Abstract
The aim of this study was to determine the effect of microwave power during the vacuum–microwave drying (VMD) on sour cherries in terms of drying kinetics, including the temperature profile of dried material, as well as on some quality factors of the finished product including phenolic compounds, antioxidant capacity, and color. The content of phenolic compounds, antioxidant activity, and color change were used as quality indicators of dried sour cherries. Sour cherries were dehydrated by convective drying (CD) at temperatures of 50 °C, 60 °C, and 70 °C and by VMD at the initial microwave power of 240 W, 360 W, and 480 W reduced to 240 W and 120 W in order to avoid a rapid increase in temperature at the critical moisture content of ca. 1 kg/kg dry mass. Control samples were prepared by freeze drying (FD). The increase in air temperature during CD as well as the increase in material temperature during VMD deteriorated dried product quality in terms of the content of phenolic compounds, antioxidant activity, and color, which was consistent with anthocyanins content. However, VMD turned out to be much better than CD and competitive to FD. The best quality of the dried product and its more attractive color were achieved at VMD at 480 W followed by drying at microwave power reduced to 120 W, which corresponds to anthocyanins content. In addition, the drying process had a positive impact on contents of quercetin and keampferol derivatives. Dried sour cherries have a long shelf life and therefore may be a fine alternative to fresh fruit all year round.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Sour cherries (Prunus cerasus L.; Rosaceae) are relatively diverse and broadly distributed around the world, including Asia, Europe, and North America. The Russian Federation, Poland, Turkey, USA, and Serbia and Montenegro are the leading world producers of sour cherries. Poland alone produces more than 770,000 tons of cherries annually despite a short cherry season, i.e., from mid-June to July. In the last few years, about 40 % of harvested sour cherries have been processed as brined (70 %), canned (12 %), frozen, and dried, or have been used for juice production (18 %) (Nosecka 2011). The processed sour cherries are mainly used in baking and cooking. Although there have been several investigations on the effects of processing on anthocyanins and polyphenolics of various fruit (Kalt et al. 2000; Skrede et al. 2000), there are no thorough studies on the impact of processing on these compounds in cherries. Because of their possible health benefits, a growing interest is being observed in the impact of processing on the antioxidant properties of these compounds.
Cherries are a very attractive fruit to consumers due to their qualities of taste and color as well as to their wealth of nutrients. Cherries are a good source of natural antioxidant substances, namely polyphenols, which are reported to have many health benefits. Cherry polyphenols include flavonoids (anthocyanins, flavan-3-ols, and flavonols) and hydroxycinnamic acids (Veres et al. 2008; Gonçalves et al. 2004). Among these compounds, special interest has been focused on anthocyanins, which are the polyphenols responsible for the red color of fruit skin and flesh, due to their strong antioxidant and anti-inflammatory, anticancer, antidiabetic, and antibacterial activities (Blando et al. 2004; Mulabagal et al. 2009).
Fresh fruit and vegetables respire and transpire after harvest, which contributes to a loss of their quality. Physiological changes cause quality to decline and shelf life of the fruit to shorten. Drying can drastically reduce or completely inactivate physiological, microbial, and enzymatic degradation and, therefore, considerably prolong shelf life. This is possible because drying reduces moisture content of the product to a level that impedes microbial growth or other reactions and also reduces product mass.
Convective drying using hot air is still widely used to produce dried products because of its low costs (Soysal et al. 2009). However, this method requires relatively long times and high temperatures to be completed, which causes degradation of important nutrients (Marfil et al. 2008; Figiel 2010) as well as color alteration (Chua et al. 2001). Another drawback of this method is shrinkage, which results from tissue collapse caused by volume reduction due to the loss of moisture and action of inner forces (Mayor and Sereno 2004).
Freeze drying (FD) has been widely used to obtain high-quality and high-value dehydrated fruit and vegetables (Huang et al. 2009). However, it is an expensive and very slow dehydration process. Its inherently low drying rates lead to a relatively low yield and to high capital and energy costs linked with the operation of refrigeration and vacuum systems (Zhang et al. 2006). High energy consumption during FD is a major problem that limits its wider application.
Microwave drying is one method which is gaining in popularity because of its inherent advantages over conventional heating such as reducing the drying time of biological material without quality loss. At an industrial level, food processing using this technique has been reported to be both cost effective and feasible (Giri and Prasad 2007; Clary et al. 2007). Microwave drying is widely used in combination with air-drying systems (Andres et al. 2004; Maskan 2001). In this combination, hot air removes water in a free state from product surface, whereas microwave energy removes water from product interior (Sham et al. 2001). Systems combining air and microwave drying not only increase drying rates but they also better retain the quality of the dry products (Sunjka et al. 2004,Torringaet al. 1996). Other benefits are ensured by vacuum conditions, which reduce the thermal oxidation of food products. Successful application of the microwave-vacuum technology in the drying of food products has been reported for many food products and herbal materials, including potato chips (Yongsawatdigul and Gunasekaran 1996; Mui et al. 2002; Nawirska et al. 2009; Figiel 2010).
However, there are still some disadvantages and limitations concerning microwave drying of plant materials. The inhomogeneity of a microwave field along with the heterogenic structure of dried material leads to the occurrence of hot spots (Holtz et al. 2010), which may result in overheating of some regions of a food product despite the application of vacuum and rotating systems. Another problem regards the costs of microwave systems and safety aspects (Vadivambal and Jayas 2007). Most of the problems might be overcome or significantly reduced by applying results obtained in a laboratory scale in the industrial conditions (Zhang et al. 2006). One of the important issues is adequately adjusting the microwave power to the current stage of drying process in order to keep the safe temperature of the dried material (Lombraña et al. 2010) and thus preserve the high quality of the final product.
The quality parameters of dried products are classified into four major groups: physical, chemical, microbial, and nutritional quality. According to our knowledge, no scientific work has as yet been reported on the effects of VMD on bioactivity of sour cherries. This method could make a significant contribution to the fruit processing industry and confer a higher quality to the finished products. Sour cherries dehydrated by means of vacuum–microwaves can be an attractive component of popular breakfast cereals such as muesli.
