Abstract
Cornelian cherry (Cornus mas L.) is a rich source of (poly)phenols such as phenolic acids, flavonoids, and anthocyanins that are known for their bioactivity. This study aimed to enhance the industrial applications of this cherry’s pulp as a potential functional food ingredient. Foam-mat drying was used to produce cherry powder from cherry pulp. The convective drying process was performed at different temperatures (50, 60, 70, and 80°C) to study the effect of drying temperature on the physical properties, flow properties, microstructure, (poly)phenol content, antioxidant properties, and bioaccessibility of foam-dried cherry pulp, in comparison to freeze-dried sample. Different drying temperatures did not affect color properties. The highest gloss was determined at high temperatures (70–80°C). According to the Carr index, the fluidity increased between 18 and 22% as the temperature increased. The total phenolic content was higher at elevated temperatures (70–80°C), yet the anthocyanin content was the highest at 60°C. The flavonoid content was not affected by different drying temperatures, as well as compared to lyophilized samples. The antioxidant activity was higher in samples dried at elevated temperatures, with no significant difference in comparison to lyophilized samples. The bioaccessibility experiment showed that the lyophilized samples had significantly greater bioaccessibility in comparison to the convective dried samples, yet further investigation is required to understand the differences in bioaccessibility and bioavailability of individual polyphenols. Overall, foam-mat drying using a convective drying system could be an economic choice to preserve acceptable amounts of phenolics and antioxidants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cornelian cherry (CC), Cornus mas L., belongs to the family of Cornaceae. This tree is native to southern Europe and southwest Asia [1]. CC fruits contain high amounts of different reducing sugars and acids. In addition, it is a rich source of ascorbic acid (vitamin C) and (poly)phenols such as anthocyanins. The amount of ascorbic acid present in CC fruit was reported to be two times greater than in oranges. The amount of anthocyanins present in CC fruit can range between 34 and 292 mg/100 g [2]. A wide range of anthocyanins have been reported in CC fruit including cyanidin-3-glucoside, cyanidin-3-rutinoside, cyanidin-3-galactoside, delphinidin-3-galactoside, pelargonidin-3-glucoside, and pelargonidin-3-galactoside [3]. In fact, CC fruits also contain other polyphenols such as gallic acid, rutin, resveratrol, chlorogenic acid, and quercetin [2].
Due to the rich phytochemical content of CC fruits, they have been reported for their ethnomedicinal applications [4]. CC fruits, flowers, leaves, and bark have been widely used in Asian folk medicine. They have been used for diarrhea, lowering blood sugar, common cold, liver diseases, and weight loss. The CC fruit extracts have cosmetic applications as well and are used as a replacement for synthetic astringents [4].
CC fruits have the potential to become a functional food ingredient due to their rich phytochemical content and reported benefits. However, despite their valuable pro-health properties, these fruits are widely used in the food production industry. Drying these fruits could potentially increase their applications in the food industry and make them more compatible with the development of functional food products. Drying can lower the moisture content of the fruit, thus preventing microbial spoilage and undesired enzymatic changes [5]. Drying can be done by natural and artificial techniques [6]. Today, many artificial drying techniques have been developed that have advantages over natural drying. Few of these developed techniques are used for drying liquid products. Foam-drying, freeze-drying, and spray-drying techniques are mostly used for drying liquid products [7].
One of the techniques that is used to produce fruit powders is foam drying. Foam drying is suitable for drying fruits which are heat-sensitive, have high sugar content, and have a viscous nature [8]. In the process, the fruit is converted from liquid to stable foam (by whipping, bubbling, or shaking), with the addition of surfactants, and then dried by different methods [9]. Advantages of this technique include compatibility with most types of juices, fast drying at lower temperatures, preservation of nutritional quality, easy reconstitution, and cost-effectiveness to produce easily reconstituted juice powders.
So far, many fruits such as cherry [10], kiwi [11], blueberry [12], mango [13], dates [14], papaya [15], bitter orange [16], raspberry [17], and guava [18] have been successfully dried by foam drying. It has been stated that since the total surface area available for drying is increased by foaming the product, the drying time is significantly shortened, and therefore, the fruits are exposed to the heat effect for a shorter period of time [14]. Foam mat drying has proven to be an effective technique for producing fruit powders while preserving both their physical characteristics and nutritional properties, ensuring originality in the content [13].
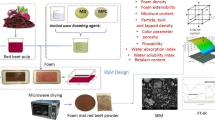
Although this technique has been widely applied to fruits, this technique has not been investigated with many fruit waste products — such as CC pulp. Thus, the current study aimed to evaluate the potential of foam drying to utilize CC waste (pulp) towards sustainable processing and production. The developed powder could potentially be a functional food ingredient used in the development of other products. The temperature at which the foam drying process is performed is the main factor considered in the study. The effect of temperature on the physical properties (color, pH, water activity, and percentage dry matter), flow properties (bulk density, tap density, Hausner ratio, Carr index, and solubility), microstructure, (poly)phenol content (phenolics, flavonoids, and anthocyanins), antioxidant properties, and bioaccessibility of foam-dried pulp.
2 Material and methods
2.1 Samples
Fresh CC fruits were purchased from the local market (Samsun, Turkey) in the summer of 2023. Fruits were washed with water after the removal of stems and leaves. The fruits were boiled in steam for 10 min. The seed was removed manually, and the fruits were juiced using a commercial juicer. The solid material left over after juicing was blended with distilled water to help remove any remaining juice. The mixture was then sieved through a 500-μm sieve, and the remaining pulp was collected for the foaming experiment.
2.2 Foaming experiment
The foamed CC pulp was prepared by mixing the foaming agent and foaming stabilizers with the pulp at room temperature. The foaming agent used was 10% egg albumen, and the foaming stabilizers were carboxyl methylcellulose (1.0%) and maltodextrin (MD) (10%). A commercial blender was used to form the foam by whipping for 5 min.
2.3 Freeze-drying
The frozen foam was dried for 48 h (until reaching a constant mass) using a freeze-dryer (Labconco Freezone 12 Plus, USA) operating at −80°C and 13 Pa. The freeze-dried samples were turned into fine powder and stored in sealed aluminum foil bags at −18°C.
2.4 Convective drying
The foams were dried with the convective system we used in our previous study [19]. In drying, air temperatures were set at 50, 60, 70, and 80°C, and the air velocity was 1.0 m/s.
2.5 Foam analysis
Foam expansion, foam density, and foam stability analyses were performed on the foam according to previously reported methods [20].
2.5.1 Foam expansion
The percentage change was determined by measuring the volume before and after foam formation.
2.5.2 Foam density
The density was determined by transferring the foam to a measuring cylinder, determining its volume and weight, and proportioning it to each other.
2.5.3 Foam stability
The foam was transferred to a measuring tape and left at room temperature for 3 h. Volume reduction was measured every 30 min.
2.6 Properties of fresh and powdered pulps
2.6.1 Color
The color of fresh and powdered pulps was analyzed using a colorimeter (MiniScan EZ 4500, Reston, VA, USA).
2.6.2 Moisture content
The moisture content was determined gravimetrically according to the standard method 934.06 [21]. In this method, samples were dried to a constant weight under vacuum at 70 °C.
2.6.3 Water activity
The water activity (aw) of fresh and powdered pulps was performed on a water activity meter (AquaLab 4TE), at 25 °C.
2.6.4 pH analysis
A digital pH meter (ORION 3-Star, USA) was used to measure the pH of the pulp and reconstituted CC pulp powder.
