Abstract
Feather is a high recalcitrance keratin-based biomass, and keratin-degrading microorganisms have been seen as beneficial tools for its biodegradation and production of valuable products with biomedical and industrial applications. In the current study, the isolated keratinolytic bacteria was identified on the base of its 16 S rDNA sequencing data as Bacillus halotolerans, and its efficiency in the hydrolysis of feather with the preservation of most of its amino acid constituent was estimated by high-performance liquid chromatography Pico-Tag (HPLC PICO-TAG) method. The produced hydrolysate possessed antioxidant activity of 2,2-Diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity of 52.3 ± 5.6 mmole TE/g without possessing any cytotoxicity to human normal skin fibroplast cell line BJ-1 estimated by MTT assay. Moreover, the isolated strain was capable for producing keratinase under submerged fermentation of feather that was optimized by combining single-variable-at-a-time optimization with the application of statistical designs (Plackett-Burman and Box-Behnken). The optimum keratinase activity was 140.83 U/mL that was enhanced by 3.2-fold of the estimated initial activity, and the purified enzyme possessed optimum activity at pH 9.5 and 70 °C with a half-life of 69.3 min. Finally, the applicability of the purified enzyme in the dehairing of bovine hide was examined in comparison to sodium sulfide (Na2S) and lime (Ca(OH)2) conventional method. The results estimated the efficiency of the examined bio-treatment process, it achieved complete dehairing after 2 h with a good skin quality as manifested by scanning electron microscope (SEM). In addition, a significant reduction of the pollution load parameters was estimated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Poultry meat is an essential source of protein for billions of people all over the world that contributes by about 36% of the total produced meat. By its consequence consumption, a huge amount of waste including hatchery, manure, mortality, feather, and abattoir waste is generated that negatively affects the environment and human health. Burning and landfilling were the commonly applied methods for the treatment of poultry wastes. In the last two decades, these wastes have been considered sources for various value-added products having the ability to participate in several industries, providing positive economic, health, and environmental impacts [1].
Feather constitutes about 5–7% of the bird total weight, and consequently, several million tons are generated per year from the poultry industry in the world [2]. Feather is a protein-based biomass that composed mainly of keratin; a protein biopolymer constitutes about 90% of the feather biomass, that has been used as a source for numerous valuable amino acids and peptides [3, 4]. However, the high recalcitrance of keratin resulted from the presence of numerous disulfide cross-linkages within its structure makes it resistant to degradation by conventional proteolytic enzymes [5]. Several physicochemical techniques including the application of acidic or alkaline hydrolysis, the hydro-thermal treatment, and the use of organic solvents and ionic liquids have been examined for the degradation of keratin, but these methods usually cause destruction or derivatization of some amino acid and consequently modify the amino acid composition of the resulted hydrolysate. Therefore, keratinolytic microorganisms and their enzymes are considered potential alternatives for the biodegradation of keratin and the production of a high nutritional protein hydrolysate [6,7,8,9,10,11].
Keratinolytic enzymes are proteolytic enzymes capable to catalyze keratin hydrolysis. In addition to their efficiency in the management of keratinous wastes, they find applications in various fields including detergent, tannery [12], and wool industry [13]. The environmentally beneficial advantages of keratinases in several industries urged the efforts for the search of keratinase-producing strains with low-cost efficient productivity [14]. Therefore, the utilization of keratinous wastes for offering the nutritional requirements for the growth of microorganisms during the fermentation process and reducing the cost of the produced keratinases has attracted the research focus [8, 15,16,17,18]. In addition, the optimization of the cultural and nutritional condition of the fermentation process has been turned to be inevitable part for the maximization of the enzyme production and consequently reducing its cost. Statistical optimization has been reported as an efficient tool for the optimization of several biotechnological process [19] in addition to the microbial production of keratinolytic enzymes [8, 20,21,22,23].
Among leather processing, dehairing is the fundamental primary step in which the application of Na2S and Ca(OH)2 is the conventional applied chemicals, causing serious environmental pollution and waste disposal problems. The environmental legislation around the globe obliges tanneries to lower their organic and inorganic effluent loading. Therefore, enzymatically based dehairing via the application of keratinases has attracted the research focus as eco-friendly alternatives [16, 24,25,26]. Herein, the keratinolytic activity of the isolated strain was examined via the fermentation of feather. In addition, the solubilized keratin hydrolysate was analyzed and examined as free radical scavenging agent. Moreover, the produced keratinase activity was determined and optimized followed by purification and characterization. Finally, the application of the produced keratinase in the dehairing process was examined in comparison to the conventional applied method with the evaluation of the pollution load resulted from this process.
2 Materials and methods
2.1 Microorganism and culture conditions
The isolated strain (Isolate no. 9) currently examined was isolated from leather industry byproducts at the department of chemistry of natural and microbial products, National Research Centre, Dokki, Giza, Egypt. It was selected on the base of the preliminary keratinolytic investigation carried out as described by Emran et al. [27], using keratin-rich byproducts (feather). Identification of the isolate was carried out by matrix-assisted lased desorption ionization-time-of-flight mass spectroscopy (MALDI-TOF MS) performed in Children’s Cancer Hospital (57,357), Cairo, Egypt, and 16 S rDNA sequencing performed in Sigma Scientific Services Co. The obtained sequencing data was submitted to the GenBank and received an accession number. Moreover, phylogenetic analysis was inferred using neighbor joining method, and MEGA x was used to construct the phylogenetic tree.
For enzyme production, feather fermentation was carried out by applying submerged technique using production medium composed of (%) milled feather, 1.0; K2HPO4, 0.14; KH2PO4, 0.07; NaCl, 0.05; and MgSO4, 0.01, and adjusted at pH 7 [27]. The fermentation was carried in a 250-mL Erlenmeyer conical flask in which inoculation of the production medium at 6% (v/v) was carried out by 48-h pre-cultured inoculum composed of (%) glucose, 1.0; peptone, 1.0; yeast extract, 0.3; and CaCl2, 0.15, adjusted at pH 7, and then incubated at 37 °C and 180 rpm for 2 days. Finally, centrifugation of the culture at 4 °C and 4500 rpm for 10 min was carried out, and the cell-free supernatant was used furtherly.