It is not obvious, however, what microwave wattage is supposed to be applied to ensure the optimal conditions of sour cherries dehydration by VMD. Therefore, the aim of this study was to determine the effects of microwave power during the VMD on sour cherries in respect to drying kinetics, including the temperature profile of dried material, as well as on some quality factors of the finished product including (1) phenolic compounds and antioxidant capacity, and (2) color.
Materials and Methods
Chemicals
Quercetin and keampferol 3-O-glucoside, cyanidin 3-rutinoside, p-coumaric acid, (+)-catechin, and (−)-epicatechin were purchased from Extrasynthese (Lyon Nord, France). Chlorogenic acid and neochlorogenic acid were supplied by TRANS MIT GmbH (Giessen, Germany). Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), 2,2′azinobis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), potassium persulfate, acetic acid, TPTZ (2,4,6-tripyridyl-1,3,5-triazine), FeCl3, acetic acid, phloroglucinol, ascorbic acid, HMF, acetonitrile, and methanol were purchased from Sigma-Aldrich (Steinheim, Germany).
Materials
Samples of sour cherry (P. cerasus) cultivar ‘Turgieniewka’ were harvested at the Research Station for Cultivar Testing in Zybiszów near Wrocław at processing maturity in July 2011 and were immediately brought to the University for further processing.
Drying experiments
Just before drying, 10 kg of sour cherries were pitted. Three methods of dehydration were used: (1) convection (convective drier designed and made at the Agricultural Engineering Institute of Wroclaw), (2) vacuum–microwave drying (VM-200; Plazmatronika S.A., Wroclaw, Poland), and (3) freeze drying (24 h; Alpha 1-4 LSC; Martin Christ GmbH, Osterode am Harz, Germany). Initial mass of dried samples was 60 g. Hot air temperatures during convective drying (CD) were 50 °C, 60 °C, and 70 °C; air velocity was 1 m/s. Hot air temperatures were measured using thermocouples located close to the dried samples. The accuracy of the temperature gauge was 0.1 °C. This allowed to control the air temperature in the range of ±1 °C by adjusting the autotransformers, which supplied heating elements with electric energy. During the vacuum–microwave drying (VMD), the initial microwave power was set to at 240 W, 360 W, and 480 W, respectively. However, the preliminary tests revealed that during drying at 360 W and 480 W, sample temperatures increased too rapidly at the critical moisture content of ca. 1 kg/kg dm (dry mass), leading to the burning of the samples. Therefore, before the samples reached the moisture content of 1 kg/kg dm, the microwave wattage was reduced to 240 W and 120 W, respectively. Microwave power was also reduced to 120 W at the initial microwave power of 240 W, which was generally safe for the product. The pressure in the VMD chamber varied between 4 and 6 kPa. During FD, the pressure was reduced to 0.960 kPa. The temperature in the drying chamber was −60 °C, while the temperature of shelves reached 26 °C. Drying kinetics for CD and VMD was determined according to sample mass losses measured during drying. The process of dehydration using all the methods mentioned above was continued until moisture content in dried samples was 0.05 kg/kg dm. In the case of FD, the samples were kept in the drying chamber for 24 h.
The moisture ratio MR was determined using the following equation:
In that equation M(t), M 0, and M e denote moisture content achieved after drying time t, initial moisture content, and equilibrium moisture content, respectively. The equilibrium moisture content M e was determined at the final stage of drying as an asymptotic value of the function fitted to the experimental points using Table Curve 2D Windows v2.03 (Nawirska et al. 2009). The moisture content of dried samples was determined by drying the previously ground samples in a vacuum dryer (SPT-200; ZEAMiL Horyzont, Krakow, Poland) for 24 h at temperature 60 °C.
Temperature Measurement During VMD
The surface mean temperature of sour cherries was measured with an infrared camera Flir i50 (Flir Systems Inc., Stockholm, Sweden) immediately after taking them out of the VM dryer. The accuracy of the camera was 0.1 °C. It can be assumed that the temperature measured in this way can help to observe the changes of the inner temperature of the samples, which occur in the time of drying process. It was supposed that the inner temperature of the samples was higher due to the volumetric heat generation. However, a direct measurement of the samples temperature using a fiber optic thermometer (Knoerzer et al. 2012) within the VMD chamber was not possible because of the technical difficulties concerning the dryer, which was used in the process.
Analysis of Polyphenols Compounds by UPLC Coupled to PDA and FL Detector
The solvent for analysis of polyphenols was prepared as described previously by Wojdyło et al. (2009). The content of polyphenols (anthocyanin, flavanolo, flavonol, and phenolic acid) and polymeric procyanidins by phloroglucinol method was measured by protocol described previously by Wojdyło et al. (2013).
Analysis of Antioxidant Capacity
The solvent for analysis was prepared as described previously by Wojdyło et al. (2009). The ABTS°+ activity of a sample was determined according to the method of Re et al. (1999). The total antioxidant potential of a sample was determined using a ferric reducing ability of plasma (FRAP) assay by Benzie and Strain (1996) as a measure of antioxidant power.
All determinations were performed in triplicate using a Shimadzu UV-2401 PC spectrophotometer (Kyoto, Japan). The results of the assay were expressed relative to micromoles of Trolox per gram of dry matter.
Color Measurement
The color of sour cherry powders was determined using an A5 Chroma-Meter (Minolta CR300, Osaka, Japan), referring to color space CIE L*a*b*. The color coordinates of the samples were determined using Illuminant D65 and 10° observer angle, and samples were measured against a white ceramic reference plate (L* = 93.80; a* = 31.58; b* = 33.23). Data were mean of five measurements. The total change in color of dried sour cherries was expressed as ΔE according to the following equation described by Šumić et al. (2013) (2):
where L *0 , a *0 , and b *0 denote the value of fresh sour cherries color.
