Abstract
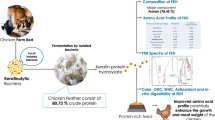
The poultry processing produces large quantities of feathers that are often discarded as waste, causing significant refuse and environmental pollution. However, utilizing microorganisms capable of degrading keratin to ferment feathers represents an effective way to repurpose feather waste. Bacillus sp. TC5 isolated from alpine lake sediments of the Hengduan Mountains achieved a feather degradation rate of about 70% after 4 days of fermentation with feathers as the only carbon and nitrogen source. The culture conditions associated with keratinase production by Bacillus sp. TC5 were optimized by Plackett-Burman design and response surface methodology (RSM), and the highest keratinase activity of 215.87 U/mL was predicted at a feather concentration of 1.72%, fermentation days of 3.68 days, and calcium concentration of 0.103%. Purified keratinase TC5 (KerTC5) has a molecular weight of approximately 30 kDa. It showed maximal activity at 55 °C and pH 9.5. Additionally, keratinase activity was enhanced by low concentrations of Mn2+ (2.5 mM). The fermentation product showed significant radical scavenging ability and reactive oxygen species (ROS) scavenging ability in human umbilical vein endothelial cells (HUVECs). Obtained results indicate that feather fermentation products were not toxic to HUVECs in the concentration range of 50 to 500 µg/mL. These results suggested that Bacillus sp. TC5 is suitable for converting the feather waste to value-added fermentation products.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article.
References
Saba M, Khan A, Ali H, Bibi A, Gul Z, Khan A, Rehman MMU, Badshah M, Hasan F, Shah AA, Khan S (2022) Microbial pretreatment of chicken feather and its co-digestion with rice husk and green grocery waste for enhanced biogas production. Front Microbiol 13:792426. https://doi.org/10.3389/fmicb.2022.792426
Qiu J, Wilkens C, Barrett K, Meyer AS (2020) Microbial enzymes catalyzing keratin degradation: classification, structure, function. Biotechnol Adv 44:107607. https://doi.org/10.1016/j.biotechadv.2020.107607
Sha Y, Chen J, Yunan LU, Ping MA, Dai D (2019) Isolation and identification of Bacillus cereus and observation of its effect on decomposition of various poultry feathers. Anim Husb Vet Med 51:39–43
Banerjee G, Ray AK (2017) Impact of microbial proteases on biotechnological industries. Biotechnol Genet Eng Rev 33:119–143. https://doi.org/10.1080/02648725.2017.1408256
Brandelli A (2008) Bacterial keratinases: useful enzymes for bioprocessing agroindustrial wastes and beyond. Food Bioproc Tech 1:105–116
Bhari R, Kaur M, Sarup Singh R (2021) Chicken feather waste hydrolysate as a superior biofertilizer in agroindustry. Curr Microbiol 78:2212–2230. https://doi.org/10.1007/s00284-021-02491-z
Lasekan A, Abu Bakar F, Hashim D (2013) Potential of chicken by-products as sources of useful biological resources. Waste Manag 33:552–65. https://doi.org/10.1016/j.wasman.2012.08.001
Nnolim NE, Udenigwe CC, Okoh AI, Nwodo UU (2020) Microbial keratinase: next generation green catalyst and prospective applications. Front Microbiol 11. https://doi.org/10.3389/fmicb.2020.580164
Hassan Mohamed A, Abol-Fotouh Deyaa, Omer Ahmed M, Tamer Tamer M, Abbas Eman (2020) Comprehensive insights into microbial keratinases and their implication in various biotechnological and industrial sectors: a review. Int J Biol Macromol 154:567–583. https://doi.org/10.1016/j.ijbiomac.2020.03.116
Brandelli A, Sala L, Kalil SJ (2015) Microbial enzymes for bioconversion of poultry waste into added-value products. Food Res Int 73:3–12
Syed DG, Lee JC, Li WJ, Kim CJ, Agasar D (2009) Production, characterization and application of keratinase from Streptomyces gulbargensis. Bioresour Technol 100:1868–71. https://doi.org/10.1016/j.biortech.2008.09.047
Park G, Lee KM, Lee YS, Kim Y, Jeon CM, Lee OM, Kim YJ, Son HJ (2023) Biodegradation and valorization of feather waste using the keratinase-producing bacteria and their application in environmentally hazardous industrial processes. J Environ Manag 346:118986. https://doi.org/10.1016/j.jenvman.2023.118986
