knocking down this lncRNA, making it a potential therapeutic target [93]. UCA1 is another lncRNA that induces glioma growth via EMT via TGF-β and functions as a competitive RNA for miR-1 and miR-203a. Tumor regression can be induced by knocking down UCA1, which leads to the downregulation of slug, a molecular effector protein [95]. The aberrant production of IL-6 promotes EMT in HCC via activating the STAT3 pathway through lncTCF7 [97]. IL-6 in the tumor microenvironment promotes lncTCF7 by activating STAT3.
MicroRNAs play a role in lncRNA-dependent gene regulation during EMT in CC metastases [98]. NEAT1 is a lncRNA that mediates EMT through invasion in CC [86]. It does this by interacting with the miR-361 and adversely impacting it in an HSP90 signaling-dependent manner. In addition, the lncRNA cardiac hypertrophy-related factor (CHRF) negatively influences the anti-metastatic factor miR-489, hence promoting metastasis and EMT [87]. The lncRNA UICLM interacted with miR-215, which promotes cellular invasion, migration, and proliferation through EMT in a ZEB2-dependent fashion [88].In hypoxic bone marrow mesenchymal stem cells (BMSCs), the EV-miRNAs miR-5100, miR-210-3p, and MiR-193a-3p interact with the lncRNA DNMT1 to promote EMT, migration, and invasion in lung cancer cells. [89]. The dissemination of malignant phenotypes among diverse populations of cancer stem cells is facilitated by the exchange of EV-RNAs within tumors. By transferring the metastatic capacity to nearby or distant weakly metastatic BC cells, EV-miR-200 from highly metastatic BC cells promotes EMT and lung colonization [99, 100]. MiR-200c is targeted, Sox2 is increased, and EV-lncRNA-Sox2ot is released from highly invasive pancreatic ductal adenocarcinoma cells, inducing EMT and stemness in less invasive recipient cells, and ultimately causing in vivo tumor metastasis [90]. To increase pancreatic cancer cell motility, invasion, EMT, and lung metastasis by secreting IL-10, TGF-β, and arginase-1, EV-miR-301a interacts with HOTAIR and MIR31HG from hypoxic pancreatic cancer cells and causes macrophage M2 polarization [101].
4.4 Vascular mimicry and interplay of miRNA-lncRNA
Similar to the embryonic vascular network, tumor tissue can receive plasma and red blood cells via microcirculatory channels made of extracellular matrix in a process known as vascular mimicry (VM) [102][102,103,104, 109]. VM is unique because it resembles embryonic vasculogenesis processes, which suggests that tumor cells can be reverted to an undifferentiated, embryonic-like phenotype to supply nutrients for tumor growth in hypoxic environments in lung, colorectal, bladder, osteosarcoma, prostate, breast, ovarian, and melanoma cancers, and to facilitate invasion and metastasis in these diseases. VM causes high levels of matrix metalloproteinase-9 (MMP-9), metalloproteinase-2 (MMP-2), vimentin (VIM), fms-like activating kinase (FAK), ephrin-A2 (EphA2), vascular endothelial growth factor (VEGF), transforming growth factor beta 1 (TGF-ß1), Dickkopf-1 (DKK-1), maspin (maspin), lamin. Here, we are clarifying the pre-clinical significance of crosstalk between miRNA and lncRNA as it relates to neovascularization and summarizing this interaction in Table 4.
Neither microRNAs nor long noncoding RNAs have been overlooked in their roles as master controllers of cancer progression in VM. In a murine model of glioblastoma, HOXA-AS2 is a lncRNA that acts as a sponge for miR-373 through MMP-9, MMP-2, or VE-cadherin in a PIP3-kinase, serine/threonine kinase, and EGFR EGFR-dependent way. Therapeutically, HOXA-AS2 knockdown can be used to reduce glioma progression [103]. Similarly, MALAT1 interacted with miR-145-5p in lung cancer patients, inducing metastasis in an aEr dependent manner, via sponging off via NEDD9 signaling for VM. Therapeutic intervention against lung cancer can be achieved through the knockdown of molecular effector molecules like Er [107].
Glioma cells migrate and invade when the lncRNA TWIST1, upon LINC00339 polymorphism, interacts with miR-539-5p, negatively impacting the miRNA for VM in an MMP-2 and MMP-14 dependent way. Therapeutically, TWIST1 knockdown can lead to glioma regression [104]. In addition, in an in-vitro investigation against breast cancer cells, TWIST1 interacted with miR-430-3p, negatively affecting the miRNA in a TP73-AS1-dependent mechanism for VM. Therapeutically, breast cancer regresses when the lncRNA TWIST1 is knocked down [105].
Similar research found that the lncRNA n339260 correlated with the expression of the pluripotency-maintaining molecules c-myc, SOX2, and Nanog, and interacted with multiple miRNAs (miR-92a-1-5p, miR-29b-1-5p, miR-520c-5p, miR-519c-5p, miR-30e-5p, and miR-31-3p) for stem-like features and VM development.
4.5 Impact of crosstalk of miRNA and lncRNA on inhibition of apoptosis
Inhibition of apoptosis is a well-coordinated cellular hallmark that occurs in a variety of physiological circumstances in cancer cells to evade the apoptotic response [108, 109]. Gene inactivation at the p53 locus results in epigenetic regulation that increases the expression of anti-apoptotic proteins and decreases the expression of pro-apoptotic proteins, making the cell resistant to a wide range of apoptotic triggers. Recent research has demonstrated that ncRNAs have a critical regulatory role in the prevention of apoptosis. Several of these RNAs’ key roles in oncogenic processes are in controlling cell apoptosis. [110]. Overexpression of lncRNA can reduce the expression of necroptosis-related proteins and block the extrinsic apoptosis pathway by downregulating membrane receptors. [111]. By modulating the expression of effector molecules involved in apoptosis signaling pathways, lncRNA and miRNA inhibits apoptosis through interacting among themselves. Herein, the pre-clinical significance of crosstalk between miRNA and lncRNA affecting inhibition of apoptosis is being elucidated, illustrated in Fig. 6, and are summarized in Table 5.
The lncRNA DLX6-AS1 negatively regulated miR-577, which in turn promoted CRC progression by inhibiting apoptosis and metastasis and elevating p-P13K and p-AKT signaling pathway levels. Tumors shrank after a surge in miR-577 expression was triggered by blocking the PI3K/mTOR signaling pathway [112]. In addition, DLX6-AS1 interacts with miR-142 in a PRR11 gene-dependent manner, suppressing apoptosis and metastasis in the process of developing lung cancer. Tumor regression and miR-142 upregulation both resulted after lncRNA inhibition knock-down. By promoting cell proliferation and inhibiting apoptosis [122, 123], miR-27b-3p contributes to the development of lung cancer. [122, 123].
In addition, DLX6-AS1 induced hepatocellular carcinoma via interacting with miR-203a, which in turn inhibited apoptosis, cellular proliferation, and metastasis in an MMP MMP-dependent manner [128, 129]. Similarly, lncRNA DLX6-AS1 induces hepatocellular cancer by suppressing apoptosis through the STAT pathway after binding with miR-424-5p in a WEE1-dependent manner. Hence, DLX6-AS1 knockdown represents a potential therapeutic strategy for the treatment of HCC. In a fibroblast growth factor Receptor 1–dependent manner, the lncRNA UCA1, when interacting with miR-216b, promotes suppression of G0/G1 cell cycle arrest, hence inhibiting apoptosis and metastasis in hepatocellular carcinoma cells [130, 131]. A similar study found that the zinc finger E-box-binding protein DLX6-AS1, upon interacting with miR-181b, promoted cell proliferation, migration, metastasis, and inhibition of apoptosis in pancreatic cancer via up-regulation of the Wnt/beta-catenin signaling pathway and the FZD4/FZD6 signaling pathway (ZEB2). Tumor regression was observed after DLX6-AS1 was knocked down, suggesting that this process could be used therapeutically. Interaction between DLX6-AS1 and miR-181b facilitated cell proliferation, migration, metastasis, and apoptosis suppression [132]. The MAP4K1 protein inhibits apoptosis and metastasis in gastric cancer, and this has been linked to its upregulation of the interaction of lncRNA DLX6-AS1 with miRNA expression [145,146,147]. Via a direct interaction with miR-204-5p, DLX6-AS1 suppressed EMT and invasion in GC cells by blocking apoptosis and metastasis in OCT1-dependent fashion [148]. MiR-199a inhibits apoptosis and metastasis in CC through an interaction with lncRNA DLX6-As1 [140]. This effect is FUS-dependent.