2.2 Enzyme activity determination
The enzyme activity was determined using the method outlined by Cai et al. [28]. Initially, feather keratin substrate was prepared according to the method described by Wawrzkiewicz et al. [29] using locally collected white feather after being washed extensively with water and detergent followed by drying at 60 °C. Briefly, 0.5 L of dimethyl sulfoxide was added to 10 g feather and heated with a reflux condenser at 100 °C for 120 min followed by acetone precipitation via the addition of duplicate volumes of cold acetone and maintenance for 48 h at 4 °C. The precipitate was collected by centrifugation at 4 °C and 4500 rpm for 10 min followed by washing with distilled water for several times, drying, and grinding. Each 1 g of the dried precipitate was dissolved in 20 mL of 0.05 M NaOH followed by pH adjustment to pH 8 using 0.1 M HCl, then completed to 200 mL by 0.1 M Tris/HCl buffer of pH 8.
After preparing keratin substrate, the enzyme activity was determined by adding 1 mL of the prepared substrate to 1 mL of the suitable dilution of the enzyme solution, and the mixture was incubated at 50 °C for 15 min, and then an equivalent volume of 15% trichloroacetic acid was added. The blank was prepared by the initial precipitation of the protein content in the enzyme solution by the addition of 2 mL of 15% trichloroacetic acid before the addition of the substrate. The mixture was then centrifuged for 10 min at 4 °C and 8000 rpm to determine the protein content of the clear supernatant in accordance to Lowry et al. [30]. The amount of the enzyme required to raise the absorbance by 0.01 under the specified procedure was estimated for each unit.
2.3 Optimization of cultural conditions
2.3.1 Single-variable-at-a-time
The influence of different variables including the incubation period, the feather concentration, and the pH of the fermentation medium on the enzyme productivity was evaluated. Moreover, the influence of the addition of different additives including corn steep liquor, lyophilized whey protein concentrate, molasses, and wheat bran (1% w/v) was evaluated with the examination of the effect of different concentration of the additive that enhance the productivity.
2.3.2 Statistical optimization
Optimization of the enzyme productivity was carried out in two sequential steps. Plackett-Burman design [31] was applied for the identification of the significant variables followed by the applying of Box-Behnken design [32] for the optimization of these variables.
Plackett-Burman design
Seven independent variables (feather concentration, wheat bran concentration, initial pH, incubation period, inoculum size, K2HPO4 concentration, and KH2PO4 concentration) were examined at low (−1) and high (+ 1) levels in which the effect of each variable was calculated as follows:
where E(Xi) is the variable effect, and Mi− and Mi+ represent the keratinase activity where the variable (Xi) adjusted at −1 and +1 values, respectively. N is the number of trials.
Box-Behnken design
By using Box-Behnken design, the variables that had a significant impact on the productivity of the enzyme were optimized. In seventeen experimental runs with five central points, three variables were investigated at three levels. The following equation was used to interpret the correlation between the keratinase activity (Y) and the variables under investigation:
ß0, ßi, ßij, and ßii are the model’s intercept, linear, cross-product, and quadratic coefficients, respectively; Xi and Xj are the coded levels of the variables under study.
2.4 Purification and characterization of the produced keratinase
The enzyme produced under the optimized conditions was fractionally precipitated using acetone or ethanol (from 20 to 90%). The enzymatic activity of each fraction was evaluated as above, and the protein content was estimated according to Lowry et al. [30]. According to Laemmli [33], the enzyme fraction with the highest specific activity was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
The influence of the pH and the temperature on the enzymatic activity and stability was estimated. By diluting the enzyme with various buffers at pH ranges of 7 to 10.5; 0.1 M phosphate buffer for pH ranges of 7 to 8, Tris/HCl buffer for pH 9, and carbonate buffer for pH ranges of 9.5 to 10.5. Moreover, the residual activity was estimated after its incubation at the optimum pH for different incubation periods up to 120 min in which the primary enzymatic activity at the zero time was 100%.
The thermal effect on the enzymatic activity was studied at different temperatures (50–90 °C) where the activation energy (Ea) of the enzyme was estimated based on the slope of Arrhenius plot as follows:
Furthermore, the enzyme thermal stability was investigated at various temperatures (70–80 °C) by tracking the remaining enzymatic activity at the optimal conditions after pre-incubation (without substrate) for varying incubation times up to 120 min. The primary enzyme activity at the zero time was 100% activity. The following estimates were made for the enzyme’s thermostability kinetic parameters:
where Kd is the thermal deactivation rate constant, T is the temperature (K), Ed is the decay activation energy (KJ mol−1), and R is the gas constant (8.3145 Jmol−1K−1).
2.5 Dehairing process
2.5.1 Bio-treatment process
In a 250-mL Erlenmeyer conical flask, the applicability of the purified keratinase in the dehairing of bovine hide (≈ 5 cm × 5 cm) was initially examined in a final volume of 50 mL containing enzyme activity of 25 U/mL completed with carbonate buffer of pH 9.5 and then incubated at 37 °C and 100 rpm. The dehairing activity was evaluated mechanically at different periodic intervals via the gentle finger scraping of the treated hide in order to remove the loosen hair. After that, different enzymatic activities ranged from 25 to 500 U were evaluated. In order to assess the efficiency of the added enzyme activities in the removal of organic matters from the extracellular matrix of the treated samples, the total organic carbon released in the dehairing solution was estimated using total organic carbon analyzer and the amount of the total protein released was estimated according to Lowry et al. [30] using the enzyme solution as the blank.
2.5.2 Conventional process
In general, conventional dehairing process was carried out using Ca(OH)2 and Na2S. Initially, the bovine hide was placed in NaCl (10%) for 15 min and then washed twice with a wetting agent (Egyptol PLM). After that, the hides were subjected to the dehairing process as follows: the hides were limed for 30 min, and then 3% of Na2S was added in two portions for 2 h. Finally, the dehaired hides were delimed with NH4Cl and washed thrice with water.
2.5.3 Scanning electron microscopy
The effect of the dehairing process on the examined samples (air-dried) was assessed via the examination of the morphological changes under SEM (high resolution field emission gun, Quanta 250, HRFEG, Czech) in which both surface and cross section views were represented in this study.