Statistical Analysis
Statistical analysis was conducted using Statistica version 9.0 (StatSoft, Krakow, Poland). Significant differences (p ≤ 0.05) between means were evaluated by one-way ANOVA and Duncan’s multiple range test. Results of tables and figures are presented as mean ± standard deviation of two independent technological determinations. All analyses were done in triplicate.
Results and Discussion
Drying Kinetics
The drying kinetics of sour cherries dehydrated by CD and VMD is shown in Figs. 1 and 2, respectively. The process of sour cherries drying was described using the Page model (3) and Table 1:
This model was reported as the best model for describing the drying kinetics of numerous plant materials such as garlic (Sharma and Prasad 2004), apples (Doymaz 2010), pomegranate arils (Kingsly and Singh 2007), and pumpkin slices (Alibas 2007).
In this study, VMD was much faster than CD as it took only 23 and 48 min at the microwave power of 480/240 W and 240/120 W, compared to CD spanning for 14 and 40 h at temperatures of 70 °C and 50 °C, respectively. In the first phase of CD, water loss was relatively fast; however, subsequent water loss was slow and drying was found to be time-consuming (Maskan 2000). Significantly faster drying ensured by the VM method results from the transformation of microwave energy into heat energy by water dipoles located in the whole volume of the material being dried. This generates a high vapor pressure in the center of the material, allowing rapid transfer of moisture to the surrounding vacuum and preventing structural collapse (Lin et al. 1998).
Temperature Profile
The temperature of the dried material is a deceiving factor influencing the bioactivity of the finished product (Zhu et al. 2010; Zubaira et al. 2011). Therefore, the temperature variation during drying requires more consideration. During CD, the temperature of the dried material does not exceed the temperature of hot air. During VMD, a mild increase in temperature was observed up to the critical moisture content of ca. 1 kg/kg dm (Fig. 3). Beyond that moisture content, sample temperatures rapidly increased, despite an earlier reduction in microwave power at the moisture content ranging from 1.06 to 1.5 kg/kg dm. It may be speculated that the course of temperature versus moisture content depends on two phenomena. The first is the generation of heat energy by water dipoles in the microwave field (Tang 2005), while the second is the absorption of that energy by water evaporating from the surface of the material. The increase in material temperature beyond the critical moisture content results from the excess of energy generated over the energy necessary for water evaporation. Naturally, the amount of water generating the energy decreases and the process of water evaporation slows down with decreasing moisture content. At these conditions, the microwave energy is transformed into heat energy mainly by water dipoles tightly closed in the plant cellular system. This creates higher internal pressure that enhances an increase in the dried sample’s temperature (Figiel 2009). The process of microwave energy transformation, depending on the distribution of water within the cellular structure, varies in individual fruits, which constitute a sample. The range of uneven heating of the fruits in the microwave field is demonstrated by the relatively large standard deviation bars concerning mean temperature beyond the moisture content 1 kg/kg dm (Fig. 3). The highest mean temperature (>75 °C) was attained by the samples dehydrated by VMD at the microwave power reduced to 240 W. At a final microwave power of 120 W, significantly lower temperatures were noted in the samples (Table 2).
Improving Nutritional Quality Using Different Drying Methods
Phenolic Compounds of Fresh and Dried Sour Cherries
The initial value of TP in the fresh samples was 7,905.77 mg/kg dm (Table 3). Similar initial values were reported by Khoo et al. (2011). The main phenolic compounds in sour cherries were anthocyanins (37 % of total polyphenolic compounds) > flavan-3-ols (28 %), especially polymeric proanthocyanidins > phenolic acid (23 %) and flavonols (11 %). Drying methods as well as parameters applied had a significant influence on components compared to the fresh samples.
The content of phenolic compounds in the samples dried by FD amounted to 6,498.34 mg/kg dm, which was higher than in the CD-dried samples, but lower than in the VMD-dried samples treated at the final microwave power of 120 W. Compared to the other analyzed methods of drying, the greatest change in polyphenols was found in the fruits dehydrated by CD. There was a clear dependency between hot air temperature and polyphenols loss. In fact, this variability led to a notable reduction in the initial value, e.g., at 50 °C, 60 °C, and 70 °C, the loss of polyphenols was 44 %, 48 %, and 49 %, respectively. This could be explained by irreversible oxidative processes proceeding during drying and a long exposure to thermal degradation of the phenolic compounds with increased heat intensity. However, statistically significant changes between 1 % and 5 % found for laboratory conditions may not be confirmed on the industrial scale. Comparable results were reported by other authors when drying aloe vera and tomato (Miranda et al. 2009, 2010), red pepper (Vega-Galvez et al. 2009), and kiwi (Kaya et al. 2009). Piga et al. (2009) established that plum and peach drying in an air-flow cabinet, at temperatures between 55 °C and 85 °C, significantly reduced contents of polyphenols, anthocyanin, and ascorbic acid when compared to fresh fruits.
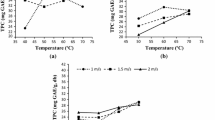
The negative effect of temperature on the content of phenolic compounds was also observed during dehydration of sour cherry samples by VMD under different conditions (Fig. 4a). The gentle final drying at 120 W instead of 240 W resulted in lower temperatures of the material (61.3 °C vs. 64.4 °C), which yielded higher contents of polyphenols in the dried product (from 7,320 to 7,587 mg/kg dm) despite the initial wattage ranging from 240 W to 480 W. However, the reduction of microwave wattage to only 240 W evoked higher temperatures of the dried material (77.1 °C compared to 87.5 °C). These higher temperatures resulted in a lower content of polyphenols in the dried product (6,195 compared to 6,526 mg/kg dm). Figure 4b demonstrates that the intensive reduction in polyphenols content was proceeding till a temperature of 77 °C. The following increase in temperature of the samples to 87 °C did not contribute to the further decrease of polyphenols content.