2.6.5 Moisture ratio
The moisture content of CC foam during the drying process was determined using the concept of moisture ratio (MR), represented by the equation:
In this equation, Mt refers to the moisture content at any given time during drying, Mi represents the initial moisture content, and Me represents the equilibrium moisture content (db) (kg water/kg dry solid). By using the moisture ratio, the changes in moisture content throughout the drying process can be quantified and analyzed [22].
2.7 Drying models
In order to select the most suitable drying model that represents the drying process of the CC foam, drying curves were fitted with 11 thin-layer drying MR models (Table 1). Curve fitting toolbox and Levenberg–Marquardt algorithm were used for data fitting in MATLAB software (R2016d). The fitting quality of the proposed models was assessed based on different statistical parameters:
-
1.
Determination of coefficient (R2):
-
2.
Reduced chi-square (χ2):
-
3.
Root mean square error (RMSE):
The best model describing thin-layer drying characteristics of CC foam will have the highest R2 values and lowest values of χ2 and RMSE [22].
2.8 Characterization of product
2.8.1 Flowability, and bulk and tapped density
The flowability of pulp powders is represented by numerically calculated Hausner ratio (HR) and Carr index (CI). The apparent and tap densities of the powders were measured in other to determine the HR and CI values [34]. Bulk density was determined by measuring the volume filled with 1 g of CC powder per 25-mL graduated cylinder by pouring it from top to bottom. On the other hand, before the volume of the powder is recorded, the impact intensity is measured after the graduated cylinder containing the powder has been struck 80 times.
2.8.2 Solubility
The solubility was determined as described by Abbasi and Azizpour [10]. For this, 100 mL of distilled water was transferred to a blender jar. Then, an amount of 1 g of CC pulp powder was added slowly to mixer running at 1500 rpm. After 5 min, the sample was centrifuged at 4300 g for 10 min. An aliquot (25 mL) of the obtained supernatant was transferred into Petri dishes and dried in a 105°C oven for 5 h. The solubility percentage was calculated according to Eq. (5):
where m2 is the final mass of the Petri dish containing the sample, and m1 is the mass of the empty Petri dish.
2.8.3 Microstructure
The microstructure of different CC pulp powders was performed using a scanning electron microscope (SEM, JEOL JSM-7001F). A small sample was briefly taken from the samples and attached to a stainless rod with double-bond tape. The assembly was then immediately sputtered with a gold/palladium target (60/40) at approximately 10 nm for 2 min using a spray coater powered by a flow of argon and plasma. Images were recorded at 10 kV and 15 kV acceleration rates.
2.8.4 Fourier transformed infrared analysis
An FTIR spectrometer (Perkin Elmer, Spectrum-Two, USA, PEService 35) was used to scan the samples and obtain the spectra of the region 4000 to 400 cm-1.
2.9 Analysis of polyphenol content
2.9.1 Extraction
An amount of 1 g of each powder sample was extracted with 50 mL of 80% methanol by ultrasound-assisted extraction. The samples were kept in an ultrasonic water bath (Soniclean™, Thermo Scientific, USA) for 20 min at 30°C with 100 W power. Then, samples were centrifuged for 15 min and 4000 rpm. The supernatant was collected for total phenolic content (TPO), total flavonoid content (TFO), and total anthocyanin content (TACN) analysis.
2.9.2 Total phenolic content
The total phenolic content (TPO) was determined by the spectrophotometric Folin-Ciocalteu method [35]. A volume of 150 μL of extract was mixed with 750 μL of 10% Folin-Ciocalteu reagent and vortexed for 1 min. Then, 600 μL of 7.5% Na2CO3 solution was added to the mixture. The mixture was vortexed again and incubated at room temperature in the dark for 2 h. Finally, the absorbance was measured at 760 nm using a UV/VIS spectrophotometer (LAMBDA™ 365, Perkin Elmer, USA). The TPO was calculated and reported as gallic acid equivalents (GAE); mg GAE/g dry weight (dw).
2.9.3 Total flavonoid content
The total flavonoid content (TF) was determined spectrophotometrically according to the AlCl3 method [35]. A volume of 1 mL of extract was mixed with 0.3 mL of 5% NaNO2. After 5 min, a volume of 0.5 mL of 5% AlCl3 was added. After waiting for 6 min, a volume of 0.5 mL of 1 M NaOH was added and vortexed. Finally, after 10 min, the absorbance was measured at 510 nm using a UV/VIS spectrophotometer (LAMBDA™ 365, Perkin Elmer, USA). The TF was calculated and reported as quercetin equivalents (QE); mg QE/g dw).
2.9.4 Total anthocyanin content
The total monomeric anthocyanin (TACN) was determined based on the pH differential method [36]. Extracts were diluted with buffers (pH 1.0 (0.025 M KCl) and 4.5 (0.4 M CH3COONa)) and their absorbance was measured at 520 and 700 nm using a UV/VIS spectrophotometer (LAMBDA™ 365, Perkin Elmer, USA). TACN was then calculated and reported as cyanidin-3-glucoside equivalents (CGE); mg CGE/g dw according to Eq. (6):
where At = (absorbance at 520 nm – absorbance at 700 nm) pH 1.0 – (absorbance at 520 nm – absorbance at 700 nm) pH 4.5; Mw (molecular weight) = 449.2 g/mol for cyanidin-3-glucoside, Df = dilution factor, l = path length in cm, and ϵ (molar extinction coefficient) = 26900 L/mol cm for cyanidin-3-glucoside.
2.10 Analysis of antioxidant potential
2.10.1 DPPH radical scavenging activity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging potential of the powders was spectrophotometrically determined [35]. A volume of 50 μL of extract was mixed with 1-mL DPPH solution (0.06 mM in 80% methanol). The solution was incubated at room temperature in the dark for 1 h. Finally, the absorbance of the solutions was measured at 517 nm using a UV/VIS spectrophotometer (LAMBDA™ 365, Perkin Elmer, USA). The radical scavenging activity was calculated using Eq. (7):
where AC is the absorbance of pure DPPH solution and AS is the absorbance of the test sample. The DPPH radical scavenging activity was calculated from a calibration curve using Trolox as a standard and results were reported as mmol Trolox equivalent (TE)/g dw.
2.10.2 Ferric-reducing antioxidant power
Ferric-reducing antioxidant power (FRAP) determination was performed according to the spectrophotometric method proposed by Zannou and Koca [35]. A volume of 50 μL of extract was mixed with 950 μL of 950 mM of FRAP solution (comprised of 100 mM acetate buffer, 10 mM FeCl3, and 10 mM TPTZ (2,4,6-tripyridyl-s-triazine)). The solution was shaken for 5 min, and the absorbance of the solutions was measured at 593 nm using a UV/VIS spectrophotometer (LAMBDA™ 365, Perkin Elmer, USA). The FRAP was calculated from a calibration curve using FeSO4 as a standard, and results were reported as mmol FeSO4 equivalent (ISE)/g dw.
2.11 Measurement of bioaccessibility
In vitro digestion of the samples was performed according to a previously published method [37]. A volume of 5 mL of each sample was combined with 20 mL of simulated gastric fluid containing 1.5 mL of pepsin (3.2 g/L). The pH of the mixture was adjusted to 1.7 using HCl (1 M). The mixture was incubated for 2 h at 37°C with continuous shaking. After 2 h, the pH of the mixture was adjusted to 7 with NaOH (1 M). A volume of 4 mL bile salt (5 g/L) and 1 mL CaCl2 (0.75 M) were added, followed by 2.5 mL of lipase (4.8 g/L). The pH of the mixture was adjusted to 7 with NaOH (1 M) and incubated for 2 h at 37°C with continuous shaking. After 2 h, an aliquot of each sample was collected and centrifuged at 4°C for 15 min at 5000×g. The TPO of supernatant was determined according to the previous method (Section 2.9.1.). The bioaccessibility was calculated according to the following equation:
where Cd and Cud are the concentrations of the TPO in the digested powder sample and the undigested powder sample, respectively.