2.5.4 Analysis of pollution load
The effluent of the dehairing process resulted from the bio-treatment process was analyzed for pollution load in comparison to the one resulted from the conventional treatment process. Standard procedures for water and wastewater analysis were followed in order to estimate the parameters under examination, which included sulfide content, total dissolved solids (TDS), chemical oxygen demand (COD), and bio-chemical oxygen demand (BOD) [34].
2.6 In-house prepared feather hydrolysate
2.6.1 Feather degradation
The degradation of feather and production of water-soluble feather hydrolysate under the fermentation conditions described in Sect. 2.1 was evaluated. The culture was initially centrifuged for 10 min at 4500 rpm and 4 °C in order to eliminate the produced bacterial biomass as well as the un-solubilized fraction of feather. After that, the cell-free culture was boiled for 10 min to terminate the hydrolytic action of the existent enzymes followed by centrifugation for 10 min at 4500 rpm and 4 °C. Finally, the clear supernatant was dried at 50 °C, and the recovery percentage was calculated as follows:
where DHW and IFW were the weight of the dried hydrolysate and the initial feather weight, respectively.
2.6.2 Protein and amino acid content
According to Lowry et al. [30], the total protein content of the sample under examination was estimated using bovine serum albumin as a standard. Next, using N-butanol:acetic acid:water (50:30:20%) as the running solution, the resultant hydrolysate (50 µL of 10% solution) was examined by TLC. The plate was then sprayed with 0.5% ninhydrin acetone solution and baked for 1 min at 100 °C [35]. Additionally, water associates HPLC PICO-TAG method [36] was used to determine the amino acid composition of the dried sample. The PICO-TAG method is applied in three stages: (i) sample hydrolysis in which the sample was weighted into 25 × 150 mm hydrolyzed tube using 6 N HCl and placed in 110 °C oven for 24 h, then the tube contents after cooling were quantitatively transferred to volumetric flask and diluted with HPLC grade water. The diluted hydrolysate was filtered through 0.45 μm sample filter (ii) pre-column derivatization with Phenylisothiocyanate and (iii) analyzed using reverse phase HPLC, and the chromatographic separation of the hydrolyzate was performed using a reverse phase Pico-Tag column (150 × 3.9 mm) with 600 E Multisolvent Delivery System and the following gradient of Pico-Tag solvent A and B (Waters Eluent A & B) at 38 °C, flow rate 1 mL/min. The detection of the PTC derivatives was carried out by ultraviolet absorption measurements using a fixed wavelength 254 nm (2489 UV/Vis Detector). Identification of the amino acids in the samples was carried out by comparison with the retention times of the standards.
2.6.3 Antioxidant activity
The generated hydrolysate capacity to scavenge DPPH was assessed in accordance with Brand-Williams et al. [37]. In summary, after standing in the dark for 30 min, the reaction mixture (4 mL) consisting of 100 µL of 10% w/v hydrolysate solution combined with methanolic solution of DPPH radical (1.1 × 10 –4 mol/L) was observed for a decrease in absorbance at 515 nm. The antioxidant activities were expressed in mmole Trolox Equivalents (TE)/g of the dry sample.
2.6.4 In vitro cytotoxicity
Based on the mitochondrial dependent reduction of yellow 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) forming purple formazan, the effect of the produced hydrolysate on the viability of human normal skin fibroplast cell line BJ-1 in the range from 0.78 to 100 µg/mL was estimated [38]. The following equation was used to compute the percentage change in the cell viability:
2.7 Statistical analysis
All experiments were carried in triplicates with duplicate measurements for each replicate, and the results were expressed as the average ± standard deviation. In addition, regression was carried via Excel data analysis, and the Box-Behnken design was analyzed via Design Expert 13.
3 Results and discussion
3.1 Microorganism
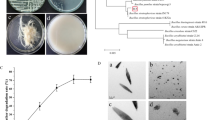
The isolated strain shown in Fig. 1A was selected on the base of its keratinolytic activity estimated by its ability for the hydrolysis of feather as shown in Fig. 1B. The isolate was a gram-positive bacillus that indicated by MALDI-TOF MS as a candidate of the genus Bacillus. On the base of the data obtained from16S rDNA sequencing, the isolate was identified as Bacillus halotolerans with identity percentage of 99.95% with Bacillus halotolerans strain DSM 8802. The obtained sequence was submitted to the NCBI under the name Bacillus halotolerans EGY5 and received accession number of PP038118. Moreover, the constructed phylogenetic tree was shown in Fig. 1C. Various Bacillus species had been reported as proteolytic enzymes producers [39,40,41,42,43], and Bacillus halotolerans had been reported as a beneficial bioproduct that can be used as a biocontrol agent possessing phytoprobiotic functions [44, 45] in addition to its ability for the production of biosurfactants [46] as well as enzymes including amylase [47] and keratinase [48] that can be exploited in various industries.
3.2 Enzyme activity
In the current study, feather was used for inducing keratinase production using the isolated Bacillus halotolerans EGY5 strain. The result indicated the production of 43.9 U/mL enzyme activity under the described fermentation conditions. The utilization of feather in the production of keratinases with promising industrial applications has attracted the research focus from the economic point of view as well as environmental protection aspects [9, 15, 16, 18].