Another lncRNA, urothelial carcinoma-associated 1 (UCA1), has been shown to interact with miR-204e5p via negative regulation to up-regulate CREB1 expression, which in turn can promote cell proliferation and treatment resistance to 5-fluorouracil via prevention of apoptosis and metastasis [113]. Inhibition of apoptosis and metastasis in CRC can also be attributed to the lncRNA HOTAIR [114,115,116], which interacts with several miRNAs including miR-218, miR-545, and miR-203a-3p via Wnt- β-catenin, EGFR signaling. More so, UCA1 regulates the cell cycle by blocking apoptosis when it interacts with miR-18a. Inhibiting the expression of the lncRNA UCA1 may prove useful in the therapeutic management of breast cancer [121]. In addition, UCA1 interacts with miR-193a, leading to lung cancer via cell proliferation, apoptosis suppression, and metastasis in an ERBB4 and HMGB1-dependent way [124]. The homeobox 3 gene promotes invasion, suppression of apoptosis, and metastasis in bladder and lung malignancies [125, 126], and UCA1 interacts with miR-144 via genes involved in pre-b cell leukemia. Similarly, UCA1 inhibits apoptosis in lung cancer by causing a G2/M cell cycle stop through a negative-influencing interaction with miR-143 [127]. Similar research has found that UCA1 promotes cervical cancer glycolysis by inhibiting apoptosis via a sponging mechanism with miR-493-5p [142]. CC is brought on by UCA1’s negative feedback mechanism with miRNA-206, which promotes cell development and inhibits apoptosis in a vascular endothelial growth factor (VEGF)-dependent manner [141]. Through interacting with miR-96, the lncRNA UCA1 promoted cellular proliferation and suppressed apoptosis and metastasis in pancreatic cancer. UCA1 promotes cell proliferation and suppresses apoptosis by interacting with miR-135a in pancreatic cancer [134].
Another lncRNA (Opa-interacting macromolecule five antisense polymer one; OIP5-AS1) interacts with microRNA-369e3p to suppress apoptosis and metastasis in colorectal cancer (CRC) cells and increase expression of dual-specificity tyrosine-regulated enzyme 1 A (Dyrk1A) RNA. OIP5-AS1 also interacted with miR-143-3p via a molecular sponge method, preventing apoptosis, cell proliferation, and metastasis in a ROCK1-dependent way [144].
Overexpression of programmed cell death 4 (PDCD4) is caused by the lncRNA MEG3, which interacts with miR-141 through a competitive binding mechanism, making CRC cells resistant to oxaliplatin [117]. Furthermore, lncRNA LIFR antisense polymer one (LIFRAS1) induced drug resistance in CRC by interacting with miR-29a in TNFAIP3 [120]. In addition, HOTAIR is another another lncRNA that, after interacting with miRNA, increases GC advancement via invasion and prevention of apoptosis. Therapeutic applications for lncRNA silencing have been proposed [149]. H19, a lncRNA, interacts with miR-675 to cause GC by blocking apoptosis and metastasis in a Runt Domain Transcription Factor 1 (RUNX1) and Calneuron 1 dependent manner [135, 136].
GAPLINC, another lncRNA, interacts with miR-211-3p in a CD44 CD44-dependent way, inducing GC by inhibiting apoptosis and metastasis via a molecular decoy mechanism [137, 138]. For this reason, knockdown of GAPLINC lncRNA could be used as a therapy for GC [150]. Similar research has shown that the lncRNA ANRIL interacts with the miRNAs miR-99a and miR-449 to prevent apoptosis and metastasis, hence promoting the development of GC. Tumors regressed once NARIL was knocked down, suggesting that this strategy could be used in therapy [139]. Through suppressing apoptosis and metastasis, the lncRNA HOXC6 interacts with the microRNA miR-361-3p to promote CC progression [143].
4.6 Impact of crosstalk of miRNA and lncRNA on metastasis
Malignant malignancies are characterized by metastasis, the spread of tumors to distant tissues [151]. Cancer cells leave their main location to circulate in the circulation, adapting to new biological circumstances in a secondary site in order to withstand pressure in blood arteries and dodge immuno-stimulation by immune cells [152, 153]. Table 6 summarizes the research on how miRNAs and lncRNAs interact with one another to regulate protein-coding genes involved in cancer metastasis [154, 155]. In this article, we are elucidating the pre-clinical relevance of crosstalk between miRNA and lncRNA impacting prevention of apoptosis (Fig. 7).
Renal cell cancer was triggered by DLX6-AS1 through the PTEN pathway and metastasis as a result of its interaction with miR-26a via a negatively impacting mechanism. Tumors may be regressed by silencing the lncRNA DLX6-AS1 [156]. In addition developing brain cancer and glioma through metastasis, DLX6-AS1 interacts with miR-107 in a BDNF-dependent manner, negatively impacting processes.
Brain cancer and glioma are the result of another miRNA-lncRNA interaction: GAS5, which interacts with miR-10b and promotes metastasis by activating mitogen-activated protein kinase kinases (MEK), protein kinase B (AKT), phosphoinositide 3-kinase (PI3K), and extracellular signal-regulated kinase (ERK), and PTEN pathway. Tumor regression may be induced by therapeutic suppression of phosphorylation of PIP3K, AKT, ERK, and MEK pathways [158]. Moreover, GAS5 interacts with miR-18a-5p to promote glioma growth via a mimic mechanism that ultimately leads to metastasis [159]. In addition, GAS5 interacts with MiR-34a in a manner that promotes the spread of renal cell carcinoma and hepatocellular cell carcinoma [160].
Prostate cancer metastasis and cellular proliferation were caused by the lncRNA UCA1 interacting with miRNA [166]. This was dependent on the transcription factors Sirt1 and ATF2. Also, UCA1 interacts with miR-182, leading to osteosarcoma via cellular proliferation and metastasis in a TIMP2-dependent manner [160, 162]. In a Ras-related toxin substrate 1–dependent way, UCA1 interacts with miR-126 via an endogenous sponge mechanism in leukemia [163].
In a Slug and ZEB2 protein-dependent way, the lncRNA MALAT1 interacts with miR-204, leading to breast cancer growth via metastasis. MALAT1 knockdown led to Slug inhibition, which in turn led to tumor regression via increased apoptosis and metastasis [164]. In addition, MALAT1 interacts with miR-1 to promote the spread of breast cancer, a process that is slug-dependent [85].
4.7 Impact of crosstalk of miRNA and lncRNA on invasion
Physical, biological, and molecular variables adapt with cancer progression via signaling pathways to enhance cytoskeletal dynamics for cell-matrix and cell-cell junction turnover, resulting in cell migration into adjacent tissue. [167, 168]. By decreasing or removing intercellular adhesion molecules, the separation of malignant cells from the tumor bulk increases invasiveness. They induce abnormally high motility by allowing the cells to penetrate the structural features of the surrounding stroma [169]. Table 7 summarizes how the interaction between miRNA and lncRNA plays a crucial role in cancer invasion, while the pre-clinical significance of crosstalk between miRNA and lncRNA affecting inhibition of apoptosis, illustrated in Fig. 8.