2.12 Statistical analysis
All experiments were performed in triplicate. IBM SPSS Statistics V22.0 (International Business Machines (IBM) Corporation, Armonk, NY, USA) software was used for statistical analysis. The analysis of variance (ANOVA) and comparison among means was determined according to Duncan’s new multiple range test at 95% confidence level.
3 Results and discussion
3.1 Properties of CC pulp
The physicochemical properties of CC pulp were examined before any drying treatment. The pulp had 91.06±1.09% moisture content, 8.00±0.09% total soluble solids, and a pH of 3.70±0.10. Moldovan and David [38] found the pH value of the CC they used in the study to be 3.12 ± 0.04. This result is similar to ours. Szczepaniak et al. [39] reported that the soluble solids content of CC juice varied between 10.7 and 19.3%. These differences are thought to be related to genotype variation. The L*, a*, and b* values of pulp were 44.29±0.41, 7.94±0.20, and 19.99±0.31, respectively. Güzel [40] stated that the % dry matter values in cranberry fruit vary between 14.43 and 27.49, the L* value in color measurement varies between 25.18 and 33.00, the a value between 9.74 and 30.26, and the b value between 2.46 and 14.41. Our results are outside these stated ranges. These differences in physical properties are thought to be due to the fact that the fruit may be different in whole and pulp form. In this study, egg white, maltodextrin, and carboxymethyl cellulose (10:10:1, w/w) obtained as a result of the preliminary study were used in a single ratio. The density, stability, and expansion values of CC foam were found as 0.55±0.01 g/cm3, 98.68±1.00%, and 67.00±0.50 %, respectively. Krasaekoopt [41] determined that the optimum concentration of egg albumin for drying yoghurt with the foam drying method was 3%. They determined the foam expansion to be between 67.6 and 70.0%. This result is consistent with our findings. Shameena Beegum [42] observed that the protein-based nature of foam stabilizers plays the role of surfactant. The kinetic stability of the foam balanced its thermodynamic instability. Additionally, in their study, they obtained the best foam expansion result from sodium caseinate. In our study, egg albumin gave positive results in terms of expansion, and its selection was also evaluated in terms of accessibility and cost. Other studies in the literature also used egg albumin to create foam stability [43].
3.2 Drying kinetics
Increasing the drying temperature resulted in a significant decrease in drying time as seen in Fig. 1. This is due to the fact that increasing the temperature will result in an increase in molecular movement, enhancing the removal rate of moisture, which leads to a reduction in the drying time. In the foam mat drying method, because of the porous structure, increasing the drying temperature greatly affects the drying rate. The porous structure of the foam mat allows water vapor to escape quickly at high drying temperatures, which increases the drying rate [10]. Kadam and Balasubramanian [44] and Abd Karim and Wai [45] reported similar results. Drying rates were generally in the decreasing rate period. In the foam drying method, the drying rate varies depending on the agents used, the chemical structure of the food, and its content [45]. As the water content decreased, a decrease was observed in the drying rate occurring in the inner regions of the foam as the outer layers dried faster. As the surface area of the food increases, the dehydration process is easy. The constants and statistical parameters obtained for the cranberry pulp foam are given in Table 2.
Kaya and Aydin [46] investigated the drying kinetics and drying behaviors of CC dried with the help of a convective dryer. They were dried in a convective dryer at 30, 40, and 50°C. As a result of the study, Two-term exponential and Henderson, which is one of the thin-layer drying models of cranberry fruit, determined that it provided the most appropriate correlation in Pabis models. In another study examining the drying kinetics of CC fruit and the degradation of vitamin C, it was determined that Henderson and Pabis, modified from thin layer drying models of samples dried at 50, 60, and 70°C, provided the most appropriate correlation [47].
In a study in which foam drying was performed at 65, 75, and 85°C with 0–9% egg white using mango pulp, the increase in egg white concentration adversely affected the drying properties. While the optimum ratio was chosen as 3% egg white, the Wangh and Singh model was found suitable for thin layer drying models at 65 and 85°C, while the logarithmic and diffusion models were found suitable for 75°C [48]. Azizpour et al. [49] found that the moisture content decreased with increasing drying time in the foam drying of shrimp. Among the drying models, they determined that the Midilli model gave the highest R2, the lowest RMSE, and X2 values. In our study, it has been determined that the Aghabashlo model, which is one of the thin layer drying models, is the most suitable model for CC pulp foam drying by giving high R2, low RMSE, and X2 values.
3.3 Physical properties
The physicochemical characteristics of CC pulp powder are given in Table 3 and the powders prepared using foam-mat drying are shown in Fig. 2. The observed L* value of powder ranged from 63.15±0.06 to 66.35±1.32, a* value ranged from 27.46±1.98 to 31.57±0.61, and b* value ranged from 4.10±0.96 to 5.38±0.06 at different drying methods. In general, as the drying temperature increased, the lightness value increased and the redness decreased. Sahu et al. [50] found that as the drying temperature of foam-dried jamun pulp increased, the lightness, redness, and yellowness values decreased. The researchers attributed this change in color to the fact that when the drying temperature is increased, the reducing sugars bind with an amino group protein, causing the Maillard reaction and the breakdown of the total anthocyanin content.
It has been determined in various studies that foaming agents and foam stabilizers affect color parameters. Gao et al. [12] reported that egg white powders added to the foam had a diluting effect on the color in their study on blueberry. They observed that L*, a*, and b* values tended to decrease by increasing the carboxymethyl cellulose stabilizer from 0 to 1%. Ng and Sulaiman [51] investigated the physicochemical properties of powders produced from red beet using egg albumin, maltodextrin, and fish gelatin. They reported that control samples of beets without maltodextrin showed lower L*, a*, and b* values than those containing 10% egg albumin. They reported that the increase in L* value was due to the white color of maltodextrin. In a study on tomatoes, it was observed that a* values increased with increasing drying temperature, egg albumin, and carboxymethyl cellulose concentration. It is thought that non-enzymatic reactions are effective in the structural degradation of various pigment compounds [52]. Hajiaghaei and Sharif [53] carried out foam drying in red beet and quince fruit with 0–10% maltodextrin. They observed that the color results of the powders obtained by hot air and freeze-drying methods were different from each other, and the L*, a*, and b* values of the freeze-dried ones were lower, which is consistent with our data. Çalışkan Koç et al. [54] foam dried taro fruit using different methods. They observed that the use of egg white powder accelerated the browning reaction, the L* value increased, and the a* and b* values decreased compared to freeze-drying and hot air. These results were inconsistent with our study. In the present study, the b* (yellowness) value of the powders obtained by the freeze-drying method is lower than the convective-dried ones. There was no statistically significant difference between a* values (p>0.05).
Depending on the drying method applied, the samples obtained were characterized by water activity ranging from 0.2094 to 0.3272, which is shown in Table 3. The lowest water activity was determined in the freeze dried powder. It can be assumed that the obtained products were microbiologically stable.