3.3 Optimization of cultural conditions
3.3.1 Single-variable-at-a-time
In general, the microbial production of enzymes is significantly influenced by the cultural and nutritional fermentation conditions. In the current study, the influence of some variables was optimized by single-variable-at-a-time examination, and the results were illustrated in Table 1. By examining the effect of the incubation period on the fermentation of feather and production of keratinase, an activity of 41.55 U/mL was achieved after 1 day that increased to 43.91 U/mL after 2 days. While by further increase up to 5 days, a slight reduction on the activity was estimated. Similar behavior was observed for Bacillus sp. CN2 keratinase in which the peak for production was 2 days [8]. However, 3 days was estimated for Bacillus anthracis MKR-9 keratinase productivity [49], 5 days for Bacillus cereus HD1 [50], and 6 days for Bacillus sp. MD24 [51]. Moreover, the effect of feather concentration on the enzyme productivity at the optimum incubation period (2 days) was examined, and the results estimated that the keratinase productivity was higher at low concentrations of feather in which the peak was estimated at concentration of 1%. Similar behavior was reported for the fermentation of feather using various Bacillus sp [27, 49]. In addition, the keratinase productivity in the current study was higher at neutral and alkaline pH with a gradual decrease in the productivity by decreasing the pH in the acidic region. This result is in line with earlier studies that showed Bacillus sp. produced keratinase more favorably at neutral to alkaline pH levels [27, 49, 50]. Keratinase can be produced via the fermentation of feather as the sole carbon and nitrogen source, but the addition of other carbon and nitrogen sources could influence the enzyme productivity [50]. Therefore, the addition of other byproducts rich in nutrients with feather was examined in the current study. Based on the obtained results, the highest keratinase activity (93.11 U/mL) was achieved by the fermentation of 1% feather with the addition of 4% wheat bran in the described production medium. Wheat bran is a low-cost substrate that has been used in the production of various value-added products since it is considered an ample source of various micro- and macro-nutrients including carbohydrates, proteins, phenolic acids, and flavonoids [52]. It had been used under submerged fermentation in the production of various bacterial enzymes including xylanase, alkaline protease [53], keratinolytic protease [13], glucanase [54], and amylase [55].
3.3.2 Statistical optimization
Plackett-Burman design
The influence of the examined variables on the keratinase productivity was examined by applying Plackett-Burman design in which each variable was examined at two levels (−1 and + 1), and the results were shown in Table 2. The applied design’s overall significance was demonstrated by the ANOVA data, where the p-value (less than 0.05) was 0.000129 and the F value was 21.88865. Moreover, the regression indicated that the coefficient of determination (R2) was 0.95 manifesting its good fitness since it was higher than 0.8 as reported by Joglekar and May [56]. Furthermore, it was estimated that the feather and wheat bran concentrations as well as the incubation period were the significant variables, with the remaining variables being non-significant (Supplementary Table 1). Each variable’s main effect was computed, and the supplementary Fig. (1) displays the representative graph. According to the results, every significant variable had a negative impact. The variable higher effect was attained at its lower level, according to the negative effect estimate. The significant variables were thus chosen for a subsequent optimization stage using the Box-Behnken design, where their zero level was the negative values estimated in the Plackett-Burman design.
Box-Behnken design
The significant variables that influence the enzyme productivity were optimized by applying the Box-Behnken design. The obtained data (Table 3) fitted a quadratic model in which the ANOVA indicated the overall significance of the applied design since the F-value was 6.1 with p-value of 0.0132, indicating that there was only 1.32% chance that the F-value could occur due to noise. In addition, the lack of fit was non-significant manifesting the fitness of the applied design. Moreover, the regression indicated that the R2 value was 0.89 indicating its good fitness, and the analysis of the model terms indicated the significant of the linear (X1, X2, X3), interactive (X1 × 3), and quadratic (X12) terms while the others were non-significant (Supplementary Table 2). This model was expressed by the following second-order equation:
where Y expressed the predicted keratinase activity and X1, X2, and X3 were the concentration of feather, concentration of wheat bran, and the incubation period, respectively.
By plotting of the normal plot of residual (Supplementary Fig. 2), the plot estimated that the residuals were normally distributed since the plotted points approximately followed the straight line, manifesting the suitability of the applied model for the experiment. Moreover, the response surface 3D and the contour plots that visualize the influence of the examined variables as well as their interactive effects on the keratinase productivity were shown in Fig. 2.
Response surface 3D and contour plots possessing the interactive effect between each two variables on the keratinase productivity while the third variable was maintained at the central value in which R1 was the keratinase activity. X1 , X2, and X3 were feather concentration, wheat bran concentration, and the incubation period, respectively
Validation of the model was performed using the predicted parameters in which the predicted optimal activity (136.85 U/mL), with the best model desirability of 0.94, resulted from the fermentation of 0.367% of feather and 4% of wheat bran for 3 days agreed with the obtained experimental result (140.83 U/mL). In general, the composition of the fermentation medium and the cultural conditions were significant contributors in the disparity of the keratinase activity among various microorganisms. In the current study, it can be concluded that the optimized keratinase activity (140.83 U/mL) was 3.2-fold higher than the estimated initial activity (43.9 U/mL). This result was higher than the optimized result (92.9 U/mL) reported for Bacillus haynesii ALW2 keratinase production [27] but quite high as 195.05 U/mL reported by Lai et al. [8] for Bacillus sp. CN2.
3.4 Purification and characterization of the produced keratinase
The fractional precipitation of the culture-free cell applying acetone or ethanol was examined. The results estimated that the 70% ethanol-precipitated fraction exhibited specific activity of 32.5 U/mg protein, with 3.3 purification fold and 7.7% recovered activity yield. Surprisingly, the SDS-PAGE for the precipitated enzyme shown in Fig. 3A indicated that the precipitated enzyme was purified with a single band profile at approximately 48 KDa.
A The SDS-PAGE of the produced enzyme in which lane (1) was the crude enzyme and lane (2) was the purified enzyme, B the effect of the pH on the enzyme activity, C the effect of the temperature on the enzyme activity, D Arrhenius plot for the enzyme thermal activation, E thermal stability study, and F Arrhenius plot for the enzyme thermal inactivation
The enzymatic activity of the purified keratinase had been examined at various pH values (7–10.5). The optimal enzymatic activity was estimated at pH 9.5 at which the enzyme retained its complete activity up to 2 h. Additionally, the enzyme retained 94 and 81% of its optimum activity at pH 10 and 10.5, respectively (Fig. 3B). This result agreed with the reported results for bacterial keratinases, indicating alkaline conditions as the optimal for keratinase activity [15, 57,58,59,60].
The keratinase activity had been estimated at various temperatures ranged from 50 to 90 °C. The experimental data indicated that the enzyme optimum activity was 70 °C that decreased by about 19 and 44% at 80 and 90 °C, respectively (Fig. 3C). On the base of Arrhenius plot illustrated in Fig. 3D, the estimated thermal activation value was 15.5 KJ mol−1. The enzyme optimum temperature agreed with that reported by Priyanka et al. [60] for Bacillus cereus FC1365 keratinase. On the other hand, it was higher than 30 °C reported for Pseudomonas geniculata H10 keratinase [58], 45 °C reported for the enzyme produced by Bacillus subtilis ES5 [15], 55 °C reported for Pseudomonas aeruginosa [59] and Bacillus sp. P45 keratinolytic proteases [61], and 60 °C reported for Ectobacillus sp. JY-23 keratinase [57].