Fontoura R, Daroit DJ, Correa AP, Meira SM, Mosquera M, Brandelli A (2014) Production of feather hydrolysates with antioxidant, angiotensin-I converting enzyme- and dipeptidyl peptidase-IV-inhibitory activities. N Biotechnol 31:506–13. https://doi.org/10.1016/j.nbt.2014.07.002
Oluba OM, Obi CF, Akpor OB, Ojeaburu SI, Ogunrotimi FD, Adediran AA, Oki M (2021) Fabrication and characterization of keratin starch biocomposite film from chicken feather waste and ginger starch. Sci Rep 11:8768. https://doi.org/10.1038/s41598-021-88002-3
Callegaro K, Brandelli A, Daroit DJ (2019) Beyond plucking: feathers bioprocessing into valuable protein hydrolysates. Waste Manag 95:399–415. https://doi.org/10.1016/j.wasman.2019.06.040
Liao B, Yan X, Zhang J, Chen M, Li Y, Huang J, Lei M, He H, Wang J (2019) Microbial community composition in alpine lake sediments from the Hengduan Mountains. Microbiologyopen 8(9):e00832. https://doi.org/10.1002/mbo3.832
Hassan MA, Bakhiet EK, Hussein HR et al (2019) Statistical optimization studies for polyhydroxybutyrate (PHB) production by novel Bacillus subtilis using agricultural and industrial wastes. Int J Environ Sci Technol 16:3497–3512. https://doi.org/10.1007/s13762-018-1900-y
Gradisar H, Friedrich J, Krizaj I, Jerala R (2005) Similarities and specificities of fungal keratinolytic proteases: comparison of keratinases of Paecilomyces marquandii and Doratomyces microsporus to some known proteases. Appl Environ Microbiol 71:3420–3426. https://doi.org/10.1128/AEM.71.7.3420-3426.2005
Liu D, Yang X, Huang J, Wu R, Wu C, He H, Li H (2015) In situ demonstration and characteristic analysis of the protease components from marine bacteria using substrate immersing zymography. Appl Biochem Biotechnol 175:489–501. https://doi.org/10.1007/s12010-014-1287-2
Liu D, Huang J, Wu C, Liu C, Huang R, Wang W, Yin T, Yan X, He H, Chen L (2019) Purification, characterization, and application for preparation of antioxidant peptides of extracellular protease from Pseudoalteromonas sp. H2. Molecules 24:3373 https://doi.org/10.3390/molecules24183373
Ismail SA, Abou Taleb M, Emran MA, Mowafi S, Hashem AM, El-Sayed H (2022) Benign felt-proofing of wool fibers using a keratinolytic thermophilic alkaline protease. J Nat Fibers 19(10):3697–3709. https://doi.org/10.1080/15440478.2020.1848721
Prajapati S, Koirala S, Anal AK (2021) Bioutilization of chicken feather waste by newly isolated keratinolytic bacteria and conversion into protein hydrolysates with improved functionalities. Appl Biochem Biotechnol 193:2497–2515. https://doi.org/10.1007/s12010-021-03554-4
Latafat Siddiqui MH, Ashish Vimal A, Bhargava P (2024) Isolation and screening of keratinolytic bacteria from feather dumping soil near in Lucknow and Kanpur city, North region of Indian. Biocatal and Biotransfor 42(1):68–76. https://doi.org/10.1080/10242422.2023.2235053
Yahaya RSR, Phang LY, Normi YM, Abdullah JO, Ahmad SA, Sabri S (2022) Feather-degrading Bacillus cereus HD1: genomic analysis and its optimization for keratinase production and feather degradation. Curr Microbiol 79:166. https://doi.org/10.1007/s00284-022-02861-1
Jeong JH, Jeon YD, Lee OM et al (2010) Characterization of a multifunctional feather-degrading Bacillus subtilis isolated from forest soil. Biodegradation 21:1029–1040. https://doi.org/10.1007/s10532-010-9363-y
Lai Y, Wu X, Li W and Wang L (2023) Insights into the keratin efficient degradation mechanism mediated by Bacillus sp. CN2 based on integrating functional degradomics. Biotechnol Biofuels Bioprod 16(1):1-14. https://doi.org/10.1186/s13068-023-02308-0
Su C, Gong JS, Qin J, Li H, Li H, Xu ZH, Shi JS (2020) The tale of a versatile enzyme: molecular insights into keratinase for its industrial dissemination. Biotechnol Adv 45:107655. https://doi.org/10.1016/j.biotechadv.2020.107655
El Salamony DH, Hassouna MSE, Zaghloul TI, Abdallah HM (2024) Valorization of chicken feather waste using recombinant Bacillus subtilis cells by solid-state fermentation for soluble proteins and serine alkaline protease production. Bioresour Technol 393:130110. https://doi.org/10.1016/j.biortech.2023.130110