The analysis of the content of each phenolic compound in dried sour cherry samples showed that only hydroxycinnamic acid was more stable during drying than the remaining polyphenols. The content of phenolic acids in the dried samples ranged from 74 % to 99 % of the initial value of the phenolic acids. The highest content was determined in the samples of sour cherries dried by VMD at 360/120 W, 240/120 W, and 480/120 W (more than 97 % of the initial phenolic acids) and the lowest in the samples dried by CD (less than 88 % of the initial phenolic acids).
The most significant changes in the content of polyphenolics were found in anthocyanins as well as in flavan-3-ol compounds such as (+)-catechin, (−)-epicatechin, and polymeric procyanidins, a major component of flavan-3-ols of the sour cherries. After drying, the content of flavan-3-ols was similar to that of a raw sample but only for samples dried by VMD at a microwave power reduced to 120 W. A much lower content of proanthocyanidins was determined in the samples dried by other methods. In dried sour cherries obtained by CD, the content of flavan-3-ols was only 806.63, 818.39, and 681.83 mg/kg dm at 50 °C, 60 °C, and 70 °C, respectively. The content of (−)-epicatechin and (+)-catechin decreased to ~10 % and that of polymeric procyanidins to ~50 %. The procyanidin content in dried sour cherry samples is very important because it plays the main role as radical scavenging compounds (Lu and Foo 2000). (−)-Epicatechin was the most predominant individual compound in sour cherries and in polymeric proanthocyanidin. After the drying process, a higher content of (−)-epicatechin was observed especially for VMD samples: 360 W/240 W > 240/240 W >> 480 W/240 W ≥ 240 W/120 W. The higher content of (−)-epicatechin was due to the depolymerization effect of proanthocyanidins in sour cherries and their conversion into elementary units. A similar effect has previously been observed in strawberry monomeric and polymeric procyanidins (Wojdyło et al. 2009) and in blueberry products (Brownmiller et al. 2009).
In the drying process of sour cherries, a similar situation has also been observed for flavonols. The flavonol glycosides in cherries included quercetin3-O-rutinoside and 3-O-glucoside, kaempferol-3-O-rutinoside, and isorhamnetin-3-O-rutinoside. Generally, it was observed that the flavonoid content of processed sour cherries compared with those of the fresh samples decreased, with the exception of flavonols (Table 3). Quercetin glycosides are mostly concentrated in the skin (Prior et al. 1998). The content of these compounds in the fresh sample was 885.41 mg/kg dm and was lower than in the dried sour cherries, with the exception of the FD-treated samples. In drying samples, the higher the temperature, especially for CD methods, the higher the content of flavonols measured. The probable effect of temperature and the power of microwaving in causing such a change is the breakdown of the cells in the skin and the extraction of these compounds on the outside. This effect is beneficial for human health. Quercetin has powerful antioxidant properties and protects the body from free radical, which can damage DNA and induce aging, heart disease, and cancer (Yu et al. 2011). The phenolic compounds content of freeze-dried mulberry leaves (Katsube et al. 2009) and the anthocyanins content of freeze-dried apple peel (Wolfe and Liu 2003) were much higher than the respective contents in the non-dried material. This behavior may be due to the fact that a large percentage of phenolic compounds are bound to cellular structures, and drying treatments release bound phytochemicals from the matrix to make them more accessible in extraction. This is in line with results reported by Piga et al. (2003) and Del Caro et al. (2004), and is possibly due to the liberation of phenolic compounds from the matrix during the process. Chism and Haard (1996) have claimed that fruits and vegetables normally have a high content of phenolic compounds in their outer parts, as phenolics are metabolic intermediates and usually accumulate in the vacuoles. Another possible explanation is that the polyphenols in an intermediate state of oxidation can exhibit higher radical scavenging efficiency than the non-oxidized ones, although a subsequent loss in the antioxidant properties has been found for advanced enzymatic oxidation steps (Nicoli et al. 1999).
The color of the sour cherries is an important quality attribute and is modulated by anthocyanins. Dehydration processes often affect color quality in foods, which reflects a loss of anthocyanins and other phytochemicals specific to fruits, vegetables, and herbs (Lin et al. 1998; Yousif et al. 2000). The anthocyanin content of the investigated sour cherry ‘Turgieniewka’ was 2,923.84 mg/kg dm. Sour cherries have a relatively high anthocyanin content compared with red berries and other fruits (Abby et al. 2007; Obon et al. 2011; Borges et al. 2010). In the case of sour cherries, cyanidin-3-O-glucosylrutinoside, cyanidin-3-O-rutinoside, and cyanidin-3-O-sophoroside were the main anthocyanin fractions. This is consistent with findings of other authors (Chaovanalikit and Wrolstad 2004; Kim et al. 2005; Khoo et al. 2011; Obon et al. 2011). Rubinskiene et al. (2005) demonstrated that cyanidin-3-O-rutinoside showed the highest stability to the effect of thermal treatment at 95 °C temperature in black currant. In strawberry, anthocyanin degradation occurs as soon as strawberries are processed into juice or concentrate and continues during storage. This degradation of anthocyanins is greater in concentrates compared to juices (Garzon and Wrolstad 2002). Similar trends were reported for anthocyanins in sour cherry (Cemeroğlu et al. 1994).
The freeze drying of sour cherries and vacuum–microwaving resulted in the greatest retention of anthocyanins (from 70 % to 90 % retention of the original unprocessed extract) (Table 2). The results suggest a direct relationship between the length and intensity of heat treatment and the degree of anthocyanins loss. This agrees with the results reported by Kwok et al. (2004). Cherries dehydrated by CD were characterized by the lowest anthocyanins content, that is, <60 % of fresh fruits. It is clear that the sour cherries dried with higher power (VMD = 240/240 W, 360/240 W, and 480/240 W) had a lower content of anthocyanins and other polyphenols than those dried with a final lower power during dehydration (VMD = 240/120 W, 360/120 W, and 480/120 W). These features of VMD result in reduced oxidation and produce a relatively greater retention of fruits anthocyanins as compared with the conventional hot-air drying (CD).