3.4 Flowability, cohesiveness, and solubility
The fluidity and stickiness of the powders were evaluated as the Carr index (CI) and Hausner ratio (HR). The flowability is used to determine the free flow properties of the powder. A suitable powder flow is important for both consumers and manufacturers [53]. The density, flow properties and solubility of powders are given in Table 4.
According to the CI, it was observed that the fluidity was between 18 and 22% and increased as the temperature increased (Table 4). Results between 15 and 20% were rated as “good” as classified by Hajiaghaei and Sharif [53]. The stickiness of the powders, HR, results were between 0.77 and 0.81. In Hajiaghaei and Sharif [53], samples with (<1.2) values according to their classification were evaluated as “low” adhesiveness. Sahu et al. [50] observed that fluidity values increased as the drying temperature increased for foam-dried jamun pulp, in agreement with our findings. They stated that the increased value in flowability with higher temperature of drying may be due to the reduction in moisture content.
A higher HR means the powder is more sticky and less free-flowing. The cohesiveness of dust particles is mostly affected by interparticle forces as well as other factors such as mechanical interlocking [53]. Furthermore, the solubility of powders is an important factor in the production of fruit powders. Fruit powders with poor solubility provoke challenges during processing resulting in economic losses. For food powders to be useful and functional, they must have high solubility in the aqueous phase [55]. The solubility values of the convective dried ones were close to the lyophilized powders. The lowest solubility value was obtained in those dried at high temperatures.
3.5 Scanning electron microscopy
SEM was used to examine the morphological structure of dried CC powders. It is observed that the appearance of the powders obtained by lyophilization is smoother than those obtained convectively (Fig. 3). The fact that the powders obtained by lyophilization appear more uniform than those obtained by the convective method may indicate a possible more homogeneous water removal process. It has been observed that the structure at 60 and 70°C is rougher and more complex than at 50°C and 80°C. The rough and complex structures at 60 and 70°C may indicate that drying processes in certain temperature ranges affect the intrinsic properties of the material. It has been observed that the most suitable samples according to the morphological structure are the samples dried at 50 and 80°C, and by lyophilization. The fact that the most suitable samples according to morphological structure are those lyophilized and dried at 50 and 80°C may suggest that these temperatures provide optimal water removal conditions for the material. Seerangurayar et al. [14] found that in the particle morphology of palm powders obtained by foam drying using maltodextrin and gum Arabic, using an agent is necessary to obtain a fluid powder and the particles appear smoother and larger depending on the sugar content. The wrinkling on the surface is directly proportional to the concentration ratios of the agents used [56].
3.6 FTIR spectra of powder samples
FTIR spectra of cranberry fruit are shown in Fig 4. Three characteristic regions are observed in the spectrum between 3600 and 2500, 2000 and 1300, and 1350 and 700 cm−1. The bands in the 3400–3000 cm−1 region are typically connected to various hydroxyl (OH) stretching vibrations. The OH stretching vibrations witnessed can be greatly influenced by hydrogen bonding, so they depend on the strength of the hydrogen bonds. The range of 3000–2900 cm−1 was assigned to the symmetrical and asymmetric stretching vibration of the C-H band and the stretching vibration of the N-H band, corresponding to both the alkyl and aromatic groups, respectively [57]. In the second region, absorption bands for carboxyl and ester groups can be found between 1640 and 1720 cm−1 [58]. The presence of amide Ι (stretch vibrations of C=O and C-N groups) and amid II (mainly from N-H bending) at absorbing wavelengths between 1700–1500 cm−1 and 1500–1300 cm−1 proves the presence of the protein. The variation in the band region between 1400 and 1100 cm−1 is due to the OCH, CCH, and COH bending vibrational modes of carbohydrates [59, 60]. Peaks related to phenolic compounds were found in regions between 1600 and 900 cm−1. For example, the bond around 1640 cm−1 predominantly belongs to the C-C bond vibrations of phenolic compounds [61]. In the third region, C-O-H bonds and C-O, C-O-C glycosidic bonds are found in the region between 1150 and 700 cm−1 [62]. The last spectral region, that is, the part below 900 cm−1, is the fingerprint responsible for the crystal field showing the conformational changes in the material.
3.7 Polyphenol content
CC fruit contains a significant amount of antioxidant anthocyanins and phenolic compounds [39]. Total phenolics, total flavonoids, and total anthocyanins of CC pulp used in this study were determined as 32.81±0.22 mg GAE/g, 12.83±0.56 mg ECE/g, and 4.69±1.23 mg CGE/g in dry matter, respectively. In the literature, total phenolics for CC fruit have been reported as 2.61–26.96 mg/g [63,64,65,66], total flavonoids as 0.54–6.69 mg/g [65, 67], and total anthocyanins as 1.07–8.50 mg/g [63, 65, 68]. Table 5 shows the TPO, TF, and TACN, and antioxidant activity of foam-dried CC.
The TPO of CC powders was found to range from 15.97 to 16.33 mg GAE/g powder. The TPO value of the lyophilized sample has a higher value than the convective dried ones. Of the convective dried ones, the samples dried at 50°C and 60°C had a lower TPO value than the other temperatures. High temperatures can reduce TPO values by accelerating oxidative degradation. The TF of CC powders is shown in Table 5 ranging from 0.94 to 1.29 mg QE/g powder. Total flavonoid values were lowest in lyophilized samples, while the highest was measured in samples dried at 80°C. Danielewski et al. [69] reported that the TPO in CC extract was 25.46 mg GAE/g and TF was 18.51 mg QE/g. Gupta et al. [70] showed that foam mat drying protects phenolic compounds in jujube juice and increases antioxidant activity. In addition, increased foaming agent concentration helped preserve phenolic compounds. Brar et al. [71] investigated the effect of process parameters on the drying of peach fruit by foam mat drying method. They determined that the most suitable process parameters to obtain the highest TPC of dried peach powder were 12% peach concentration, 900 rpm whipping speed, 50°C drying temperature, and 360 min drying time. Jakkranuhwat and Kunchansombat [72] found that the processing of purple-fleshed sweet potato powder with foam drying resulted in a decrease in TPC and TACN with an increase in temperature and volume change parameters during the foam drying process.
Anthocyanins play an important role in determining the antioxidant capacity of the samples. They are bioactive substances that degrade at high temperatures. The lyophilized sample had the highest TACN at 0.53 mg/g, while the lowest anthocyanin content was found in samples dried at 80°C with 0.32 mg/g. As the drying temperature increased, the TACN decreased. The TACN of samples dried at 50°C is significantly lower than at other temperatures. However, samples dried at 60°C and 70°C gave better results in terms of TACN than at other temperatures. This shows that certain temperature conditions have a complex effect on the degradation processes of anthocyanins and that sometimes an increase in temperature can increase color stability, contrary to expectations. This may be due to the fact that anthocyanins have a dynamic chemical structure that can enter into complex reactions and turn into different products depending on temperature. Tepić Horecki et al. [73] compared the TACN of CC for different drying methods. They calculated the TACN in fresh fruits as 14.70 mg/g. They determined the highest anthocyanin content in samples dried under vacuum at low temperature (40°C), significant anthocyanin loss was detected in samples dried at high temperatures (60–80°C) and in the sun. Zia and Alibas [4] dried CC fruits at 50, 70, and 90°C and found their TACN to be 1.05, 2.62, and 0.63 mg/g, respectively. Our results agree with these results, high temperature caused anthocyanin loss. Kitchen et al. [74] investigated the effects of drying temperature on anthocyanins of vacuum belt dried blueberry pulp powder and found that anthocyanins decreased with increasing drying temperature. Darniadi et al. [75] examined the individual anthocyanin profiles of blueberry powders obtained by foam mat freeze-drying and spray-drying methods and observed higher levels of anthocyanin compounds in blueberry powder obtained by foam mat freeze-drying method. Reis et al. [76] stated that foam mat drying can increase the stability of anthocyanins in plant foods, maintain the integrity of anthocyanins and increase the anthocyanin content. Darniadi et al. [77] investigated the effects of different matrix materials and stabilizers (trehalose, β-lactoglobulin, and bovine serum albumin) for foam drying processes based on blueberry juice. They showed that the matrix materials and stabilizers used during foam drying processes can have a significant effect on the stability and composition of anthocyanins.