LncRNA UCA1 interacts with miR-129 via RCC invasion via the SOX4 pathway. The lncRNA UCA1 knockdown caused cancer regression and might potentially be employed for therapeutic interventions [170]. Furthermore, in gastrointestinal cancer progression, UCA1 promotes invasion, cell migration, and apoptosis by interacting with the two miR-203 and miR-7-5p in a zinc finger and homeobox2 homeobox2-dependent way via EGFR [171, 172]. Additionally, UCA1 interacts with miR-196a-5p for bladder cancer through boosting cancer cell invasion by targeting Fascin homologs [173, 174]. UCA1 aided in the regulation of glutamine metabolism and the production of reactive oxygen species (ROS) in bladder cancer cells [175, 176]. Similarly, UCA1 interacted with miR-195 to promote mitochondrial function in ADP-ribosylation factor-like protein 2 dependent ways [177]. UCA1 also interacted with an anti-miR-185-5p gene to increase melanoma cells proliferation, migration, and invasion via control of the Wnt-β-catenin signaling pathway [178, 179]. FOXM1, also known as miR-507, is a tumor suppressor that interacts with the lncRNA UCA1, promoting accelerated melanoma development by targeting the G2/M phase [180, 181]. UCA1 also interacted with miR-122-5p to promote breast cancer growth via invasion and cellular proliferation by up-regulating its target genes via RNA binding protein and inhibiting the HER3 kinase UTR [182]. LncRNA UCA1 inhibits miR-204e5p expression by a similar mechanism that can up-regulate CREB1 expression, resulting in cell proliferation and 5-fluorouracil resistance [113]. UCA1 also aided pancreatic cancer growth by decreasing miR-135a expression [134].
MIIP binds to histone deacetylase 6 (HDAC6) and inhibits cell motility by lowering protein stability, resulting in a positive feedback loop between FOXO1 and GAS5 [190]. TIMP2 is a lncRNA that interacts with miR-182 to drive osteosarcoma invasion in an MMP MMP-dependent way [162].
DLX6-AS1 is another lncRNA that, upon association with miR-129-5p, functions in the formation of OS via the Wnt-beta signaling pathway by overexpression of Delta Like Non-Canonical N-Lept 1. (DLK1). By decreasing the Wnt-beta signaling pathway, DLX6-AS1 and DLK1 hindered self-renewal and stability [183]. Furthermore, DLX6-AS1 increases invasion in lung malignancies via negative regulation via crosstalk with miR-142. The lncRNA knockdown produced tumor regression and can thus be employed therapeutically. The lncRNA DLX6-AS1 is up-regulated in human liver cells and tissues after interacting with miR-203a, promoting invasion in human hepatocellular carcinoma in an MMP2 MMP2-dependent manner. By knocking off the lncRNADLX6-AS1, it suppressed cell proliferation and invasion [128, 129].
Moreover, DLX6-AS1 interacts with miR-424-5p via the STAT3 signaling pathway, promoting invasion in WEE1-dependent hepatocellular cancer [191]. When DLX6-AS1 interacted with miR-181b, it promoted pancreatic invasion via an up-regulated Wnt-β-catenin FZD4/FZD6 signaling pathway that was dependent on the zinc finger E-box-binding protein (ZEB2). The suppression of lncRNA reduced the levels of ZEB2, a regulatory protein involved in the induction of invasion in pancreatic cancer [132]. DLX6-AS1 also interacts with miR-497-1 via an endogenous sponge mechanism in a FZD4 dependent way, inducing pancreatic cancer cells, migration, and invasion [133]. MiR-199a interacts with the lncRNA DLX6-As1 in a FUS-dependent way to negatively influence tumor growth via invasion in CC [188]. The MAP4K1 protein upregulates the interaction of lncRNA DLX6-AS1 with miR-204-5p expression in gastric cancer (GC), promoting invasion [145,146,147]. DLX6-AS1 reduction inhibited tumor proliferation and MAP4K-dependent GC cell stability in an OCT1, MMP9, and SLG SLG-dependent manner. The ERK1 pathway inhibited GC cell growth by downregulating OCT1. As a result, it could be a potential therapeutic target for cancer [148]. OCT1 knock-down decreased DLX6-AS1 expression, which suppressed the expression of cell markers SLUG, MMP2, and N-cadherin [147]. As a result of these discoveries, it is possible that inhibiting lncRNA leads to therapeutic treatments for cancer progression.
The lncRNA HOTAIR interacts with miR-218, miR-545, and miR-203a-3p via a negative impacting mechanism, producing up-regulation of EGFR, β-catenin, Groucho related gene 5 (GRG5), and VOPP1 pathways, resulting in chemotherapy resistance and invasion in CRC [114, 115]. Furthermore, HOTAIR was revealed to be capable of positively modulating human epithelial growth factor receptor 2 (HER2) in GC by competing for miR-331-3p binding, a miRNA with different target specificities for both HOTAIR and HER2. When the lncRNA HOTAIR was knocked out, cell invasion and survival were reduced, but cell invasion, migration, and proliferation were boosted when HOTAIR was expressed ectopically in the GC [187].
LncRNAs bind to miR-34a and disrupt miRNA target gene expression, increasing CRC proliferation, migration, and invasion. LncRNAs such as lnc34a, which is prevalent in gastric adenocarcinoma, colon cancer stem cells (CCSCs), and short nucleolar RNA host gene 7 (SNHG7) bind to miR-34a and produce miRNA dysregulation [36, 184, 185]. Furthermore, Opa-interacting macromolecule five antisense polymer one (OIP5-AS1) interacts with miR-369e3p, leading to an increase in the expression of dual-specificity tyrosine-regulated enzyme 1 A (Dyrk1A) RNA in CRC cells [119]. OIP5-AS1 interacted adversely with miR-143-3p, inducing CC development via cellular proliferation, invasion, and metastasis [144].
Additionally, the lncRNA LIFR antisense polymer one (LIFRAS1), when interacting with miR-29a in a tumor gangrene issue (TNFAIP3) dependent manner, has a function in treatment resistance in CRC [120]. The lncRNA H19-derived from miR-675 may play an oncogenic role in gastric carcinogenesis by enhancing cell proliferation, metastasis, invasion, and migration by modifying specific miR-675 targets for poor prognosis [135, 136]. GAPLINC is another lncRNA that interacts with miR-211-3p by negatively impacting the mechanism in a CD44 oncogene-dependent manner, causing cellular proliferation and invasion [137, 138, 150]. The interaction of lncRNA HOXC6 and miR-361-3p induces upregulation, which leads to CC development [143]. The interaction of lncRNA GAS5 and miRNA 21 has been described for its role in cancer as an oncogene targeting protein-coding genes for CC advancement via invasion and metastasis via the PTEN pathway in a PDCD4 and Spry1-dependent manner [189].
5 Perspectives and Expert Opinion
Cancer progression depends on extend of various factors (such as, angiogenesis, neovascularization, vascular mimicry, invasion, metastasis, epithelial-mesenchymal transition and inhibition of apoptosis) where miRNA and lncRNA interplay found critical. The inhibition of the lncRNA via knockdown or silencing typically inhibit the tumor progression. Therefore, the miRNA involved in the crosstalk could be utilized as a biomarker for the early detection of respective cancer. The downstream effector molecules of the signaling pathway could be used as potential therapeutic target sites for future drug discovery and utilized for tumor regression by targeting them via specific inhibitors. This review discussed various possibilities, where the knockdown of the lncRNA has been reported to cause tumor regression. But this method of knockdown or silencing of lncRNA for therapeutics poses a significant challenge on a large scale. In the future, we can therefore employ genome editing technologies such as ZFNs, TALENs, CRISPR to delete the necessary gene upon delivery by viral or non-viral vectors to regulate tumor progression [192, 193]. Because of the different types of cancers, tailored treatments supported by green nanotechnology could be used in clinical settings alongside mass production. We have also discussed a wide range of applications for miRNA as a diagnostic biomarker. Thus, it is important to consider conducting a clinical or pre-clinical evaluation of these biomarkers. Furthermore, only a select few scenarios are illustrated addressed in pre-clinical investigations where signals of interplay must be considered. Hence, research into signaling networks should be of interest.