Antioxidant Capacity of Fresh and Dried Sour Cherries
In this study, it was observed that the polyphenols content and antioxidant capacity of the sour cherries were changing under different drying conditions in the same way. It may therefore be concluded that the polyphenols content of raw materials is largely responsible for their antioxidant capacity (Table 4). The FD process produced significantly higher ABTS scavenging activities and FRAP reducing power, compared with other dehydration methods, which accounted for 95 % and 98 % of the original activity found in fresh sour cherries, respectively. The CD-processed sour cherries had the lowest retained affinity to scavenge ABTS radical and FRAP values compared with the fresh fruits. A greater decrease in the antioxidant capacity was observed at 70 °C and 60 °C than at 50 °C. Sour cherries dried at 70 °C and 60 °C contained only 51 % and 57 %, compared to 75 % at 50 °C (ABTS), and 52 % and 54 %—against 68 % of reducing power (FRAP), respectively, of the initial total antioxidant capacity. Drying processes and, in particular, high temperatures (i.e., 60 °C and 70 °C) and long exposure to drying might destroy some of the phenolic compounds and thus result in the loss of the antioxidant activity. A similar effect was previously obtained for apple cubes (Wojdyło et al. 2007). However, Larrauri et al. (1997) did not notice differences in the antioxidant activity between freeze-dried grape peel and that dried at 60 °C in a forced-air oven, but when the drying temperature increased (100 °C or 140 °C), a decrease of 28 % and 50 %, respectively, was observed in the antioxidant capacity of the samples.
The VMD samples retained similar or higher ABTS radical scavenging activity when the power of final drying was 120 W irrespective of the initial power (240 W, 360 W, and 480 W). When the power of final drying was 240 W, antioxidant potency decreased compared to the counterpart or the fresh sample (about 74–89 %).
This study demonstrated that the VMD method enabled obtaining high-quality products, with the additional advantage of reduced processing times, compared to the other analyzed methods, i.e., CD and FD. Those results could be affected by many complex factors. The lowest antioxidant capacity of the samples dried by convection resulted from intensive oxidation that occurred during their relatively long exposure to hot air. The VM drying effectively reduced this oxidation effect mainly due to the significantly shorter processing time at a lower air pressure. The antioxidant capacity of the VMD-treated sour cherries was additionally facilitated by the relatively low temperature registered for the higher reduction of microwave power (up to 120 W instead of 240 W) at the final stage of dehydration (Figs. 5a and 6a). The negative impact of temperature on the antioxidant activity of VMD cherry fruits is presented in Figs. 5b and 6b. However, the application of a lower microwave power increased the time of VM final drying and in this way could intensify degradation of the compounds responsible for the antioxidant capacity. Therefore, the initial VMD with the highest powers at the safe temperatures resulted in the cooling effect of intensive evaporation of water from wet material (Figiel 2010), and eventually decreased the total drying time and contributed to a high antioxidant capacity.
Astonishingly lower antioxidant capacity of sour cherries after VM final drying at 240 W could be caused by a lower content of phenolic compounds and an unfavorable profile of various Maillard reaction products (MRPs), which affect the antioxidant capacity (Yilmaz and Toledo 2005). The content of hydroxymethylfurfural (HMF) in dried sour cherries is presented in Fig. 7. A higher content of HMF was noted in the VMD samples than in the CD and FD ones. However, Piga et al. (2003) claimed that the increase in the antioxidant capacity due to HMF and MRPs may not compensate for the destruction of phenolic compounds. In all cases, the lower contents of bioactive compounds in vegetable samples, as a consequence of drying treatment, were attributed to the thermal and oxidative degradation of these compounds. Yet, the mechanism of antioxidant capacity changes during VMD of sour cherries needs more consideration, especially with regard to MRPs.
Color Parameters of Fresh and Dried Sour Cherries
Product color is the other quality parameter that needs to be maintained during fruits drying. The chromatic parameters L*, a*, and b* of fresh sour cherries were 28.40, 16.50, and 2.35, respectively. All color values of all dried samples decreased significantly in comparison to the fresh sour cherries, except of FD drying (Table 5). Park and Kim (2007) confirmed that FD was the most suitable drying method for maintaining vegetable and fruits color quality. Drying using CD method resulted in positive a* values, indicating less red hues, and negative b* values, indicating blue hues. The color of this sample was correlated with a loss of anthocyanins during drying. The color parameters of VMD dried samples did not differ significantly, but these samples were more red than those treated with the CD method. A more attractive color was obtained and higher anthocyanins content was ensured when a lower power was used for final drying. The value of coordinate a* decreased compared to the fresh samples and denoted a more red chroma, which is indicative of the browning reaction (Bahloul et al. 2009). Consumers prefer visible quality, i.e., the color of the product; therefore, ΔE values can be very important for the dried product, which expresses the human eye’s ability to discriminate between colors of a sample. According to Šumić et al. (2013), if ΔE between two samples is less than 1.0, it is assumed that the difference would not be perceptible. The total color change (ΔE) of the dried samples varied from 75.46 to 82.94 units, depending on applied drying temperature and power. In our study, ΔE values among all dried samples were greater than FD (ΔE = 7.49 units), which indicated that these samples were darker than the fresh samples.
Therefore, although anthocyanins are lost due to the exposure to high temperatures, the pigments, which are retained, are concentrated in a reduced volume. Conditions of drying (high temperatures and presence of oxygen) may promote the activity of polyphenolic oxidase, resulting in browning that characterizes many dehydrated food materials (Howard et al. 1996).