It is important to highlight that further studies are needed to characterize the polyphenol profile of the products formed and assess the effect of drying temperature on specific phenolic acids, flavanoids, and anthocyanins that are known for their remarkable bioactive properties.
3.8 Antioxidant activity
Antioxidant activities of dried samples were performed with two different methods, and the results are presented in Table 5. DPPH and FRAP values of CC pulp used in this study were determined as 311.60±16.70 mmol/g, and 228.10±20.40 mmol/g in dry matter, respectively. FRAP and DPPH values of cornelian cherries were previously reported as being between 41 and 149 μmol/g dw [63, 78], and 33.41 and 39.89 μmol Trolox/g in fresh weight [79], respectively.
As can be seen, the DPPH and FRAP values of the samples varied significantly depending on the drying method and temperature (p>0.05). High-temperature convective drying had a higher DPPH free radical scavenging effect. Lyophilization minimizes oxidative degradation of phenolic compounds, especially anthocyanins, by allowing water to directly transition into a solid state under low temperature and vacuum; this contributes to the maintenance of high antioxidant activity and long shelf life of the samples. The significant protection of the phenolic compound, especially anthocyanin content, of the lyophilized samples, led to high antioxidant activity. Drying at 60 and 80°C had the highest DPPH free radical removal effect. However, the differences in other temperature levels are not statistically significant. The lowest DPPH free radical scavenging effect was found in the powders produced by the convective drying process at 50°C. Cosmulescu et al. [67] found that the DPPH free radical scavenging effect in various genotypes of cranberry ranged from 1.24 to 2.71 mmol Trolox/100 g. According to this study, it has been shown that there is a significant difference in the antioxidant capacity of CC under different conditions and the use of different methods also affects the results of the analysis. Petkova and Ognyanov [80] stated that the lowest antioxidant activity was in dried CC (46–48 mM TE/g), therefore, storing CC as a compote has a promising antioxidant potential. Wojdyło et al. [81] stated that drying fruit at high temperatures can reduce the antioxidant activity, and they found that cherries dried at 70°C had lower antioxidant capacity than those dried at lower temperatures. Zia and Alibas [4] found a decrease in the antioxidant activities of DPPH, ABTS, and FRAP as the drying temperature increased. They noted that the samples dried at 50°C had higher antioxidant activity. Tepić Horecki et al. [73] stated that as the drying temperature increases, the antioxidant activity decreases. Skrovankova et al. [82] found that the antioxidant activity in CC could increase with drying time, and the increase measured after drying was approximately 1.3 times higher than before drying.
FRAP data show that lyophilization and drying at 80°C resulted in high antioxidant activity. Lyophilization provided the best preservation of antioxidant compounds in CC pulps. FRAP values increased when the phenolics were high. In other words, FRAP values were better preserved due to the better preservation of phenolic compounds. In addition, higher FRAP values at 70–80°C may be an indication that a mild Maillard reaction occurs when these temperatures are increased, and as a result, new antioxidant compounds are formed. The Maillard reaction is a complex reaction that occurs as a result of the reaction between proteins and carbohydrates, and the compounds formed as a result of this reaction are known to have antioxidant properties. Zielinska and Zielinska [83] determined the antioxidant activities of dried CC with different drying methods. They determined that the antioxidant activities of the samples dried by the microwave-vacuum drying method were better preserved than those dried by the conventional drying method. Oszmiański et al. [84] convective and freeze-dried CC and found that convective-dried ones showed higher FRAP values. Michalska et al. [85] investigated the effect of different drying conditions (temperature, airflow) on the antioxidant properties of black currant pulp, which was ground into powder, and found that the powders dried at low temperature (50°C) had high antioxidant capacity.
Although the methods used to assess the antioxidant power of the developed samples are valid, accurate, and quantitative; yet they have some limitations. These methods may not represent the biological system in which the polyphenols would act as antioxidants. The complexity and biological interactions that happen in the human body are not considered in these assays. In addition, different polypehnols or other antioxidants may have different mechanisms of action—such as regulating cellular antioxidant pathways which are not considered in these assays.
3.9 Bioaccessibility of product
The highest bioaccessibility of total phenolic compounds was obtained in the samples prepared by the lyophilization process (Table 6). In the drying processes at other temperature levels, the bioaccessibility of total phenolics was lower. This result shows that the lyophilization process prevents the loss of phenolic compounds in CC and the bioactive components of the fruit are better preserved. However, based only on the total phenolic content, it is not possible to say that CC foam drying provides less bioactive component protection compared to drying processes at other temperature levels. The bioaccessibility of flavonoids and anthocyanins was not investigated in the current study which is considered one of the limitations. A previous study [86] investigated the effect of foam drying on red sorghum at different temperatures (60–80°C) on bioaccessibility. The results showed that foam drying at high temperatures reduced the bioaccessibility of proanthocyanidins in sorghum extract. This is because oxidative damage at high temperatures reduces antioxidant capacity. However, no reduction in bioaccessibility of proanthocyanidins was observed when foam drying at low temperatures was applied. It has been reported that foam drying had higher bioaccessibility than other drying methods [75].
It is important to highlight that the determination of the bioaccessibility of compounds by in vitro digestion has limitations and may not reflect the true gastrointestinal conditions. The bioaccessibility of polyphenols is affected by the gut microbiota, and those microorganisms contribute to the breakdown of large polyphenols into smaller compounds that are more bioaccessible and can pass through the intestinal wall into the blood stream. The experimental method used did not consider the gut microbiota. In addition, the product formed can potentially be a functional food ingredient, thus it would be incorporated into a product. The new matrix (product formed) will be different in comparison to the raw material (CC powder), and this can influence the bioaccessibility of polyphenols. Thus, further experiments are required to assess the bioaccessibility of the formulated powder.
4 Conclusions
In the present study, the CC powder was produced by the foam mat-drying method, a method that requires a shorter drying time with a relatively lower processing temperature. The Aghbashlo model best described the drying behavior of cranberry pulp foams. After the lyophilized samples, the lowest bulk density, tap density, and CI values were determined in those dried at 50°C. The highest phenolic content was preserved at high temperatures, and anthocyanin preservation was at 60°C. The highest conservation of FRAP and DPPH values was determined at 80°C. With convective drying, values close to lyophilization were obtained in terms of both physical and antioxidant properties. The highest bioaccessibility value was determined in the lyophilized samples, and there was no statistical difference depending on the drying temperatures in the convective dried samples (p>0.05). When evaluated in terms of the temperatures used in drying, it can be said that a different temperature is better for each of the investigated parameters. Considering the convenience and cost, it was concluded that CC pulp can be used in convective dryers for foam drying. This powder can be used in many food products, such as beverages, sweets, and can be a convenient agent for synthetic additives.