6 Conclusion
In conclusion, we mentioned the interaction that occurs between microRNA and long noncoding RNA, thats affects cellular functions (such as neovascularization, VM, EMT, and angiogenesis). In addition, this paper discusses a pre-clinical research on various type of malignancies encompasses the role of crosstalk between lncRNA and miRNA. This paper also focuses on developing targeting strategies for immunomodulating the cancer through cellular signaling pathways. Based on the crossplay features of lncRNAs and miRNAs, critical for cellular functions, novel cancer theranostics can be developed, such as new biomarkers or selective targeted therapy (by silencing lncRNA or knocking down miRNA levels). We extensively reviewed the essential roles of microRNA and long noncoding RNA in gene regulation so that cell-specific therapies can be developed.
Abbreviations
- AGO:
-
Argonaute
- ATG7:
-
Autophagy Related 7
- BC:
-
Breast Cancer
- CAFs:
-
Cancer associated fibroblasts
- CCAT1:
-
Colon Cancer Associated Transcript 1
- CRC:
-
Colorectal Cancer
- DLK1:
-
Protein delta homolog 1
- DLX6-AS1:
-
Distal-less homeobox 6 antisense 1
- EC:
-
Esophageal cancer
- EMT:
-
Epithelial mesenchymal transition
- FAK:
-
Focal adhesion kinase
- FOXM1:
-
Forkhead box protein M1
- FUS:
-
Fused in sarcoma
- GAPLINC:
-
Gastric Adenocarcinoma Associated, Positive CD44 Regulator, Long Intergenic Noncoding RNA
- GAS5:
-
Growth arrest-specific
- GC:
-
Gastric cancer
- GIC:
-
Gastrointestinal Cancer
- HCC:
-
Hepatocellular Carcinoma
- HDAC4:
-
Human Histone Deacetylase 4
- HGF:
-
Hepatocyte Growth Factor
- HIF-1:
-
Hypoxia-inducible factor 1
- HMGA2:
-
High Mobility Group AT-Hook 2
- HSP90:
-
Heat shock protein 9
- LC:
-
Lung Cancer
- LncRNAs:
-
Long noncoding RNAs
- LIFR:
-
Leukemia inhibitory factor receptor
- MALAT1:
-
Metastasis Associated Lung Adenocarcinoma Transcript 1
- MAP4K:
-
Mitogen-activated protein kinase
- MEG3:
-
Maternally expressed gene 3
- miRNAs:
-
microRNAs
- MMP9:
-
Matrix metallopeptidase 9
- MRE:
-
MicroRNA Response Element
- NSCLC:
-
Non-small-cell lung carcinoma
- OCT1:
-
Octamer-binding transcription factor 1
- OC:
-
Ovarian Cancer
- PC:
-
Pancreatic Cancer
- PTEN:
-
Phosphatase and tensin homolog
- SLUG:
-
Zinc-finger transcription factor SNAI2
- SOX:
-
SRY-Box Transcription Factor
- STAT3:
-
Signal transducer and activator of transcription 3
- TC:
-
Tongue cancer
- TGF:
-
Transforming growth factor
- TME:
-
Tumor Microenvironment
- UCA1:
-
Urothelial carcinoma associated 1
- UICLM:
-
Upregulated in colorectal cancer liver metastasis
- WTAPP1:
-
Wilms tumor 1 associated protein pseudogene 1
- XIST:
-
X-inactive specific transcript
- ZEB2:
-
Zinc finger E-Box binding homeobox 2
References
D. Hanahan, R.A. Weinberg, Cell 144, 646–674 (2011)
R. Bhattacharjee, T. Dey, L. Kumar, S. Kar, R. Sarkar, M. Ghorai, S. Malik, N.K. Jha, B. Vellingiri, K.K. Kesari,. Dey, J.M. Pérez de la Lastra and A. Biomed. Pharmacother 153, 113345 (2022). https://doi.org/10.1016/j.biopha.2022.113345
R. Bhattacharjee, S. Ghosh, A. Nath, A. Basu, O. Biswas, C.R. Patil, C.N. Kundu, Crit. Rev. Oncol. Hematol 177, 103753 (2022). https://doi.org/10.1016/j.critrevonc.2022.103753
R. Bhattacharjee, L. Kumar, A. Dhasmana, T. Mitra, A. Dey, S. Malik, B. Kim, R. Gundamaraju, Frontiers in oncology 12, (2022)
R. Bhattacharjee, P. Mitra, N. Gupta, S. Sharma, V.K. Singh, N. Mukerjee, A. Dhasmana, R. Gundamaraju, Adv. Cancer Biology - Metastasis 5, 100050 (2022). https://doi.org/10.1016/j.adcanc.2022.100050
R. Bhattacharjee, S.S. Das, S.S. Biswal, A. Nath, D. Das, A. Basu, S. Malik, L. Kumar, S. Kar, S.K. Singh, V.J. Upadhye, D. Iqbal, S. Almojam, S. Roychoudhury, S. Ojha, J. Ruokolainen, N.K. Jha, K.K. Kesari, Crit. Rev. Oncol. Hematol 174, 103675 (2022). https://doi.org/10.1016/j.critrevonc.2022.103675
X. Qi, Y. Lin, J. Chen, B. Shen, Brief. Bioinform 21, 441–457 (2020)
A.D. Campos-Parra, E. López-Urrutia, L.T. Orozco Moreno, C. López-Camarillo, T. Meza-Menchaca, G. Figueroa, L.P. González, Bustamante Montes and C. Pérez-Plasencia, Int. J.Mol. Sci 19, 2711 (2018)
B.D. Adams, A.L. Kasinski, F.J. Slack, Curr. Biol 24, R762–R776 (2014)
A. Sinha, R. Bhattacharjee, B. Bhattacharya, A. Nandi, R. Shekhar, A. Jana, K. Saha, L. Kumar, S. Patro, P.K. Panda, Biomed. Pharmacother 159, 114269 (2023)
M.B. Clark, R.L. Johnston, M. Inostroza-Ponta, A.H. Fox, E. Fortini, P. Moscato, M.E. Dinger, J.S. Mattick, Genome Res 22, 885–898 (2012)
J.E. Wilusz, H. Sunwoo, D.L. Spector, Genes Dev 23, 1494–1504 (2009)
A.D. Campos-Parra, G.C. Mitznahuatl, A. Pedroza-Torres, R.V. Romo, F.I.P. Reyes, E. López-Urrutia and C. Pérez-Plasencia, Int. J. Mol. Sci. 18, 1182 (2017)
T.R. Mercer, M.E. Dinger, J.S. Mattick, Nat. Rev. Genet 10, 155–159 (2009)
J.K. Dhanoa, R.S. Sethi, R. Verma, J.S. Arora, C.S. Mukhopadhyay, J. Anim. Sci. Technol. 60, 1–10 (2018)
J.R. Prensner, M.K. Iyer, O.A. Balbin, S.M. Dhanasekaran, Q. Cao, J.C. Brenner, B. Laxman, I.A. Asangani, C.S. Grasso, H.D. Kominsky, Nat. Biotechnol 29, 742–749 (2011)