Conclusion
The study revealed that bioactivity of dried sour cherries is better maintained by a low drying temperature, a short processing time, and limited contact of the material with oxygen present in the air. In this sense, vacuum–microwave drying is much better than convective drying and competitive with freeze drying. High power levels at the beginning of VMD do not contribute to a high temperature of the dried material because relatively large amounts of evaporating water require lots of energy. In addition, the total drying time is reduced. Therefore, it is recommended to dry sour cherries with a possibly high microwave power if a certain critical temperature level is not exceeded. When approaching this level, the power of emitted microwaves should be immediately reduced to the value that ensures the maintenance of a sufficiently low temperature and provides a high bioactivity in the dried product. This study revealed that VMD at 480 W up to a moisture content of ca. 1 kg/kg dm followed by drying at microwave power reduced to 120 W ensured the highest content of phenolic compounds, high antioxidant capacity, and attractive color, which was compatible with anthocyanins content. Additionally, this procedure had a positive influence on contents of the compounds from the quercetin derivatives.
Considering the maximum content of total phenolics, especially anthocyanins, the maximum antioxidant capacity in dried sour cherries, as well as the minimum color change of the samples, the optimum drying conditions were established for the conventional drying method at temperature of 50 °C and for the microwave drying at power reduction to 120 W.
References
Abby, K., Ekeberg, D., & Skrede, G. (2007). Characterization of phenolic compounds in strawberry (Fragaria × ananasa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. Journal of Agricultural and Food Chemistry, 55, 4395–4406.
Alibas, I. (2007). Microwave, air and combined microwave–air-drying parameters of pumpkin slices. LWT, 40, 1445–1451.
Andres, A., Bilbao, C., & Fito, P. (2004). Drying kinetics of apple cylinders under combined hot air–microwave dehydration. Journal of Food Engineering, 63, 71–78.
Bahloul, N., Boudhrioua, N., Kouhila, M., & Kechaou, N. (2009). Effect of convective solar drying on colour, total phenols and radical scavenging activity of olive leaves (Olea europaea L.). International Journal of Food Science and Technology, 44, 2561–2567.
Benzie, I. F. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry, 239, 70–76.
Blando, F., Gerardi, C., & Nicoletti I. (2004). Sour cherry (Prunus cerasus L.) anthocyanins as ingredients for functional foods. Journal of Biomedicine and Biotechnology, 2004, 253–258.
Borges, G., Degeneve, A., Mullen, W., & Crozier, A. (2010). Identification of flavonoid and phenolic antioxidants in black currants, blueberries, raspberries, red currants, and cranberries. Journal of Agricultural and Food Chemistry, 58, 3901–3909.
Brownmiller, C., Howard, L. R., & Prior, R. L. (2009). Processing and storage effects on procyanidin composition and concentration of processed blueberry products. Journal of Agricultural and Food Chemistry, 57, 1896–1902.
Cemeroğlu, B., Velioğlu, S., & Işik, S. (1994). Degradation kinetics of anthocyanins in sour cherry juice and concentrate. Journal of Food Science, 59, 1216–1218.
Chaovanalikit, A., & Wrolstad, R. E. (2004). Anthocyanins and polyphenolic composition of fresh and processed cherries. Journal of Food Science, 69, FST73–FST83.
Chism, G. W., & Haard, N. F. (1996). Characteristics of edible plant tissues. In O. R. Fennema (Ed.), Food chemistry (pp. 943–1011). New York: Marcel Dekker.
Chua, K. J., Mujumdar, A. S., Hawlader, M. N. A., Chou, S. K., & Ho, J. C. (2001). Batch drying of banana pieces—effect of stepwise change in drying air temperature on drying kinetics and product colour. Food Research International, 34, 721–731.
Clary, C. D., Meijia-Meza, E., Wang, S., & Petrucci, V. E. (2007). Improving grape quality using microwave vacuum drying associated with temperature control. Journal of Food Science, 72(1), E23–E28.
Del Carlo, A., Piga, A., & Corda, G. (2004). Effect of drying conditions and storage period on polyphenolic content, antioxidant capacity, and ascorbic acid of prunes. Journal of Agricultural and Food Chemistry, 52, 4780–4784.
Doymaz, I. (2010). Effect of citric acid and blanching pre-treatments on drying and rehydration of Amasya red apples. Food and Bioproducts Processing, 88, 124–132.
Figiel, A. (2009). Drying kinetics and quality of vacuum–microwave dehydrated garlic cloves and slices. Journal of Food Engineering, 94, 98–104.
Figiel, A. (2010). Drying kinetics and quality of beetroots dehydrated by combination of convective and vacuum–microwave methods. Journal of Food Engineering, 98(4), 461–470.
Garzon, G. A., & Wrolstad, R. E. (2002). Comparison of the stability of pelargonidin based anthocyanins in strawberry juice and concentrate. Journal of Food Science, 67, 1288–1299.
Giri, S. K., & Prasad, S. (2007). Drying kinetics and rehydration characteristics of microwave–vacuum and convective hot-air dried mushrooms. Journal of Food Engineering, 78, 512–521.
Gonçalves, B., Landbo, A.-K., Knudsen, D., Silva, A. P., Moutinho-Pereira, J., Rosa, E., et al. (2004). Effect of ripeness and postharvest storage on the phenolic profiles of cherries (Prunus avium L.). Journal of Agricultural and Food Chemistry, 52, 523–530.
Holtz, E., Ahrné, L., Rittenauer, M., & Rasmuson, A. (2010). Influence of dielectric and sorption properties on drying behaviour and energy efficiency during microwave convective drying of selected food and non-food inorganic materials. Journal of Food Engineering, 97, 144–153.
Howard, L. R., Braswell, D. D., & Aselage, J. (1996). Chemical composition and color of strained carrots as affected by processing. Journal of Food Science, 61, 327–330.
Huang, L. L., Zhang, M., Yan, W. Q., Mujumdar, A. S., & Sun, D. F. (2009). Effect of coating on post-drying of freeze-dried strawberry pieces. Journal of Food Engineering, 92, 107–111.
Kalt, W., Mcdonald, J. E., & Donner, H. (2000). Anthocyanins, phenolics, and antioxidant capacity of processed lowbush blueberry products. Journal of Food Science, 65, 390–393.