References
Czerwińska ME, Melzig MF (2018) Cornus mas and Cornus officinalis—analogies and differences of two medicinal plants traditionally used. Front Pharmacol 9:894. https://doi.org/10.3389/fphar.2018.00894
Tontul I, Eroğlu E, Topuz A (2018) Convective and refractance window drying of cornelian cherry pulp: effect on physicochemical properties. J Food Process Eng 41:12917. https://doi.org/10.1111/jfpe.12917
Tural S, Koca I (2008) Physico-chemical and antioxidant properties of cornelian cherry fruits (Cornus mas L.) grown in Turkey. Sci Hortic 116:362–366. https://doi.org/10.1016/j.scienta.2008.02.003
Zia MP, Alibas I (2021) The effect of different drying techniques on color parameters, ascorbic acid content, anthocyanin and antioxidant capacities of cornelian cherry. Food Chem 364:130358. https://doi.org/10.1016/j.foodchem.2021.130358
Buljat AM, Jurina T, Jurinjak Tušek A, Valinger D, Gajdoš Kljusurić J, Benković M (2019) Applicability of foam mat drying process for production of instant cocoa powder enriched with lavender extract. Food Technol Biotechnol 57:159–170. https://doi.org/10.17113/ftb.57.02.19.6064
Hossain MA, Mitra S, Belal M, Zzaman W (2021) Effect of foaming agent concentration and drying temperature on biochemical properties of foam mat dried tomato powder. Food Res 5:291–297. https://doi.org/10.26656/fr.2017.5(1).372
Sangamithra A, Swamy GJ, Prema RS, Priyavarshini R, Chandrasekar V, Sasikala S (2014) An overview of a polyhouse dryer. Renew Sustain Energy Rev 40:902–910. https://doi.org/10.1016/j.rser.2014.08.007
Qadri OS, Srivastava AK, Yousuf B (2020) Trends in foam mat drying of foods: special emphasis on hybrid foam mat drying technology. Crit Rev Food Sci Nutr 60:1667–1676. https://doi.org/10.1080/10408398.2019.1588221
Çalışkan Koç G, Tekgül Y, Yüksel AN, Khanashyam AC, Kothakota A, Pandiselvam R (2022) Recent development in foam-mat drying process: influence of foaming agents and foam properties on powder properties. J Surfactant Deterg 25:539–557. https://doi.org/10.1002/jsde.12608
Abbasi E, Azizpour M (2016) Evaluation of physicochemical properties of foam mat dried sour cherry powder. LWT - Food Sci Technol 68:105–110. https://doi.org/10.1016/j.lwt.2015.12.004
Bogusz R, Smetana S, Wiktor A, Parniakov O, Pobiega K, Rybak K, Nowacka M (2022) The selected quality aspects of infrared-dried black soldier fly (Hermetia illucens) and yellow mealworm (Tenebrio molitor) larvae pre-treated by pulsed electric field. Innov Food Sci Emerg Technol 80:103085. https://doi.org/10.1016/j.ifset.2022.103085
Gao R, Xue L, Zhang Y, Liu Y, Shen L, Zheng X (2022) Production of blueberry pulp powder by microwave-assisted foam-mat drying: effects of formulations of foaming agents on drying characteristics and physicochemical properties. LWT 154:112811. https://doi.org/10.1016/j.lwt.2021.112811
Chaux-Gutiérrez AM, Santos AB, Granda-Restrepo DM, Mauro MA (2017) Foam mat drying of mango: effect of processing parameters on the drying kinetic and product quality. Drying Technol 35:631–641. https://doi.org/10.1080/07373937.2016.1201486
Seerangurayar T, Manickavasagan A, Al-Ismaili AM, Al-Mulla YA (2017) Effect of carrier agents on flowability and microstructural properties of foam-mat freeze dried date powder. J Food Eng 215:33–43. https://doi.org/10.1016/j.jfoodeng.2017.07.016
Kandasamy P, Varadharaju N, Kalemullah S, Maladhi D (2014) Optimization of process parameters for foam-mat drying of papaya pulp. J Food Sci Technol 51:2526–2534. https://doi.org/10.1007/s13197-012-0812-y
Süfer Ö, Pandiselvam R, Kaya YY (2023) Drying kinetics, powder properties, and bioactive components of bitter orange (Citrus aurantium L.) dried by microwave-assisted foam-mat approach. Biomass Convers Biorefin:1–13. https://doi.org/10.1007/s13399-023-04477-2
Ozcelik M, Ambros S, Heigl A, Dachmann E, Kulozik U (2019) Impact of hydrocolloid addition and microwave processing condition on drying behavior of foamed raspberry puree. J Food Eng 240:83–91. https://doi.org/10.1016/j.jfoodeng.2018.07.001
Qadri OS, Srivastava AK (2017) Microwave-assisted foam mat drying of guava pulp: drying kinetics and effect on quality attributes. J Food Process Eng 40:12295. https://doi.org/10.1111/jfpe.12295
Hassan AM, Zannou O, Pashazadeh H, Ali Redha A, Koca I (2022) Drying date plum (Diospyros lotus L.) fruit: assessing rehydration properties, antioxidant activity, and phenolic compounds. J Food Sci 87:4394–4415. https://doi.org/10.1111/1750-3841.16322
Munawar N, Azhar N, Rohaizad NS, Hussain N (2020) Characterization of watermelon rind powder using foam mat drying with different types of foaming agents and temperatures. Asian J Fundam Appl Sci 1:1–10
AOAC (2010) Association of Official Analytical Chemists Official Methods of Analysis of the Association of Official Analytical Chemists, 18th edn. AOAC International, Washington DC
Izadi Z, Mohebbi M, Shahidi F, Varidi M, Salahi MR (2020) Cheese powder production and characterization: a foam-mat drying approach. Food Bioprod Process 123:225–237. https://doi.org/10.1016/j.fbp.2020.06.019
Ayensu A (1997) Dehydration of food crops using a solar dryer with convective heat flow. Solar Energy 59:121–126. https://doi.org/10.1016/S0038-092X(96)00130-2
Page G (1949) Factors influencing the maximum rates of air-drying shelled corn in thin layer, MSc Thesis. PU Department of Mechanical Engineering, West Lafayette, USA
Henderson SM (1961) Grain drying theory, I. Temperature effect on drying coefficient. J Agr Eng Res 6:169–173. https://doi.org/10.20809/seisan.13.2_76
Lahsasni S, Kouhila M, Mahrouz M, Jaouhari JT (2004) Drying kinetics of prickly pear fruit (Opuntia ficus indica). J Food Eng 61:173–179. https://doi.org/10.1016/S0260-8774(03)00084-0
Sharaf-Eldeen YI, Blaisdell JL, Hamdy MY (1980) A model for ear corn drying. Trans ASAE 23:1261–1265. https://doi.org/10.13031/2013.34757
Wang CY, Singh RP (1978) A single layer drying equation for rough rice. Sci. Educ 4:115–124
Yaldız O, Ertekin C (2001) Thin layer solar drying some different vegetables. Drying Technol 19(3):583–596
Sacilik K et al J Food Eng 76:362–368. https://doi.org/10.1016/j.jfoodeng.2005.05.031
Chandra PK, Singh RP (1995) Applied numerical methods for food and agricultural engineers. CRC Press, Boca Raton, pp 163–167
Midilli Kucuk H, Yapar Z (2002) A new model for single-layer drying. Drying Technol 20:1503–1513. https://doi.org/10.1081/DRT-120005864
Aghbashlo M, Kianmehr MH, Khani S, Ghasemi M (2009) Mathematical modelling of thin-layer drying of carrot. Int Agrophys 23:313–317