R.A. Gupta, N. Shah, K.C. Wang, J. Kim, H.M. Horlings, D.J. Wong, M.-C. Tsai, T. Hung, P. Argani, J.L. Rinn, Nature 464, 1071–1076 (2010)
Y. Qian, L. Shi, Z. Luo, Front. Med. 7, 902 (2020)
X. Meng, A. Li, B. Yu, S. Li, Comput. Struct. Biotechnol. J. 19, 2567–2574 (2021). https://doi.org/10.1016/j.csbj.2021.04.062
J. Xu, M. Wu, Y. Sun, H. Zhao, Y. Wang, J. Gao, BioMed Research International 2020 (2020)
S. Ergun, S. Oztuzcu, Tumour Biol. 36, 3129–3136 (2015) https://doi.org/10.1007/s13277-015-3346-x
K. Neelakandan, P. Babu, S. Nair, Curr. Cancer Drug Targets 12, 716–740 (2012). https://doi.org/10.2174/156800912801784875
Y. Li, X. Wang, Mol. Med. Rep. 13, 1463–1469 (2016). https://doi.org/10.3892/mmr.2015.4711
P.K. Lo, B. Wolfson, X. Zhou, N. Duru, R. Gernapudi, Q. Zhou, Brief. Funct. Genomics 15, 200–221 (2016). https://doi.org/10.1093/bfgp/elv055
K. Takayama, S. Inoue, Brief. Funct. Genomics 15, 257–265 (2016). https://doi.org/10.1093/bfgp/elv057
Y.J. Xu, Y. Du, Y. Fan, Clin. Transl. Onc. 18, 660–665 (2016) https://doi.org/10.1007/s12094-015-1448-y
S. Jalali, D. Bhartiya, M.K. Lalwani, S. Sivasubbu, V. Scaria, PloS One 8, e53823 (2013) https://doi.org/10.1371/journal.pone.0053823
M.D. Paraskevopoulou, A.G. Hatzigeorgiou, Methods in molecular biology. (Clifton, N.J) 1402, 271–286 (2016). https://doi.org/10.1007/978-1-4939-3378-5_21
E.A. Gibb, C.J. Brown, W.L. Lam, Mol. Cancer 10, 38 (2011). https://doi.org/10.1186/1476-4598-10-38
E. López-Urrutia, L.P. Bustamante Montes, D. Ladrón de Guevara, C. Cervantes, Pérez-Plasencia, A.D. Campos-Parra, Front. Oncol 9, (2019) https://doi.org/10.3389/fonc.2019.00669
L. Salmena, L. Poliseno, Y. Tay, L. Kats, P.P. Pandolfi, Cell 146, 353–358 (2011)
B.P. Lewis, C.B. Burge, D.P. Bartel, Cell 120, 15–20 (2005). https://doi.org/10.1016/j.cell.2004.12.035
P. Furió-Tarí, S. Tarazona, T. Gabaldón, A.J. Enright, A. Conesa, Nucleic Acids Res 44, W176-W180 (2016) https://doi.org/10.1093/nar/gkw443
B. Sun, C. Liu, H. Li, L. Zhang, G. Luo, S. Liang, M. Lü, Oncol. Lett. 19, 595–605 (2020). https://doi.org/10.3892/ol.2019.11182
R. Sen, S. Ghosal, S. Das, S. Balti, J. Chakrabarti, The Scientific World Journal 2014, 896206 (2014) https://doi.org/10.1155/2014/896206
L. Wang, P. Bu, Y. Ai, T. Srinivasan, H.J. Chen, K. Xiang, S.M. Lipkin, X. Shen, Elife 5, e14620 (2016)
W.H. Majoros, U. Ohler, BMC Genom 8, 1–9 (2007)
Y. Wang, Z. Xu, J. Jiang, C. Xu, J. Kang, L. Xiao, M. Wu, J. Xiong, X. Guo, H. Liu, Dev. Cell 25, 69–80 (2013)
Z. Fang, J. Zhao, W. Xie, Q. Sun, H. Wang, B. Qiao, Cancer Med 6, 2897–2908 (2017). https://doi.org/10.1002/cam4.1253
Y. Zhou, X. Wang, J. Zhang, A. He, Y.L. Wang, K. Han, Y. Su, J. Yin, X. Lv, H. Hu, Oncotarget 8, 18260–18270 (2017). https://doi.org/10.18632/oncotarget.15353
U.A. Ørom, R. Shiekhattar, Curr. Opin. Genet. Dev. 21, 194–198 (2011) https://doi.org/10.1016/j.gde.2011.01.020
A. Keniry, D. Oxley, P. Monnier, M. Kyba, L. Dandolo, G. Smits, W. Reik, Nat. Cell Biol 14, 659–665 (2012). https://doi.org/10.1038/ncb2521
L.Da Sacco, A. Masotti, Int. J. Mol. Sci 14, 480–495 (2012). https://doi.org/10.3390/ijms14010480
L. Guo, Y. Zhao, S. Yang, H. Zhang, F. Chen, Biomed. Res. Int 2014, 345605 (2014) https://doi.org/10.1155/2014/345605
Z. Zhang, L. Sun, Y. Zhang, G. Lu, Y. Li, Z. Wei, J. Cell. Physiol 233, 8630–8638 (2018). https://doi.org/10.1002/jcp.26611
P.D. Hsu, D.A. Scott, J.A. Weinstein, F.A. Ran, S. Konermann, V. Agarwala, Y. Li, E.J. Fine, X. Wu, O. Shalem, T.J. Cradick, L.A. Marraffini, G. Bao, F. Zhang, Nat. Biotechnol 31, 827–832 (2013). https://doi.org/10.1038/nbt.2647
Y. Lu, X. Zhao, Q. Liu, C. Li, R. Graves-Deal, Z. Cao, B. Singh, J.L. Franklin, J. Wang, H. Hu, Nat. Med 23, 1331–1341 (2017)
Z. He, Y. Wang, G. Huang, Q. Wang, D. Zhao, L. Chen, Arch. Biochem. Biophys 623, 1–8 (2017)
M. Cui, M. Chen, Z. Shen, R. Wang, X. Fang, B. Song, J. Cell. Biochem 120, 6926–6936 (2019)
Y. Wang, Z. Hou, D. Li, Mol. Med. Rep 18, 3068–3076 (2018)
D. Li, C. Cui, J. Chen, Z. Hu, Y. Wang, D. Hu, Mol. Med. Rep 18, 3059–3067 (2018)
H. Liu, R. Li, L. Guan, T. Jiang, Onco Targets Ther. 11, 7197 (2018)
P. Cao, Z. Deng, M. Wan, W. Huang, S.D. Cramer, J. Xu, M. Lei, G. Sui, Mol. Cancer 9, 1–12 (2010)
S. Sander, L. Bullinger, K. Klapproth, K. Fiedler, H.A. Kestler, T.F. Barth, P. Möller, S. Stilgenbauer, J.R. Pollack, T. Wirth, Blood, The Journal of the American Society of Hematology 112, 4202–4212 (2008)
L. Benetatos, E. Voulgaris, G. Vartholomatos, E. Hatzimichael, Int. J. Cancer 133, 267–274 (2013)
M. Sun, W.L. Kraus, Endocr. Rev 36, 25–64 (2015). https://doi.org/10.1210/er.2014-1034
L. Zhang, F. Yang, J. Yuan, S. Yuan, W. Zhou, X. Huo, D. Xu, H.-s. Bi, F. Wang and S.-h. Sun, Carcinogenesis 34, 577–586 (2013)
G.A. Calin, C. Liu, M. Ferracin, T. Hyslop, R. Spizzo, C. Sevignani, M. Fabbri, A. Cimmino, E.J. Lee, S.E. Wojcik, Cancer cell. 12, 215–229 (2007)
G.A. Calin, M. Ferracin, A. Cimmino, G. Di Leva, M. Shimizu, S.E. Wojcik, M.V. Iorio, R. Visone, N.I. Sever, M. Fabbri, N. Engl. J. Med 353, 1793–1801 (2005)
R. Ronca, M. Benkheil, S. Mitola, S. Struyf, S. Liekens, Med. Res. Rev 37, 1231–1274 (2017)