In general, thermal denaturation of the enzyme is attributed to the alteration in its secondary, tertiary, or quaternary structure without the breaking of the covalent bonds that implying the loss of the efficiency of any enzymatic process, reflecting the importance of studying this parameter. Therefore, thermal stability of the purified keratinase at its optimum temperature and up to 80 °C had been evaluated. On the base of the obtained results (Fig. 3E), the enzyme half-life at its optimum temperature was 69.3 min and the D-value was 230.3 min (Table 4). The D-value was high at its optimum temperature and decreased at higher temperatures indicating the rapid inactivation of the enzyme at high temperatures with the need for more prolonged pre-incubation period at its optimum temperature to reach 90% enzyme inactivation. Herein, the estimated T1/2 and D-values were extremely higher than that reported for keratinolytic protease produced by Bacillus sp. P45; T1/2, and D-values at its optimum activity conditions (55 °C and pH 7.5) were 5.1 and 17 min, respectively [61].
Based on Arrhenius plot illustrated in Fig. 3F, the estimated thermal inactivation energy value (Ed) was 203.5 KJ mol−1 for temperatures ranging from 70 to 80 °C. The Ed value estimated for the purified enzyme was higher than that reported for Bacillus keratinases [27, 61], reflecting its increased stability. The Ed value represents the energy barrier that have to be overcome in order to inactivate the enzyme. The high Ed value indicated the increased thermal stability of the enzyme with the reduction in its unfolding rate [62].
3.5 Dehairing process
3.5.1 Effect of enzyme activity
Application of the purified enzyme in the dehairing of bovine hide was initially examined at 37 °C and 100 rpm using enzyme activity of 25 U/mL adjusted using carbonate buffer of pH 9.5 to a final volume of 50 mL. The result estimated an approximate complete dehairing after 2 h (Fig. 4). The dehairing period was extremely lower than 16 h reported for Bacillus subtilis ES5 keratinase [16], 40 h reported for Bacillus cereus L10 keratinase [21], and 48 h reported for Bacillus tropicus keratinolytic protease [9].
Jayanthi et al. [63] reported that the removal of organic matters from the extracellular matrix of the skin during the dehairing process builds up the total organic carbon and the total soluble protein content of the resulted waste water that can be used to assess the efficiency of the applied enzymatic process. Therefore, in the current study, the total organic carbon as well as the total soluble protein content was estimated in the effluent resulted after the dehairing process using different enzyme activities. The results indicated that the content of the total organic carbon increased gradually by increasing the added enzyme activity up to the use of 125 U/mL, and after that, no increase was observed while for the total protein content, a slight increase was observed by increasing the enzyme activity more than 125 U/mL (Table 5).
3.5.2 Scanning electron microscopy
SEM analyses was carried out to assess the grain surface and the cross-section of the dehaired skin samples in order to gain an insight to estimate the effect of the applied enzyme on the interior fibers and grain surface through the hierarchy of the structure. In the current study, surface quality of the enzymatically dehaired skin treated with enzyme activity of 125 U/mL was evaluated in comparison to the conventionally dehaired skin under SEM. The results shown in Fig. 5 indicated complete dehairing of the skin in both methods in which the enzymatically dehaired skin was cleaner and smother since precipitation of chemicals was clearly observed on the surface of the conventionally dehaired skin although it was washed for several times. Moreover, the cross-section view estimated comparable fiber orientation and compactness of the enzymatically dehaired skin with the conventionally dehaired one.
3.5.3 Analysis of pollution load
The liming/dehairing process generates a significant amount of solid waste, composite tanning effluent, and airborne emissions and odors. The current practice of discharging tannery effluent directly into residential sewer networks without any treatment has a detrimental effect on both human health and the environment. Therefore, in the current study, the pollution load parameters of the dehairing resulted effluents including COD, BOD, TDS, and sulfide content were determined.
Liming/dehairing effluent characteristics
Table 6 displays the primary attributes of the effluent produced by the traditional process. With an average of 24,750 mg O2/L, the COD concentration ranged from 22,750 to 26,800 mg O2/L. Additionally, the average BOD concentration was 3950 mg O2/L, the average TDS species concentration was 3844 mg/L, and the average sulfide concentration was 3425 mg/L.
Characteristics of the enzymatically resulted effluent
It was clear from Table 6 that the COD, TDS, and the sulfide content of the effluent resulted from the enzymatically dehaired hide decreased by 61, 32, and 99%. This result could be attributed to the fact that enzymes mostly acted at the hair root with the intact removal of the hair while Na2S dissolved the hair, causing an increase in the pollution load parameters [16, 24,25,26].
3.6 In-house prepared hydrolyzate
Feather is a poultry byproduct that composed mainly of keratin. It can be hydrolyzed using various physical, chemical, and biological treatments, but the latter is the advantageous method since it is the one that have the ability to produce keratin hydrolysate with a high nutritional value [6, 9, 11]. On the other hand, the chemical and the physical treatments destroy certain amino acids and decrease the quality of the produced polypeptides [64]. In the current study, the bio-degradation of feather and production of bioactive water-soluble hydrolysate under the above fermentation conditions using Bacillus halotolerans EGY5 was evaluated. Initially, the amount of the dried hydrolysate was determined, and consequently, the calculated recovery percentage was 37.5%. The total protein content of the dried sample was estimated on the base of the Lowry method, and it was 8.48 mg/g. TLC analysis estimated the presence of bands corresponding to free amino acids in addition to bands with molecular weight higher than cystine (Supplementary Fig. 3). In addition, HPLC PICO-TAG method for the determination of the amino acid composition indicated the preservation of most of the amino acid constituent of feather (Supplementary Fig. 4 and Table 7). Similarly, Sironi et al. [11] estimated the preservation of amino acid composition of Bacillus sp. B4 feather hydrolysate in which glutamic acid, histidine, leucine, lysine, and valine were the most abundant amino acid. In addition, Liya and Umesh [9] estimated the efficiency of Bacillus tropicus LS 27 in the degradation of feather keratin and solubilization of amino acids in which considerable amounts of cysteine, isoleucine, lysine, methionine, phenylalanine, proline, and valine were detected. In the current study, aspartic and glutamic acids were the most abundant amino acids. In general, feather hydrolysates found applications in the production of various products including crop, energy, pharmaceutical, and animal feed products [3] with a great scope on the commercialization of its use as a fertilizer [65]. In addition, aspartic and glutamic acids had been reported as antioxidant enhancers in crop production [66, 67].