Lu J, Zhao Y, Hu R, Cheng Y, Qin J, Yang J, Fang Y, Lyu M, Wang S (2022) Screening and characteristics of marine Bacillus velezensis Z-1 protease and its application of enzymatic hydrolysis of mussels to prepare antioxidant active substances. Molecules 27:6570. https://doi.org/10.3390/molecules27196570
Zhu Y, Yu J, Jiao C, Tong J, Zhang L, Chang Y, Sun W, Jin Q, Cai Y (2019) Optimization of quercetin extraction method in Dendrobium officinale by response surface methodology. Heliyon 5:e02374. https://doi.org/10.1016/j.heliyon.2019.e02374
Radha S, Gunasekaran P (2007) Cloning and expression of keratinase gene in Bacillus megaterium and optimization of fermentation conditions for the production of keratinase by recombinant strain. J Appl Microbiol 103:1301–10. https://doi.org/10.1111/j.1365-2672.2007.03372.x
Almahasheer AA, Mahmoud A, El-Komy H, Alqosaibi AI, Aktar S, AbdulAzeez S, Borgio JF (2022) Novel feather degrading keratinases from Bacillus cereus group: biochemical, genetic and bioinformatics analysis. Microorganisms 10(1):93. https://doi.org/10.3390/microorganisms10010093
Laskar A, Rodger EJ, Chatterjee A, Mandal C (2011) Modeling and structural analysis of evolutionarily diverse S8 family serine proteases. Bioinformation 7:239–45
Haq Iu, Akram F, Jabbar Z (2020) Keratinolytic enzyme-mediated biodegradation of recalcitrant poultry feathers waste by newly isolated Bacillus sp. NKSP-7 under submerged fermentation. Folia Microbiol 65:823–834. https://doi.org/10.1007/s12223-020-00793-6
Liaqat I, Ali S, Butt A, Durrani AI, Zafar U, Saleem S, Naseem S, Ahsan F (2022) Purification and characterization of keratinase from Bacillus licheniformis dcs1 for poultry waste processing. J Oleo Sci 71:693–700. https://doi.org/10.5650/jos.ess21426
Emran MA, Ismail SA, Abdel-Fattah AM (2020) Valorization of feather via the microbial production of multi-applicable keratinolytic enzyme. Biocatal agric biotechnol 27:101674. https://doi.org/10.1016/j.bcab.2020.101674
Derhab N, Mabrouk ME, El-Metwally MM, Mohammed YM (2023) Thermostable keratinase from Bacillus cereus L10: optimization and some potential biotechnological applications. Biomass Convers Biorefinery 1-17. https://doi.org/10.1007/s13399-023-04887-2
Sharma C, Timorshina S, Osmolovskiy A, Misri J, Singh R (2022) Chicken feather waste valorization into nutritive protein hydrolysate: role of novel thermostable keratinase from Bacillus pacificus RSA27. Front Microbiol 13:882902. https://doi.org/10.3389/fmicb.2022.882902
Wu WL, Chen MY, Tu IF, Lin YC, EswarKumar N, Chen MY, Ho MC, Wu SH (2017) The discovery of novel heat-stable keratinases from Meiothermus taiwanensis WR-220 and other extremophiles. Sci Rep 7:4658. https://doi.org/10.1038/s41598-017-04723-4
Hamiche S, Mechri S, Khelouia L, Annane R, El Hattab M, Badis A, Jaouadi B (2019) Purification and biochemical characterization of two keratinases from Bacillus amyloliquefaciens S13 isolated from marine brown alga Zonaria tournefortii with potential keratin-biodegradation and hide-unhairing activities. Int J Biol Macromol 122:758–769. https://doi.org/10.1016/j.ijbiomac.2018.10.174
Pawar VA, Prajapati AS, Akhani RC, Patel DH, Subramanian RB (2018) Molecular and biochemical characterization of a thermostable keratinase from Bacillus altitudinis RBDV1. 3 Biotech 8:107. https://doi.org/10.1007/s13205-018-1130-5
Kumar CG, Takagi H (1999) Microbial alkaline proteases: from a bioindustrial viewpoint. Biotechnol Adv 17:561–94. https://doi.org/10.1016/S0734-9750(99)00027-0
Zaraî Jaouadi N, Rekik H, Ben Elhoul M, Zohra Rahem F, Gorgi Hila C, Slimene H, Ben Aicha A, Badis A, Toumi S, Bejar B. Jaouadi (2015) A novel keratinase from Bacillus tequilensis strain Q7 with promising potential for the leather bating process. Int J Biol Macromol 79:952–964. https://doi.org/10.1016/j.ijbiomac.2015.05.038