D.R. Senger, G.E. Davis, Cold Spring Harbor Perspect. Biol. 3, a005090 (2011)
N. Naoyo, Y. Hirohisa, N. Takashi, K. Toshiharu, K. Masamichi, Vasc Health Risk Manag 2, 213–219 (2006)
H. Cai, X. Liu, J. Zheng, Y. Xue, J. Ma, Z. Li, Z. Xi, M. Bao, Y. Liu, Oncogene 36, 318–331 (2017)
C.-Z. Zhang, Biochem. Biophys. Res. Commun 489, 404–412 (2017)
H. Quan, M. Liang, N. Li, C. Dou, C. Liu, Y. Bai, W. Luo, J. Li, F. Kang, Z. Cao, Cell. Physiol. Biochem 46, 401–417 (2018)
R. Dong, G. Liu, B. Liu, G. Chen, K. Li, S. Zheng, K. Dong, Cell Death Dis 7, e2278–e2278 (2016)
Z. Wang, R. Wang, K. Wang, X. Liu, Can. J. Physiol. Pharmacol 96, 909–915 (2018)
P. Jia, H. Cai, X. Liu, J. Chen, J. Ma, P. Wang, Y. Liu, J. Zheng, Y. Xue, Cancer Lett 381, 359–369 (2016). https://doi.org/10.1016/j.canlet.2016.08.009
W.D. Li, D.M. Zhou, L.L. Sun, L. Xiao, Z. Liu, M. Zhou, W.B. Wang, X.Q. Li, Stem Cells 36, 1863–1874 (2018)
H. Liu, Y. Kato, S.A. Erzinger, G.M. Kiriakova, Y. Qian, D. Palmieri, P.S. Steeg, J.E. Price, BMC cancer 12, 1–11 (2012)
Y. Takahashi, T. Koyanagi, Y. Suzuki, Y. Saga, N. Kanomata, T. Moriya, M. Suzuki, Y. Sato, Mol. Cancer Res 10, 1135–1146 (2012)
M. Sholley, G. Ferguson, H. Seibel, J. Montour, J. Wilson, Lab. Invest 51, 624–634 (1984)
Y.F. Ping, X.W. Bian, Stem cells 29, 888–894 (2011)
N. Stahnke, Hormone Res. Paediatrics 38, 56–62 (1992)
G. Bergers, L.E. Benjamin, Nat. Rev. Cancer 3, 401–410 (2003)
L. Deng, S.-B. Yang, F.-F. Xu, J.-H. Zhang, J. experimental Clin. cancer Res. 34, 1–10 (2015)
J. Li, X. Liu, S. Zang, J. Zhou, F. Zhang, B. Sun, D. Qi, X. Li, J. Kong, D. Jin, Cancer Lett 492, 71–83 (2020)
H. Zhao, R. Peng, Q. Liu, D. Liu, P. Du, J. Yuan, G. Peng, Y. Liao, Arch. Biochem. Biophys. 610, 1–7 (2016). https://doi.org/10.1016/j.abb.2016.09.014
Y. Ma, P. Wang, Y. Xue, C. Qu, J. Zheng, X. Liu, J. Ma, Y. Liu, Tumor Biology 39, 1010428317694326 (2017)
J.H. Yoon, K. Abdelmohsen, M. Gorospe, Semin. Cell Dev. Biol 34, 9–14 (2014). https://doi.org/10.1016/j.semcdb.2014.05.015
J. Liz, M. Esteller, Biochim. Biophys. Acta 1859, 169–176 (2016) https://doi.org/10.1016/j.bbagrm.2015.06.015
C. Yang, L.G. Di Wu, X. Liu, Y. Jin, D. Wang, T. Wang, X. Li, Oncotarget 7, 13479 (2016)
J.H. Tsai, J.L. Donaher, D.A. Murphy, S. Chau, J. Yang, Cancer cell. 22, 725–736 (2012)
W.-C. Liang, W.-M. Fu, C.-W. Wong, Y. Wang, W.-M. Wang, G.-X. Hu, L. Zhang, L.-J. Xiao, D.C.-C. Wan, J.-F. Zhang, M.M.-Y. Waye, Oncotarget 6, 22513–22525 (2015). https://doi.org/10.18632/oncotarget.4154
C. Jin, B. Yan, Q. Lu, Y. Lin, L. Ma, Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine 37, 7383–7394 (2016). https://doi.org/10.1007/s13277-015-4605-6
D. Xu, P. Dong, Y. Xiong, J. Yue, Y. Konno, K. Ihira, N. Kobayashi, Y. Todo, H. Watari, Cells 9, 632 (2020)
Y. Tao, T. Han, T. Zhang, C. Ma, C. Sun, Oncotarget 8, 36410 (2017)
D.-L. Chen, Y.-X. Lu, J.-X. Zhang, X.-L. Wei, F. Wang, Z.-L. Zeng, Z.-Z. Pan, Y.-F. Yuan, F.-H. Wang, H. Pelicano, P.J. Chiao, P. Huang, D. Xie, Y.-H. Li, H.-Q. Ju, R.-H. Xu, Theranostics 7, 4836–4849 (2017). https://doi.org/10.7150/thno.20942
X. Zhang, B. Sai, F. Wang, L. Wang, Y. Wang, L. Zheng, G. Li, J. Tang, J. Xiang, Mol. Cancer 18, 1–15 (2019)
Z. Li, P. Jiang, J. Li, M. Peng, X. Zhao, X. Zhang, K. Chen, Y. Zhang, H. Liu, L. Gan, H. Bi, P. Zhen, J. Zhu, X. Li, Oncogene 37 (2018) https://doi.org/10.1038/s41388-018-0237-9
L. Zhang, Z. Yang, W. Huang, J. Wu, Cell Death Dis 10, 168 (2019). https://doi.org/10.1038/s41419-019-1423-6
Y. An, X. Chen, Y. Yang, F. Mo, Y. Jiang, D.-l. Sun and H.-h. Cai. Cancer Cell Int 18, 143 (2018). https://doi.org/10.1186/s12935-018-0643-7
C. Liao, Z. Long, X. Zhang, J. Cheng, F. Qi, S. Wu, T. Huang, Int. J. Biol. Sci 16, 1–11 (2020). https://doi.org/10.7150/ijbs.39461
C. Fang, L. Xu, W. He, J. Dai, F. Sun, Cell. cycle 18, 3288–3299 (2019)
Z. Li, H. Liu, Q. Zhong, J. Wu, Z. Tang, FEBS Open. Bio 8, 1855–1865 (2018)
M. Nishioka, N. Venkatesan, K. Dessalle, A. Mogas, S. Kyoh, T.Y. Lin, P. Nair, C.J. Baglole, D.H. Eidelman, M.S. Ludwig, Q. Hamid, Respir. Res 16, 72 (2015). https://doi.org/10.1186/s12931-015-0232-4
J. Wu, J. Zhang, B. Shen, K. Yin, J. Xu, W. Gao, L. Zhang, J. experimental Clin. cancer research: CR 34, 116 (2015). https://doi.org/10.1186/s13046-015-0229-3
T. Brabletz, R. Kalluri, M.A. Nieto, R.A. Weinberg, Nat. Rev. Cancer 18, 128–134 (2018). https://doi.org/10.1038/nrc.2017.118
A.M. Roccaro, A. Sacco, P. Maiso, A.K. Azab, Y.-T. Tai, M. Reagan, F. Azab, L.M. Flores, F. Campigotto, E. Weller, J. Clin. Investig 123, 1542–1555 (2013)
M.T. Le, P. Hamar, C. Guo, E. Basar, R. Perdigão-Henriques, L. Balaj, J. Lieberman, J. Clin. Investig 124, 5109–5128 (2014). https://doi.org/10.1172/jci75695
X. Wang, G. Luo, K. Zhang, J. Cao, C. Huang, T. Jiang, B. Liu, L. Su, Z. Qiu, Cancer Res 80, 922–922 (2020)
A.J. Maniotis, R. Folberg, A. Hess, E.A. Seftor, L.M. Gardner, J. Pe’er, J.M. Trent, P.S. Meltzer, M.J. Hendrix, Am. J. Pathol 155, 739–752 (1999)
Y. Gao, H. Yu, Y. Liu, X. Liu, J. Zheng, J. Ma, W. Gong, J. Chen, L. Zhao, Y. Tian, Cell. Physiol. Biochem 45, 131–147 (2018)