Kaleem MA, Alam MZ, Khan M, Jaffery SHI, Rashid B (2021) An experimental investigation on accuracy of Hausner Ratio and Carr Index of powders in additive manufacturing processes. Metal Powder Report 76:50–54. https://doi.org/10.1016/j.mprp.2020.06.061
Zannou O, Koca I (2020) Optimization and stabilization of the antioxidant properties from Alkanet (Alkanna tinctoria) with natural deep eutectic solvents. Arab J Chem 13:6437–6450. https://doi.org/10.1016/j.arabjc.2020.06.002
Lee J, Durst RW, Wrolstad RE, Eisele T, Giusti MM, Hach J, Hofsommer H, Koswig S, Krueger DA, Kupina, Martin SK, Martinsen BK, Miller TC, Paquette F, Ryabkova A, Skrede G, Trenn U, Wightman JD (2005) Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int 88:1269–1278. https://doi.org/10.1093/jaoac/88.5.1269
Salvia-Trujillo L, McClements DJ (2016) Enhancement of lycopene bioaccessibility from tomato juice using excipient emulsions: influence of lipid droplet size. Food Chem 210:295–304. https://doi.org/10.1016/j.foodchem.2016.04.125
Moldovan B, David L (2020) Influence of different sweeteners on the stability of anthocyanins from cornelian cherry juice. Foods 9:1266. https://doi.org/10.3390/foods9091266
Szczepaniak OM, Kobus-Cisowska J, Kusek W, Przeor M (2019) Functional properties of Cornelian cherry (Cornus mas L.): a comprehensive review. Eur Food Res Technol 245:2071–2087. https://doi.org/10.1007/s00217-019-03313-0
Güzel N (2021) Morphometric and physico-chemical properties of cornelian cherry (Cornus mas L.) grown in Çorum, Turkey. Akademik Gıda 19:373–380
Krasaekoopt W, Bhatia S (2012) Production of yogurt powder using foam-mat drying. AU J Technol 15:166–171
Shameena Beegum PP, Manikantan MR, Anju KB, Vinija V, Pandiselvam R, Jayashekhar S, Hebbar KB (2022) Foam mat drying technique in coconut milk: effect of additives on foaming and powder properties and its economic analysis. J Food Process Preserv 46:17122. https://doi.org/10.1111/jfpp.17122
Valentová K, Ulrichová J (2003) Smallanthus sonchifolius and Lepidium meyenii-prospective Andean crops for the prevention of chronic diseases. Biomedical Papers 147:119–130. https://doi.org/10.5507/bp.2003.017
Kadam DM, Balasubramanian S (2011) Foam mat drying of tomato juice. J Food Process Preserv 35:488–495. https://doi.org/10.1111/j.1745-4549.2010.00492.x
Abd Karim A, Wai CC (1999) Foam-mat drying of starfruit (Averrhoa carambola L.) puree. Stability and air drying characteristics. Food Chem 64:337–343. https://doi.org/10.1016/S0308-8146(98)00119-8
Kaya A, Aydin O (2008) Kurutma havası sıcaklıgının kızılcıgın kuruma süresi ve sorpsiyon egrisine etkisinin deneysel incelenmesi. Isı Bilimi ve Tekniği Dergisi/Journal of Thermal Science & Technology
Polatoğlu B, Beşe AV (2017) Kızılcık meyvesinin (Cornus mas L.) konvektif kurutulması: kuruma kinetiği ve C vitamini bozulması. Niğde Ömer Halisdemir Üniversitesi Mühendislik Bilimleri Dergisi 6:406–414. https://doi.org/10.28948/ngumuh.341200
Wilson RA, Kadam DM, Chadha S, Sharma M (2012) Foam mat drying characteristics of mango pulp. Int J Food Sci Nutr Eng 2:63–69. https://doi.org/10.5923/j.food.20120204.03
Azizpour M, Mohebbi M, Khodaparast MHH, Varidi M (2013) Foam-mat drying of shrimp: characterization and drying kinetics of foam. Agric Eng Int: CIGR Journal 15:159–165
Sahu I, Mohapatra M, Panda MK, Nayak R, Pal US, Rayaguru K, Dash SK (2022) Foam mat drying of Indian blackberry (SyzygiumcuminiiL.) fruit pulp: optimization of process parameters and its powder characteristics. https://doi.org/10.21203/rs.3.rs-2273109/v2
Ng ML, Sulaiman R (2018) Development of beetroot (Beta vulgaris) powder using foam mat drying. Lwt 88:80–86. https://doi.org/10.1016/j.lwt.2017.08.032
Belal M, Hossain MA, Mitra S, Zzaman W (2023) Effect of foaming agent concentration and foam stabilizer on the foaming capacity and physical properties of tomato powder at dried at different temperature: foam mat dried tomato powder. J Microbiol Biotechnol Food Sci 12:4741. https://doi.org/10.55251/jmbfs.4741
Hajiaghaei M, Sharifi A (2022) Physicochemical properties of red beetroot and quince fruit extracts instant beverage powder: effect of drying method and maltodextrin concentration. J Food Qual 2022:1–8. https://doi.org/10.1155/2022/7499994
Çalışkan Koç G, Yüksel AN, Baş E, Erdoğan SL (2020) Foam mat drying of taro (Colocasia esculenta): the effect of ultrasonic pretreatment and drying techniques on the drying behavior, flow, and reconstitution properties of taro flour. J Food Process Eng 43:13516. https://doi.org/10.1111/jfpe.13516
Fauziyah B, Yuwono M, Isnaeni I, Nahdhia N, Sholihah F (2022) Isolation and characterization of sugarcane (Saccharum officinarum l.) bagasse cellulose hydrolyzed with acid variation. Trop J Nat Product Res 6:856–862
Ekpong A, Phomkong W, Onsaard E (2016) The effects of maltodextrin as a drying aid and drying temperature on production of tamarind powder and consumer acceptance of the powder. Int Food Res J 23:300–308
Durgawati Balasubramanian P, Sutar PP (2023) Development of a novel non-water infrared refractance window drying method for Malabar spinach: optimization of process parameters using drying kinetics, mass transfer, and powder characterization. Drying Technol 41:1620–1635. https://doi.org/10.1080/07373937.2023.2169865
Pancerz M, Ptaszek A, Sofińska K, Barbasz J, Szlachcic P, Kucharek M, Łukasiewicz M (2019) Colligative and hydrodynamic properties of aqueous solutions of pectin from cornelian cherry and commercial apple pectin. Food Hydrocoll 89:406–415. https://doi.org/10.1016/j.foodhyd.2018.10.060
Athmaselvi KA, Kumar C, Balasubramanian M, Roy I (2014) Thermal, structural, and physical properties of freeze dried tropical fruit powder. J Food Process. https://doi.org/10.1155/2014/524705
Kumar PS, Keran DA, Pushpavalli S, Shiva KN, Uma S (2022) Effect of cellulose and gum derivatives on physicochemical, microstructural and prebiotic properties of foam-mat dried red banana powder. Int J Biol Macromol 218:44–56. https://doi.org/10.1016/j.ijbiomac.2022.07.071
Sarabandi K, Jafari SM, Mahoonak AS, Mohammadi A (2019) Application of gum Arabic and maltodextrin for encapsulation of eggplant peel extract as a natural antioxidant and color source. Int J Biol Macromol 140:59–68. https://doi.org/10.1016/j.ijbiomac.2019.08.133
Moniri H, Farahmandfar R, Motamedzadegan A (2020) Investigation of hot air and foam-mat dried cress seed gum by FT-IR, zeta potential, steady shear viscosity, dynamic oscillatory behavior, and other physical properties. Food Sci Nutr 8:2143–2155. https://doi.org/10.1002/fsn3.1514