Hassan MA, Haroun BM, Amara AA, Serour EA (2013) Production and characterization of keratinolytic protease from new wool-degrading Bacillus species isolated from Egyptian ecosystem. Biomed Res Int 175012 https://doi.org/10.1155/2013/175012
Hassan MA, Taha TH, Hamad GM, Hashem M, Alamri S, Mostafa YS (2020) Biochemical characterisation and application of keratinase from Bacillus thuringiensis MT1 to enable valorisation of hair wastes through biosynthesis of vitamin B-complex. Int J Biol Macromol 153:561–572. https://doi.org/10.1016/j.ijbiomac.2020.03.032
Callegaro K, Welter N, Daroit DJ (2018) Feathers as bioresource: microbial conversion into bioactive protein hydrolysates. Process Biochemistry 75:1–9
Liya SM and Umesh M (2023) Bioconversion of chicken feather waste into feather hydrolysate by multifaceted keratinolytic Bacillus tropicus LS27 and new insights into its antioxidant and plant growth-promoting properties. Biomass Convers Biorefinery 1-11. https://doi.org/10.1007/s13399-023-04664-1
Chen S, Wang Y, Zhang H, Chen R, Lv F, Li Z, Jiang T, Lin D, Zhang H, Yang L (2019) The antioxidant MitoQ protects against CSE-induced endothelial barrier injury and inflammation by inhibiting ROS and autophagy in human umbilical vein endothelial cells. Int J Biol Sci 15:1440–1451. https://doi.org/10.7150/ijbs.30193
Zhou X, Yang J, Zhou M, Zhang Y, Liu Y, Hou P, Zeng X, Yi L, Mi M (2019) Resveratrol attenuates endothelial oxidative injury by inducing autophagy via the activation of transcription factor EB. Nutr Metab 16:1–12. https://doi.org/10.1186/s12986-019-0371-6
Moussa Z, Darwish DB, Alrdahe SS, Saber WIA (2021) Innovative artificial-intelligence-based approach for the biodegradation of feather keratin by Bacillus paramycoides, and cytotoxicity of the resulting amino acids. Front Microbiol 12:731262. https://doi.org/10.3389/fmicb.2021.731262
Song SE, Jo HJ, Kim YW, Cho YJ, Kim JR, Park SY (2016) Delphinidin prevents high glucose-induced cell proliferation and collagen synthesis by inhibition of NOX-1 and mitochondrial superoxide in mesangial cells. J Pharmacol Sci 130:235–43. https://doi.org/10.1016/j.jphs.2016.03.005
Rizwan H, Pal S, Sabnam S, Pal A (2020) High glucose augments ROS generation regulates mitochondrial dysfunction and apoptosis via stress signalling cascades in keratinocytes. Life Sci 241:117148. https://doi.org/10.1016/j.lfs.2019.117148
Funding
The work was supported by the National Natural Science Foundation of China (31900038 and 31500048); the Natural Science Foundation of Hunan Province, China (2021JJ30029); Shandong Science and Technology SMEs Innovation Capacity Improvement Project (2022TSGC1016); Research Fund of The State Key Laboratory of Coal Resources and safe Mining (SKLCRSM22KF020); The Independent Exploration and Innovation project for postgraduates of Hunan Province (CX20220357, CX20220316); the Independent Exploration and Innovation project for postgraduates of Central South University (2022ZZTS0996); and the Open Sharing Fund for the Large-scale Instruments and Equipments of Central South University (CSUZC202244).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Qing Liu, Xue Li, BingQiang Liao, and Juan Zhou. Analysis was performed by Xun Xiao, BingQiang Liao, Yidan Chen, WenZhao Li, and Fei Bian. The first draft of the manuscript was written by Xun Xiao and HaiLian Rao. Hailun He edited the first draft and is the corresponding author. Tong Li made major revisions to the revised manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
The research included in the current submission does not involve human or animal subjects, or involves pathological reports, etc.
Consent to participate
All authors agree to participate in this study.
Consent for publication
All authors agree to the publication of this research in the journal. Manuscript is approved by all authors for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, X., Liao, B., Li, T. et al. Fermentation of feathers with Bacillus sp. TC5 to simultaneous obtain keratinase and antioxidant-rich peptide products. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05679-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05679-y