Katsube, T., Tsurunaga, Y., Sugiyama, M., Furuno, T., & Yamasaki, Y. (2009). Effect of air-drying temperature on antioxidant capacity and stability of polyphenolic compounds in mulberry (Morus alba L.) leaves. Food Chemistry, 113(4), 964–969.
Kaya, A., Aydin, O., & Kolayli, S. (2009). Effect of different drying conditions on the vitamin C (ascorbic acid) content of Hayward kiwifruits (Actinidia deliciosa Planch). Food and Bioproducts Processing, 88, 165–173.
Khoo, G. M., Clausen, M. R., Pedersen, B. H., & Larsen, E. (2011). Bioactivity and total phenolic content of 34 sour cherry cultivars. Journal of Food Composition and Analysis, 24(6), 772–776.
Kim, D. K., Heo, H. J., Kim, Y. J., Yang, H. S., & Lee, C. Y. (2005). Sweet and sour cherry phenolics and their protective effects on neuronal cells. Journal of Agricultural and Food Chemistry, 53, 9921–9927.
Kingsly, A. R. P., & Singh, D. B. (2007). Drying kinetics of pomegranate arils. Journal of Food Engineering, 79, 741–744.
Knoerzer, K., Murphy, A. B., Fresewinkel, M., Sanguansri, P., & Coventry, J. (2012). Evaluation of methods for determining food surface temperature in the presence of low-pressure cool plasma. Innovative Food Science and Emerging Technologies, 15, 23–30.
Kwok, B. H. L., Hu, C., Durance, T., & Kitts, D. D. (2004). Dehydration techniques affect phytochemical contents and free radical scavenging activities of Saskatoon berries (Amelanchier alnifolia Nutt.). Journal of Food Science, 69(3), S122–S126.
Larrauri, J. A., Rupérez, P., & Saura-Calixto, F. (1997). Effect of drying temperature on the stability of polyphenols and antioxidant activity of red grape pomace peels. Journal of Agricultural and Food Chemistry, 45(4), 1390–1393.
Lin, T. M., Durance, T. D., & Scaman, C. H. (1998). Characterization of vacuum microwave, air and freeze-dried carrot slices. Food Research International, 31, 111–117.
Lombraña, J. I., Rodríguez, R., & Ruiz, U. (2010). Microwave-drying of sliced mushroom. Analysis of temperature control and pressure. Innovative Food Science and Emerging Technologies, 11, 652–660.
Lu, Y., & Foo, L. Y. (2000). Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chemistry, 68, 81–85.
Marfil, P. H. M., Santos, E. M., & Telis, V. R. N. (2008). Ascorbic acid degradation kinetics in tomatoes at different drying conditions. LWT—Food Science and Technology, 41, 1642–1647.
Maskan, M. (2000). Microwave/air and microwave finish drying of banana. Journal of Food Engineering, 44, 71–78.
Maskan, M. (2001). Drying, shrinkage and rehydration characteristics of kiwi fruits during hot air and microwave drying. Journal of Food Engineering, 48, 177–182.
Mayor, L., & Sereno, A. M. (2004). Modeling shrinkage during convective drying of food materials: a review. Journal of Food Engineering, 61, 373–386.
Miranda, M., Maureira, H., Rodriguez, K., & Vega-Galvez, A. (2009). Influence of temperature on the drying kinetics, physicochemical properties, and antioxidant capacity of Aloe Vera (Aloe barbadensis Miller) gel. Journal of Food Engineering, 91, 297–304.
Miranda, M., Vega-Galvez, A., & Garcia, P. (2010). Effect of temperature on structural properties of Aloe vera (Aloe barbadensis Miller) gel and Weibull distribution for modelling drying process. Food and Bioproducts Processing, 88, 138–144.
Mui, W. Y. W., Durance, T. D., & Scaman, C. H. (2002). Flavor and texture of banana chips dried by combinations of hot air, vacuum, and microwave processing. Journal of Agricultural and Food Chemistry, 50, 1883–1889.
Mulabagal, V., Lang, G. A., DeWitt, D. L., Dalavoy, S. S., & Nai, M. G. (2009). Anthocyanin content, lipid peroxidation and cyclooxygenase enzyme inhibitory activities of sweet and sour cherries. Journal of Agricultural and Food Chemistry, 57(4), 1239–1246.
Nawirska, A., Figiel, A., Kucharska, A. Z., Sokół-Łętowska, A., & Biesiada, A. (2009). Drying kinetics and quality parameters of pumpkin slices dehydrated using different methods. Journal of Food Engineering, 94(1), 14–20.
Nicoli, M. C., Anese, M., & Parpinel, M. (1999). Influence of processing on the antioxidant properties of fruit and vegetables. Trends in Food Science & Technology, 10(3), 94–100.
Nosecka, B. (2011). Pozycja polskich owoców, warzyw i ich przetworów na rynku unijnym i światowym. Przemysł FermentacyjnyiOwocowo-Warzywny, 7(8), 6–9.
Obon, J. M., Diaz-Garcia, M. C., & Castellar, M. R. (2011). Red fruit juice quality and authenticity control by HPLC. Journal of Food Composition and Analysis, 24, 760–771.
Park, J. H., & Kim, C. S. (2007). The stability of color and antioxidant compounds in paprika (Capsicum annuum L.) powder during the drying and storing process. Food Science and Biotechnology, 16(2), 187–192.
Piga, A., Del Caro, A., & Corda, G. (2003). From plums to prunes: influence of drying parameters on polyphenols and antioxidant activity. Journal of Agricultural and Food Chemistry, 51, 3675–3681.
Piga, A., Romeo, F. V., Poiana, M., Del Caro, A., Sanguinetti, A. M., & Piscopo, A. (2009). Effect of drying temperature on polyphenolic content and antioxidant activity of apricots. European Food Research and Technology, 228(3), 441–448.
Prior, R. L., Cao, G., Martin, A., Sofic, E., McEwen, J., O’Brien, C., et al. (1998). Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. Journal of Agricultural and Food Chemistry, 46, 2686–2693.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine, 26(9/10), 1231–1237.