Moreover, the antioxidant activity of the produced hydrolysate was determined, and the result estimated that the resulted hydrolysate possessed DPPH free radical scavenging activity of 52.3 ± 5.6 mmole TE/g. In general, the antioxidant activity of protein hydrolysates is strongly correlated to their aromatic and the hydrophobic content of amino acids [68]. In addition, Prajapati et al. [69] reported that the cysteine content present in feather hydrolysates has been reported as a potent free radical hydrogen doner achieving antioxidant activity. Alahyaribeik et al. [70] indicated that feather hydrolysate did not induce any cytotoxicity on normal cell line. Herein, in order to cancel the possibility of the cytotoxicity that might be induced by any bacterial secondary metabolite, the impact of the produced hydrolysate at different concentrations on the viability of human normal skin fibroplast cell line BJ-1 was evaluated. The result estimated that the produced hydrolysate did not possess any negative effect on the growth of the cells.
4 Conclusion
The current study is concerned with the valorization of feather biomass. Initially, the isolated keratinolytic bacteria that identified as Bacillus halotolerans have been estimated as an efficient tool for the degradation of feather with the preservation of its amino acid constituent, suggesting its food, medical, and agricultural applications. Moreover, the produced hydrolysate was examined as antioxidant agent, and it possessed DPPH free radical scavenging activity of 52.3 ± 5.6 mmole TE/g without possessing any cytotoxicity to human normal skin fibroplast cell line. In addition, the isolated strain was capable for the production of keratinase with optimum activity of 140.83 U/mL. The purified enzyme was alkalophilic and thermophilic with a half-life of 69.3 min at 70 °C. Finally, the efficiency of the produced keratinase in the dehairing process was manifested as an eco-friendly alternative to the conventional chemical method, and it achieved complete dehairing after 2 h and possessed a good skin quality with a significant reduction in the pollution load parameters.
Data availability
All the information found in the following manuscript is fundamental.
References
Zhang L, Ren J, Bai W (2023) A review of Poultry Waste-to-Wealth: Technological Progress, modeling and Simulation studies, and Economic-Environmental and Social Sustainability. Sustainability 15(7):5620
Onifade AA, Al-Sane NA, Al-Musallam AA, Al-Zarban S (1998) A review: potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Bioresour Technol 66(1):1–11
Callegaro K, Brandelli A, Daroit DJ (2019) Beyond plucking: feathers bioprocessing into valuable protein hydrolysates. Waste Manag 95:399–415
da Silva RR (2024) Keratin hydrolysate improves the production of commercially valuable metabolites. Biologia 79:1071–1073
Brandelli A (2008) Bacterial keratinases: useful enzymes for bioprocessing agroindustrial wastes and beyond. Food Bioprocess Technol 1:105–116
Goda DA, Bassiouny AR, Abdel Monem NM, Soliman NA, Abdel-Fattah YR (2021) Feather protein lysate optimization and feather meal formation using YNDH protease with keratinolytic activity afterward enzyme partial purification and characterization. Sci Rep 11(1):14543
Latafat SMH, Ashish, Vimal A, Bhargava P (2024) Isolation and screening of keratinolytic bacteria from feather dumping soil near in Lucknow and Kanpur city, north region of Indian. Biocatal Biotransfor 42(1):68–76
Lai Y, Wu X, Li W, Wang L (2023) Insights into the keratin efficient degradation mechanism mediated by Bacillus sp. CN2 based on integrating functional degradomics. Biotechnol Biofuels Bioprod 16(1):1–14
Liya SM, Umesh M, Nag A, Chinnathambi A, Alharbi SA, Jhanani GK, Shanmugam S, Brindhadevi K (2023) Optimized production of keratinolytic proteases from Bacillus tropicus LS27 and its application as a sustainable alternative for dehairing, destaining and metal recovery. Environ Res 221:115283
Liya SM, Umesh M (2023) Bioconversion of chicken feather waste into feather hydrolysate by multifaceted keratinolytic Bacillus tropicus LS27 and new insights into its antioxidant and plant growth-promoting properties. Biomass Convers Biorefinery 1–11
Sironi PB, Mazotto AM, de Lima MF, Nogueira RI, Miguel ÂSM, Vermelho AB (2022) Hydrolyzed feather keratin obtained by microbial fermentation encapsulated with maltodextrin–A sustainable approach to increase digestible protein in feed. Biocatal Agric Biotechnol 40:102297
Akram F, ul Haq I, Jabbar Z (2020) Production and characterization of a novel thermo-and detergent stable keratinase from Bacillus sp. NKSP-7 with perceptible applications in leather processing and laundry industries. Int J Biol Macromol 164:371–383
Ismail SA, Abou Taleb M, Emran MA, Mowafi S, Hashem AM, El-Sayed H (2022) Benign felt-proofing of wool fibers using a keratinolytic thermophilic alkaline protease. J Nat Fibers 19(10):3697–3709
Rajput N, Sharma H, Bajwa J (2023) Potential role of keratinase in the environmental remediation. Mater Today Proc
Alamnie G, Gessesse A, Andualem B (2023) Production of surfactant-stable keratinolytic protease from B. subtilis ES5 and its application as a detergent additive. Biocatal Agric Biotechnol 50:102750
Alamnie G, Gessesse A, Bitew M, Dawud N, Andualem B, Girma A (2023) Production and biochemical characterization of keratinase enzyme from Bacillus subtilis ES5 and its potential application in leather dehairing process: a clean leather tanning process. Biotechnol Biotechnol Equip 37(1):2288691
Al-Bedak OAHM, Moharram AM, Hussein NAG, Taha DM, Stephenson SL, Ameen F (2023) Microbial exploitation of feather wastes for sustainable production of keratinase and collagenase enzymes by Didymella Keratinophila AUMC 15399 in submerged fermentation. Fermentation 9(6):507