J. Guo, H. Cai, X. Liu, J. Zheng, Y. Liu, W. Gong, J. Chen, Z. Xi, Y. Xue, Mol. Therapy-Nucleic Acids 10, 170–186 (2018)
W. Tao, W. Sun, H. Zhu, J. Zhang, Biochem. Biophys. Res. Commun 504, 629–634 (2018)
X. Zhao, B. Sun, T. Liu, B. Shao, R. Sun, D. Zhu, Y. Zhang, Q. Gu, X. Dong, F. Liu, Cancer Sci 109, 3197–3208 (2018)
H. Lu, Y. He, L. Lin, Z. Qi, L. Ma, L. Li, Y. Su, Tumor Biology 37, 1683–1691 (2016)
J. Lopez, S. Tait, Br. J. Cancer 112, 957–962 (2015)
J.L. Koff, S. Ramachandiran, L. Bernal-Mizrachi, Int. J. Mol. Sci 16, 2942–2955 (2015)
M. Soudyab, M. Iranpour, S. Ghafouri-Fard, Arch. Iran. Med 19, 0–0 (2016)
N. Jiang, X. Zhang, X. Gu, X. Li, L. Shang, Cell. Death Discovery 7, 1–11 (2021)
F.R. Zhou, Z.P. Pan, F. Shen, L.Q. Huang, J.H. Cui, K. Cai, X.L. Guo, Eur. Rev. Med. Pharmacol. Sci. 23, 3742–3748 (2019). https://doi.org/10.26355/eurrev_201905_17800
Z. Bian, L. Jin, J. Zhang, Y. Yin, C. Quan, Y. Hu, Y. Feng, H. Liu, B. Fei, Y. Mao, Sci. Rep 6, 1–12 (2016)
Z. Xiao, Z. Qu, Z. Chen, Z. Fang, K. Zhou, Z. Huang, X. Guo, Y. Zhang, Cell. Physiol. Biochem 46, 1275–1285 (2018)
X. Huang, S. Lu, Mol. Cell. Biochem 431, 45–54 (2017)
P. Li, X. Zhang, L. Wang, L. Du, Y. Yang, T. Liu, C. Li, C. Wang, Mol. Therapy-Nucleic Acids 8, 356–369 (2017)
H. Wang, H. Li, L. Zhang, D. Yang, Biomed. Pharmacother 106, 1607–1615 (2018)
Y. Zou, S. Yao, X. Chen, D. Liu, J. Wang, X. Yuan, J. Rao, H. Xiong, S. Yu, X. Yuan, F. Zhu, G. Hu, Y. Wang, H. Xiong, Eur. J. Cell Biol 97, 369–378 (2018). https://doi.org/10.1016/j.ejcb.2018.04.005
K. Liu, H. Yao, Y. Wen, H. Zhao, N. Zhou, S. Lei, L. Xiong, Biochim. Biophys. Acta - Mol. Basis Disease 1864, 2871–2880 (2018)
K. Liu, H. Yao, Y. Wen, H. Zhao, N. Zhou, S. Lei, L. Xiong, Biochimica Biophysica Acta. Mol. basis disease 1864, 2871–2880 (2018). https://doi.org/10.1016/j.bbadis.2018.05.020
X. Li, Y. Wu, A. Liu, X. Tang, Tumour Biol. 37, 14733–14743 (2016). https://doi.org/10.1007/s13277-016-5348-8
Y. Huang, R. Ni, J. Wang, Y. Liu, Biomed. Pharmacother 109, 1851–1859 (2019)
W. Sun, L. Zhang, R. Yan, Y. Yang, X. Meng, OncoTargets Ther. 12, 3945 (2019)
H. Liang, M. Liu, X. Yan, Y. Zhou, W. Wang, X. Wang, Z. Fu, N. Wang, S. Zhang, Y. Wang, J. Biol. Chem 290, 926–940 (2015)
D. Li, H. Li, Y. Yang, L. Kang, Oncol. Res 26, 537 (2018)
Y. Guo, L. Ying, Y. Tian, P. Yang, Y. Zhu, Z. Wang, F. Qiu, J. Lin, FEBS J 280, 4531–4538 (2013)
S. Guo, P. Yang, X. Jiang, X. Li, Y. Wang, X. Zhang, B. Sun, Y. Zhang, Y. Jia, Oncotarget 8, 644 (2017)
D. Li, X. Tang, M. Li, Y. Zheng, J. Cell. Biochem 120, 12290–12299 (2019)
L. Zhang, X. He, T. Jin, L. Gang, Z. Jin, Biomed. Pharmacother 96, 884–891 (2017)
F. Wang, H.-Q. Ying, B.-S. He, Y.-Q. Pan, Q.-W. Deng, H.-L. Sun, J. Chen, X. Liu, S.-K. Wang, Oncotarget 6, 7899 (2015)
G. Zhu, X. Liu, Y. Su, F. Kong, X. Hong, Z. Lin, Oncol. Res 27, 55 (2018)
Y. An, X. Chen, Y. Yang, F. Mo, Y. Jiang, D.-l. Sun and H.-h. Cai. Cancer Cell Int 18, 1–12 (2018)
J. Yang, Z. Ye, D. Mei, H. Gu, J. Zhang, Cancer Manage. Res. 11, 4209–4221 (2019). https://doi.org/10.2147/cmar.s194453
X. Zhang, F. Gao, L. Zhou, H. Wang, G. Shi, X. Tan, Oncol. Res 25, 1529 (2017)
H. Li, B. Yu, J. Li, L. Su, M. Yan, Z. Zhu, B. Liu, Oncotarget 5, 2318 (2014)
M. Zhuang, W. Gao, J. Xu, P. Wang, Y. Shu, Biochem. Biophys. Res. Commun 448, 315–322 (2014)
D. Naor, S. Nedvetzki, I. Golan, L. Melnik, Y. Faitelson, Crit. Rev. Clin. Lab. Sci 39, 527–579 (2002)
R. Marhaba, M. Zöller, J. Mol. Histol 35, 211–231 (2004)
E. Zhang, R. Kong, D. Yin, L. You, M. Sun, L. Han, T.-p. Xu, R. Xia, J.-s. Yang and W. De, Oncotarget 5, 2276 (2014)
X. Wang, Y. Lin, J. Liu, Mol. Med. Rep 19, 1248–1255 (2019)
Q. Yan, Y. Tian, F. Hao, Oncology Research Featuring Pre-clinical and Clinical Cancer Therapeutics, (2021)
F. Wu, D. Zhou, Y. Cui, G. Shen, Y. Li, F. Wei, Int. J. Clin. Exp. Pathol. 11, 3943–3951 (2018)
J. Xu, B. Yang, L. Wang, Y. Zhu, X. Zhu, Z. Xia, Z. Zhao, L. Xu, Cell Prolif 53, e12823 (2020)
L. Song, L. Wang, X. Pan, C. Yang, Brazilian Journal of Medical and Biological Research 53, (2020)
X. Fu, Y. Tian, W. Kuang, S. Wen, W. Guo, Experimental and therapeutic medicine 17, 4715–4722 (2019)
Q. Wu, J. Ma, W. Meng, P. Hui, Cancer Biol. Ther 21, 17–25 (2020)
Y. Liang, C.-D. Zhang, C. Zhang, D.-Q. Dai, Gastric Cancer 23, 212–227 (2020)
K. Vázquez-Arreguín, D. Tantin, Biochim. Biophys. Acta (BBA)-Gene Regulatory Mechanisms 1859, 792–804 (2016)
J. Zhang, W. Qiu, H. Zhu, H. Liu, J. Sun, Y. Chen, H. Shen, C.-l. Qian and Z.-y. Shen. Cell Death Dis 11, 780 (2020). https://doi.org/10.1038/s41419-020-02946-4
Y. Hu, J. Wang, J. Qian, X. Kong, J. Tang, Y. Wang, H. Chen, J. Hong, W. Zou, Y. Chen, Cancer Res 74, 6890–6902 (2014)
G.P. Gupta, J. Massagué, Cell 127, 679–695 (2006)
Maitra A. Molecular envoys pave the way for pancreatic cancer to invade the liver. Nature 567, 181–182 (2019). https://doi.org/10.1038/d41586-019-00710-z
J. Massagué, A.C. Obenauf, Nature 529, 298–306 (2016)
Z. Shi, Q. Wei, J. She, Critical Reviews™ in Eukaryotic Gene Expression 24, (2014)
X. Shen, P. Qi, X. Du, Mod. Pathol 28, 4–13 (2015)