Pantelidis GE, Vasilakakis M, Manganaris GA, Diamantidis GR (2007) Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem 102:777–783. https://doi.org/10.1016/j.foodchem.2006.06.021
Rop O, Mlcek J, Kramarova D, Jurikova T (2010) Selected cultivars of cornelian cherry (Cornus mas L.) as a new food source for human nutrition. Afr J Biotechnol 9:1205–1210. https://doi.org/10.5897/AJB09.1722
Hassanpour H, Yousef H, Jafar H, Mohammad A (2011) Antioxidant capacity and phytochemical properties of cornelian cherry (Cornus mas L.) genotypes in Iran. Sci Hortic 129:459–463. https://doi.org/10.1016/j.scienta.2011.04.017
Moldovan B, Popa A, David L (2016) Effects of storage temperature on the total phenolic content of Cornelian Cherry (Cornus mas L.) fruits extracts. J Appl Bot Food Qual 89:208–211. https://doi.org/10.5073/JABFQ.2016.089.026
Cosmulescu SN, Trandafir I, Cornescu F (2019) Antioxidant capacity, total phenols, total flavonoids and colour component of cornelian cherry (Cornus mas L.) wild genotypes. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 47:390–394. https://doi.org/10.15835/nbha47111375
Klimenko S (2004) The cornelian cherry (Cornus mas L.): collection, preservation, and utilization of genetic resources. J Fruit Ornam Plant Res 12:93–98
Danielewski M, Kucharska AZ, Matuszewska A, Rapak A, Gomułkiewicz A, Dzimira S, Sozański T (2021) Cornelian cherry (Cornus mas L.) iridoid and anthocyanin extract enhances PPAR-α, PPAR-γ expression and reduces I/M ratio in aorta, increases LXR-α expression and alters adipokines and triglycerides levels in cholesterol-rich diet rabbit model. Nutrients 13:3621. https://doi.org/10.3390/nu13103621
Gupta V, Prabhakar PK, Gharde S, Nimbaria A, Sharma V, Rawat A (2021) Foam mat drying of Jujube (Ziziphus mauritiana) juice: process optimisation, physico-functional, phenolic content and antioxidant analysis. J Institution Eng (India): Series A 102:1013–1025. https://doi.org/10.1007/s40030-021-00566-5
Brar AS, Kaur P, Kaur G, Subramanian J, Kumar D, Singh A (2020) Optimization of process parameters for foam-mat drying of peaches. Int J Fruit Sci 20:1495–1518. https://doi.org/10.1080/15538362.2020.1812017
Jakkranuhwat N, Kunchansombat P (2021) Effect of foam-mat drying conditions on antioxidant activity, total phenolic compound, anthocyanin content and color of purple-fleshed sweet potato powder. CMUJ. Nat Sci 20:2021045. https://doi.org/10.12982/CMUJNS.2021.045
Tepić Horecki A, Vakula A, Pavlić B, Jokanović M, Malbaša R, Vitas J, Šumić Z (2018) Comparative drying of cornelian cherries: kinetics modeling and physico-chemical properties. J Food Process Preserv 42:13562. https://doi.org/10.1111/jfpp.13562
Kitchen KM, Kerr WL, Pegg RP (2013) Effects of drying temperature and deseeding on the anthocyanins and total phenolics of vacuum belt dried blueberry pomace powder. In: Polyphenolic-rich products made with Georgia-grown rabbiteye blueberries, pp 98–124
Darniadi S, Ifie I, Ho P, Murray BS (2019) Evaluation of total monomeric anthocyanin, total phenolic content and individual anthocyanins of foam-mat freeze-dried and spray-dried blueberry powder. J Food Meas Charact 13:1599–1606. https://doi.org/10.1007/s11694-019-00076-w
Reis FR, de Moraes ACS, Masson ML (2021) Impact of foam-mat drying on plant-based foods bioactive compounds: a review. Plant Foods Hum Nutr 76:153–160. https://doi.org/10.1007/s11130-021-00899-3
Darniadi S, Ifie I, Luna P, Ho P, Murray BS (2020) Foam-mat freeze-drying of blueberry juice by using trehalose-β-lactoglobulin and trehalose-bovine serum albumin as matrices. Food Bioproc Tech 13:988–997. https://doi.org/10.1007/s11947-020-02445-6
Yilmaz KU, Ercisli S, Zengin Y, Sengul M, Kafkas EY (2009) Preliminary characterisation of cornelian cherry (Cornus mas L.) genotypes for their physico-chemical properties. Food Chem 114:408–412. https://doi.org/10.1016/j.foodchem.2008.09.055
Dragovic-Uzelac V, Levaj B, Bursać D, Pedisić S, Radojčić I, Biško A (2007) Total phenolics and antioxidant capacity assays of selected fruits. Agric Conspec Sci 72:279–284
Petkova NT, Ognyanov MH (2018) Phytochemical characteristics and in vitro antioxidant activity of fresh, dried and processed fruits of Cornelian cherries (Cornus mas L.). Bulg Chem Commun 50:302–307
Wojdyło A, Figiel A, Lech K, Nowicka P, Oszmiański J (2014) Effect of convective and vacuum–microwave drying on the bioactive compounds, color, and antioxidant capacity of sour cherries. Food Bioproc Tech 7:829–841. https://doi.org/10.1007/s11947-013-1130-8
Skrovankova S, Sumczynski D, Mlcek J, Jurikova T, Sochor J (2015) Bioactive compounds and antioxidant activity in different types of berries. Int J Mol Sci 16:24673–24706. https://doi.org/10.3390/ijms161024673
Zielinska M, Zielinska D (2019) Effects of freezing, convective and microwave-vacuum drying on the content of bioactive compounds and color of cranberries. LWT 104:202–209. https://doi.org/10.1016/j.lwt.2019.01.041
Oszmiański J, Nowicka P, Teleszko M, Wojdyło A, Cebulak T, Oklejewicz K (2015) Analysis of phenolic compounds and antioxidant activity in wild blackberry fruits. Int J Mol Sci 16:14540–14553. https://doi.org/10.3390/ijms160714540
Michalska A, Wojdyło A, Łysiak GP, Lech K, Figiel A (2017) Functional relationships between phytochemicals and drying conditions during the processing of blackcurrant pomace into powders. Adv Powder Technol 28:1340–1348. https://doi.org/10.1016/j.apt.2017.03.002
Susanti DY, Sediawan WB, Fahrurrozi M, Hidayat M, Putri AY (2021) Encapsulation of red sorghum extract rich in proanthocyanidins: process formulation and mechanistic model of foam-mat drying at various temperature. Chem Eng Process-Process Intensification 164:108375. https://doi.org/10.1016/j.cep.2021.108375
Availability of data and materials
Data available within the article or its supplementary materials
Author information
Authors and Affiliations
Contributions
Beyza Kaba: methodology, investigation, formal analysis, writing—original draft. Yasemin Yıkılkan: methodology, investigation, formal analysis, writing—original draft. Hojjat Pashazadeh: formal analysis, data curation, writing—original draft. Ali Ali Redha: validation, writing—original draft, writing—review and editing. Ilkay Koca: conceptualization, supervision—project administration.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaba, B., Yıkılkan, Y., Pashazadeh, H. et al. Production of cornelian cherry (Cornus mas L.) pulp powder by foam-mat drying: analysis of physicochemical and antioxidant properties. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-05234-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-05234-1