Bhange K, Nath A, Singh N, Chaturvedi V, Bhatt R (2023) Statistical optimization and prediction of significant nutritional factors for keratinase production by Stenotrophomonas maltophilia Kb2 and its application as dehairing agent. Bioresour Technol Rep 23:101541
Wahba MI, Ismail SA, Hassan AA, Abdel-Aziem SH, Hassan AM, Nour SA (2024) Xylanase immobilization using activated carrier of gellan gum-agar beads: improved stability and catalytic activity for the production of antioxidant and anti-proliferative xylooligosaccharides. Biocatal Agric Biotechnol 56:103013
Abdul Gafar AI, Khayat ME, Ahmad SA, Yasid NA, Shukor MY (2020) Response surface methodology for the optimization of keratinase production in culture medium containing feathers by Bacillus sp. UPM-AAG1. Catalysts 10(8):848
Derhab N, Mabrouk ME, El-Metwally MM, Mohammed YM (2023) Thermostable keratinase from Bacillus cereus L10: optimization and some potential biotechnological applications. Biomass Convers Biorefinery 1–17
El Salamony DH, Hassouna MSE, Zaghloul TI, Abdallah HM (2024) Valorization of chicken feather waste using recombinant Bacillus subtilis cells by solid-state fermentation for soluble proteins and serine alkaline protease production. Bioresour Technol 393:130110
Sun Z, Li X, Liu K, Chi X, Liu L (2021) Optimization for production of a plant growth promoting agent from the degradation of chicken feather using keratinase producing novel isolate Bacillus pumilus JYL. Waste Biomass Valori 12:1943–1954
Ben Elhoul M, Zaraî Jaouadi N, Bouacem K, Allala F, Rekik H, Mechri S, Khemir Ezzine H, Miled N, Jaouadi B (2021) Heterologous expression and purification of keratinase from Actinomadura viridilutea DZ50: feather biodegradation and animal hide dehairing bioprocesses. ESPR 28:9921–9934
Li X, Zhang Q, Xu Z, Jiang G, Gan L, Tian Y, Shi B (2022) High-expression and characterization of a novel serine protease from Ornithinibacillus caprae L9T with eco-friendly applications. ESPR 29(24):35996–36012
Venkatachalam M, Rathinam A, Rao JR, Krishnan C (2022) Bioconversion of animal hair waste using salt-and sulphide-tolerant Bacillus sp. KLP1 and depilation using keratinase. IJEST 19(7):6389–6398
Emran MA, Ismail SA, Abdel-Fattah AM (2020) Valorization of feather via the microbial production of multi-applicable keratinolytic enzyme. Biocatal Agric Biotechnol 27:101674
Cai CG, Lou BG, Zheng XD (2008) Keratinase production and keratin degradation by a mutant strain of Bacillus subtilis. J Zhejiang Univ Sci B 9(1):60–67
Wawrzkiewicz K, Łobarzewski J, Wolski T (1987) Intracellular keratinase of Trichophyton gallinae. J Med Vet Mycol 25(4):261–268
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Boil Chem 193:265–275
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 33(4):305–325
Box GEP, Behnken DW (1960) Simplex-sum designs: a class of second order rotatable designs derivable from those of first order. Ann Math Stat 31(4):838–864
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259): 680–685
APHA (2012) Standard Methods for the examination of water and waste water. 22nd Edition, American Public Health Association, American Water Works Association, Water Environment Federation
Qiu T, Li H, Cao Y (2010) Pre-staining thin layer chromatography method for amino acid detection. AJB 9(50):8679–8681
White JA, Hart RJ, Fry JC (1986) An evaluation of the Waters Pico-Tag system for the amino-acid analysis of food materials. J Anal Methods Chem 8:170–177
Brand-Williams W, Cuvelier ME, Berset CLWT (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28(1):25–30
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Gomaa SK, Zaki RA, Wahba MI, Taleb MA, El-Refai HA, El-Fiky AF, El-Sayed H (2022) Green method for improving performance attributes of wool fibres using immobilized proteolytic thermozyme. 3 Biotech 12(10):254
Wahba MI (2022) Gum tragacanth for immobilization of Bacillus licheniformis protease: optimization, thermodynamics and application. React Funct Polym 179:105366
Khan Z, Shafique M, Jabeen N, Naz SA, Yasmeen K, Ejaz U, Sohail M (2023) Protease from Bacillus subtilis ZMS-2: evaluation of production dynamics through response surface methodology and application in leather tannery. J King Saud Univ Sci 35(4):102643
Mankge ME, Maela MP, Abrahams AM, Serepa-Dlamini MH (2024) Screening of Bacillus spp. bacterial endophytes for protease production, and application in feather degradation and bio-detergent additive. Heliyon 10(9):e30736
Moreno JF, Oulego P, Collado S, Díaz M (2024) Valorisation of waste activated sludge for protease production by Bacillus licheniformis. J Clean Prod 445:141282
Tsalgatidou PC, Thomloudi EE, Delis C, Nifakos K, Zambounis A, Venieraki A, Katinakis P (2023) Compatible Consortium of Endophytic Bacillus halotolerans strains Cal. l. 30 and Cal. f. 4 promotes Plant Growth and induces systemic resistance against Botrytis Cinerea. Biology 12(6):779
Wang D, Sun L, Yu H, Zhang C, Guan X, Wang M, Cheng R, Wang C, Xie Z (2023) Whole-genome analysis of the benzoic acid-degrading bacterium Bacillus halotolerans B28 to reveal its phytoprobiotic effects. Int Biodeterior Biodegrad 185:105668
Etemadzadeh SS, Emtiazi G, Soltanian S (2023) Production of biosurfactant by salt-resistant Bacillus in lead-supplemented media: application and toxicity. Int Microbiol 26:869–880
Rafanomezantsoa P, Gharbi S, Karkachi N, Kihal M (2023) Optimization of amylase production by the biological control agent Bacillus halotolerans RFP74 using response surface methodology. J Genet Eng Biotechnol 21(1):1–10