X. Zeng, Z. Hu, X. Ke, H. Tang, B. Wu, X. Wei, Z. Liu, Cell. Cycle 16, 2212–2219 (2017)
H. Zhang, M. Xing, J. Guo, J. Zhao, X. Chen, Z. Jiang, H. Zhang, Q. Dong, Cancer Cell Int 19, 313 (2019). https://doi.org/10.1186/s12935-019-0968-x
Y. Ding, J. Wang, H. Zhang, H. Li, Biofactors 46, 487–496 (2020)
Q. Liu, W. Yu, S. Zhu, K. Cheng, H. Xu, Y. Lv, X. Long, L. Ma, J. Huang, S. Sun, K. Wang, J. Cell. Physiol 234, 757–768 (2018). https://doi.org/10.1002/jcp.26889
E.A. Toraih, S.A. Alghamdi, A. El-Wazir, M.M. Hosny, M.H. Hussein, M.S. Khashana, M.S. Fawzy, PloS one 13, e0198231 (2018)
Z. Zhang, Z. Zhu, K. Watabe, X. Zhang, C. Bai, M. Xu, F. Wu, Y.Y. Mo, Cell Death Differ 20, 1558–1568 (2013). https://doi.org/10.1038/cdd.2013.110
Q. Li, W. Xing, X. Gong, Y. Wang, Cell. Physiol. Biochem 51, 1149–1163 (2018)
M. Sun, Y. Zheng, L. Wang, H. Zhao, S. Yang, Eur. Rev. Med. Pharmacol. Sci. 22, 2233–2245 (2018)
Y. Wang, Y. Zhou, Z. Yang, B. Chen, W. Huang, Y. Liu, Y. Zhang, Tumor Biology 39, 1010428317690998 (2017)
M. Li, M. Pan, C. You, F. Zhao, D. Wu, M. Guo, H. Xu, F. Shi, D. Zheng, J. Dou, Breast Cancer Res 22, 26 (2020). https://doi.org/10.1186/s13058-020-01264-z
C. He, X. Lu, F. Yang, L. Qin, Z. Guo, Y. Sun, J. Wu, Biosci. Rep 39, (2019) https://doi.org/10.1042/bsr20181465
P. Friedl, S. Alexander, Cell 147, 992–1009 (2011)
P. Friedl, K. Wolf, Nat. Rev. Cancer 3, 362–374 (2003)
T. Grudinskaya, A. Kovalev, K. Kovalev, T. Kuznetsova, Oncology 14, 126–129 (2012)
Q. Liu, Y. Li, W. Lv, G. Zhang, X. Tian, X. Li, H. Cheng, C. Zhu, OncoTargets and therapy 11, 2475 (2018)
P. Gong, F. Qiao, H. Wu, H. Cui, Y. Li, Y. Zheng, M. Zhou, H. Fan, Cell Death Dis 9, 1–14 (2018)
Z. Yang, X. Shi, C. Li, X. Wang, K. Hou, Z. Li, X. Zhang, Y. Fan, X. Qu, X. Che, Exp. Cell Res 368, 194–201 (2018)
J. Luo, J. Chen, H. Li, Y. Yang, H. Yun, S. Yang, X. Mao, Oncol. Lett. 14, 5556–5562 (2017)
J. Pan, X. Li, W. Wu, M. Xue, H. Hou, W. Zhai, W. Chen, Cancer Lett 382, 64–76 (2016). https://doi.org/10.1016/j.canlet.2016.08.015
H.-J. Li, X. Li, H. Pang, J.-J. Pan, X.-J. Xie, W. Chen, Jpn J. Clin. Oncol 45, 1055–1063 (2015)
R.A. Cairns, I.S. Harris, T.W. Mak, Nat. Rev. Cancer 11, 85–95 (2011)
H.-J. Li, X.-M. Sun, Z.-K. Li, Q.-W. Yin, H. Pang, J.-J. Pan, X. Li, W. Chen, Cell. Physiol. Biochem 43, 2548–2561 (2017)
X. Chen, J. Gao, Y. Yu, Z. Zhao, Y. Pan, Gene 676, 298–305 (2018)
J. He, N. Tian, Y. Yang, L. Jin, X. Feng, J. Hua, S. Lin, B. Wang, H. Li, J. Wang, Oncol. Lett. 13, 2442–2448 (2017)
Y. Wei, Q. Sun, L. Zhao, J. Wu, X. Chen, Y. Wang, W. Zang, G. Zhao, Med. Oncol 33, 1–9 (2016)
L. Jia, W. Liu, B. Cao, H. Li, C. Yin, Oncotarget 7, 36743 (2016)
Y. Zhou, X. Meng, S. Chen, W. Li, D. Li, R. Singer, W. Gu, Breast Cancer Res 20, 32 (2018) https://doi.org/10.1186/s13058-018-0959-1
R. Zhang, T. Tang, H.-M. Yu, X.-D. Yao, Biochem. Biophys. Res. Commun 507, 260–266 (2018)
Y. Li, C. Zeng, J. Hu, Y. Pan, Y. Shan, B. Liu, L. Jia, J. Hematol. Oncol 11, 1–17 (2018)
Y. Luo, J. Ouyang, D. Zhou, S. Zhong, M. Wen, W. Ou, H. Yu, L. Jia, Y. Huang, Dig. Dis. Sci 63, 890–899 (2018)
J.J. Li, X.F. Chen, M. Wang, P.P. Zhang, F. Zhang, J.J. Zhang, Clin. Exp. Pharmacol. Physiol 47, 877–885 (2020). https://doi.org/10.1111/1440-1681.13259
X. Liu, M. Sun, F. Nie, Y. Ge, E. Zhang, D. Yin, R. Kong, R. Xia,. Li, K.-h. Lu and J.-h. Mol. Cancer 13, 1–14 (2014)
X. Wang, Y. Lin, J. Liu, Mol. Med. Rep. 19, 1248–1255 (2019). https://doi.org/10.3892/mmr.2018.9729
Q. Wen, Y. Liu, H. Lyu, X. Xu, Q. Wu, N. Liu, Q. Yin, J. Li, X. Sheng, International Journal of Gynecologic Cancer 27 (2017)
Y. Wu, S.W. Song, J. Sun, J.M. Bruner, G.N. Fuller, W. Zhang, J. Biol. Chem 285, 3554–3560 (2010)
D. Li, X. Tang, M. Li, Y. Zheng, J. Cell. Biochem 120, 12290–12299 (2019). https://doi.org/10.1002/jcb.28493
R. Bhattacharjee, A. Jana, A. Nandi, A. Sinha, A. Bhattacharjee, S. Mitra, S. Kar, A. Dey, S.K. Singh, S.R. Varma, Materials & Design, 111415 (2022)
A. Nath, R. Bhattacharjee, A. Nandi, A. Sinha, S. Kar, N. Manoharan, S. Mitra, A. Mojumdar, P.K. Panda, S. Patro, A. Dutt, R. Ahuja, S.K. Verma, M. Suar, Biomed. Pharmacother 151, 113122 (2022). https://doi.org/10.1016/j.biopha.2022.113122
Acknowledgements
We thank Aalto University for providing open-access support. NP would like to acknowledge Finnish cultural foundation (Varsinais-Suomi regional fund.)
Funding
No external funding was received for the research for this article. Open Access funding provided by Aalto University.
Author information
Authors and Affiliations
Contributions
The review was designed, conceptualized, and drafted by RB, NP, LK, DK, AN, NKJ, and KKK. Review, writing, editing, and formal analysis were done by AB, SK, SM, and JR. All authors contributed to writing, editing, and finalizing the manuscript. The manuscript was modified under the supervision of KKK.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhattacharjee, R., Prabhakar, N., Kumar, L. et al. Crosstalk between long noncoding RNA and microRNA in Cancer. Cell Oncol. 46, 885–908 (2023). https://doi.org/10.1007/s13402-023-00806-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-023-00806-9