Devi S, Chauhan A, Bishist R, Sankhyan N, Rana K, Sharma N (2023) Production, partial purification and efficacy of keratinase from Bacillus halotolerans L2EN1 isolated from the poultry farm of Himachal Pradesh as a potential laundry additive. Biocatal Biotransfor 41(3):222–242
Kumar M, Bhatia D, Khatak S, Kumar R, Sharma A, Malik DK (2019) Optimization and purification of keratinase from Bacillus anthracis with dehairing application. J Pure Appl Microbiol 13(1):585–590
Yahaya RSR, Phang LY, Normi YM, Abdullah JO, Ahmad SA, Sabri S (2022) Feather-degrading Bacillus cereus HD1: genomic analysis and its optimization for keratinase production and feather degradation. Curr Microbiol 79(6):166
Suharti S, Rozaq HN, Qisti A, Alvionita M, Wonorahardjo S (2023) Keratinase production by Bacillus sp. MD24 in sub-merge and solid state fermentation. Malays J Fundam Appl Sci 19(3):460–470
Apprich S, Tirpanalan Ö, Hell J, Reisinger M, Böhmdorfer S, Siebenhandl-Ehn S, Novalin S, Kneifel W (2014) Wheat bran-based biorefinery 2: valorization of products. LWT-Food Sci Technol 56(2):222–231
Limkar MB, Pawar SV, Rathod VK (2019) Statistical optimization of xylanase and alkaline protease co-production by Bacillus spp using Box-Behnken design under submerged fermentation using wheat bran as a substrate. Biocatal Agric Biotechnol 17:455–464
Ruiwen C, Fuqiang W, Yiren X, Lai W, Jinghao M, Peng G, Ran Y, Xiaoyan L, Guangsen F (2023) Optimization of submerged fermentation conditions for glucanase production by Burkholderia pyrrocinia B1213 using Jiuzao. Emir J Food Agric 35(5):468–480
Farooq MA, Ali S, Hassan A, Sulayman R, Kaleem MA, Shahzad H, Summer M, Latif A, Tanveer T (2023) Enhanced bacterial α-Amylase production using mutant strains through submerged fermentation. Punjab Univ J Zool 38(1):99–107
Joglekar AM, May AT (1987) Product excellence through design of experiments. CFW 32(12):857
Li K, Li G, Liang Y, Zhang R, Peng S, Tan M, Ma D (2024) Structural and enzymatic characterization of a novel metallo-serine keratinase KerJY-23. Int J Biol Macromol 260(2):129659
Park G, Lee KM, Lee YS, Kim Y, Jeon CM, Lee OM, Kim YJ, Son HJ (2023) Biodegradation and valorization of feather waste using the keratinase-producing bacteria and their application in environmentally hazardous industrial processes. J Environ Manag 346:118986
Pei XD, Li F, Yue SY, Huang XN, Gao TT, Jiao DQ, Wang CH (2023) Production and characterization of novel thermo-and organic solvent–stable keratinase and aminopeptidase from Pseudomonas aeruginosa 4–3 for effective poultry feather degradation. Environ Sci Pollut Res 30(2):2480–2493
Priyanka KG, Mouneesha R, Sushma H, Prakruti A, Manjushree HK, More SS, Fasim A (2023) Isolation and biochemical properties of extremophilic keratinase from Bacillus cereus FC1365. Proc Natl Acad Sci India B 93:721–729
Lemes AC, Gautério GV, Rosa CAD, Brandelli A, Kalil SJ (2023) Two-step purification and partial characterization of keratinolytic proteases from feather meal bioconversion by Bacillus sp. P45. Processes 11(3):803
Jana A, Maity C, Halder SK, Das A, Pati BR, Mondal KC, Mohapatra PKD (2013) Structural characterization of thermostable, solvent tolerant, cytosafe tannase from Bacillus subtilis PAB2. Biochem Eng J 77:161–170
Jayanthi D, Victor JS, Chellan R, Chellappa M (2019) Green processing: minimising harmful substances in leather making. Environ Sci Pollut Res 26:6782–6790
Osman Y, Elsayed A, Mowafy AM, Abdelrazak A, Fawzy M (2017) Bioprocess enhancement of feather degradation using alkaliphilic microbial mixture. Brit Poult Sci 58(3):319–328
Sahoo S, Dash S, Rath B, Mondal KC, Mandal A (2023) Commercial initiation of feather hydrolysate as supreme fertilizer: a smart bio-cleaning strategy of poultry waste. Waste Biomass Valori 14(7):2151–2166
Huang S, Yang X, Chen G, Wang X (2023) Application of glutamic acid improved as tolerance in aromatic rice at early growth stage. Chemosphere 322:138173
Sadak MS, Sekara A, Al-Ashkar I, Habib-ur-Rahman M, Skalicky M, Brestic M, Kumar A, Sabagh AE, Abdelhamid MT (2022) Exogenous aspartic acid alleviates salt stress-induced decline in growth by enhancing antioxidants and compatible solutes while reducing reactive oxygen species in wheat. Front Plant Sci 13:987641
Zou T, He T, Li H, Tang H, Xia E (2016) The structure-activity relationship of 519 the antioxidant peptides from natural proteins. Molecules 21(1):72
Prajapati S, Koirala S, Anal AK (2021) Bioutilization of chicken feather waste by newly isolated keratinolytic bacteria and conversion into protein hydrolysates with improved functionalities. Appl Biochem Biotechnol 193:2497–2515
Alahyaribeik S, Sharifi SD, Tabandeh F, Honarbakhsh S, Ghazanfari S (2021) Stability and cytotoxicity of DPPH inhibitory peptides derived from biodegradation of chicken feather. Protein Expr Purif 177:105748
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This study was financially supported by STDF (project no. 43926).
Author information
Authors and Affiliations
Contributions
Shaymaa A. Ismail: conceptualization, data curation, formal analysis, investigation, methodology, and writing original draft. Shaimaa A. Nour: methodology. El-Shahat H. A. Nashy: investigation, methodology, data curation, and writing—review and editing. Azza M. Abdel-Fattah: conceptualization, project administration, methodology, and funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ismail, S.A., Nour, S.A., Nashy, ES.H.A. et al. Economic production of eco-friendly dehairing keratinase and antioxidant feather hydrolyzate using Bacillus halotolerans. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05865-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05865-y