Rubinskiene, M., Viskelis, P., Jasutiene, I., Viskeliene, R., & Bobinas, C. (2005). Impact of various factors on the composition and stability of black currant anthocyanins. Food Research International, 38, 867–871.
Sham, P. W. Y., Scaman, C. H., & Durance, T. D. (2001). Texture of vacuum microwave dehydrated apple chips as affected by calcium pretreatment, vacuum level, and apple variety. Journal of Food Science, 66(9), 1341–1347.
Sharma, G. P., & Prasad, S. (2004). Effective moisture diffusivity of garlic cloves undergoing microwave–convective drying. Journal of Food Engineering, 65(4), 609–617.
Skrede, G., Wrolstad, R. E., & Durst, R. W. (2000). Changes in anthocyanins and polyphenolics during juice processing of highbush blueberries (Vaccinium corymbosum L.). Journal of Food Science, 65, 357–364.
Soysal, Y., Ayhan, Z., Esturk, O., & Arıkan, M. F. (2009). Intermittent microwave convective drying of red pepper: drying kinetics, physical (colour and texture) and sensory quality. Biosystems Engineering, 103, 446–455.
Šumić, Z., Tepić, A., Vidović, S., Jokić, S., & Malbaša, R. (2013). Optimization of frozen sour cherries vacuum drying process. Food Chemistry, 136, 55–63.
Sunjka, P. S., Rennie, T. J., Beaudry, C., & Raghavan, G. S. V. (2004). Microwave–convective and microwave–vacuum drying of cranberries: a comparative study. Drying Technology, 22(5), 1217–1231.
Tang, J. (2005). Dielectric properties of foods. In H. Schubert & M. Regier (Eds.), The microwave processing of foods (pp. 22–40). Boca Raton: CRC.
Torringa E.M., Van Dijk E.J. & Bartels P.V. (1996) Microwave puffing of vegetables: modelling and measurements. In: Proceedings of the 31st Microwave Power Symposium, pp. 16–19. International Microwave Power Institute, Manassas.
Vadivambal, R., & Jayas, D. S. (2007). Changes in quality of microwave-treated agricultural products—a review. Biosystems Engineering, 98, 1–16.
Vega-Galvez, A., Di Scala, K., & Rodrıguez, K. (2009). Effect of air-drying temperature on physico-chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capsicum annum, L. var. Hungarian). Food Chemistry, 117, 647–653.
Veres, Z., Holb, I., Nyeki, J., Szabo, Z., Szabo, T., Remenyik, J., et al. (2008). Antioxidant and anthocyanin contents of sour cherry cultivars. Acta Horticulturae (ISHS), 795, 787–792.
Wojdyło, A., Figiel, A., & Oszmiański, J. (2007). Influence of temperature and time of apple drying on phenolic compounds content and their antioxidant activity. Polish Journal of Food and Nutrition Sciences, 57(4C), 601–605.
Wojdyło, A., Figiel, A., & Oszmianski, J. (2009). Effect of drying methods with application of vacuum microwaves on the bioactive compounds, color, and antioxidant activity of strawberry fruits. Journal of Agricultural and Food Chemistry, 57(4), 1337–1343.
Wojdyło, A., Oszmiański, J., & Bielicki, P. (2013). Polyphenolic composition, antioxidant activity, and polyphenol oxidase (PPO) activity of quince (Cydonia oblonga Miller) varieties. Journal of Agricultural and Food Chemistry. doi:10.1021/jf304969b.
Wolfe, K. L., & Liu, R. H. (2003). Apple peels as a value-added food ingredient. Journal of Agricultural and Food Chemistry, 51(6), 1676–1683.
Yilmaz, Y., & Toledo, R. (2005). Antioxidant activity of water-soluble Maillard reaction products. Food Chemistry, 93, 273–278.
Yongsawatdigul, J., & Gunasekaran, S. (1996). Microwave–vacuum drying of cranberries. Part II: quality evaluation. Journal of Food Processing and Preservation, 20, 145–156.
Yousif, A. N., Durance, T. D., Scaman, C. H., & Girard, B. (2000). Headspace volatiles and physical characteristics of vacuum–microwave, air, and freeze-dried oregano (Lippia berlandieri Schauer). Journal of Food Science, 65, 926–930.
Yu, C. P., Wu, P. P., Hou, Y. C., Lin, S. P., Tsai, S. Y., Chen, C. T., et al. (2011). Quercetin and rutin reduced the bioavailability of cyclosporine from neoral, an immunosuppressant, through activating p-glycoprotein and CYP 3A4. Journal of Agricultural and Food Chemistry, 59, 4644–4648.
Zhang, M., Tang, J. M., Mujumdar, A. S., & Wang, S. (2006). Trends in microwave related drying of fruits and vegetables. Trends in Food Science and Technology, 17, 524–534.
Zhu, Y., Pan, Z., McHugh, T. H., & Barrett, D. M. (2010). Processing and quality characteristics of apple slices processed under simultaneous infrared dry-blanching and dehydration with intermittent heating. Journal of Food Engineering, 97, 8–16.
Zubaira, M., Nyboma, H., Lindholmb, C., & Rumpunena, K. (2011). Major polyphenols in aerial organs of greater plantain (Plantago major L.), and effects of drying temperature on polyphenol contents in the leaves. Scientia Horticulturae, 128, 523–529.
Acknowledgments
This work was supported by the NCN Poland, project no. UMO-2011/01/B/NZ9/07139. The authors would like to thank Piotr Laskowski from Research Station for Cultivar Testing in Zybiszów near Wroclaw for providing the fruit materials.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Wojdyło, A., Figiel, A., Lech, K. et al. Effect of Convective and Vacuum–Microwave Drying on the Bioactive Compounds, Color, and Antioxidant Capacity of Sour Cherries. Food Bioprocess Technol 7, 829–841 (2014). https://doi.org/10.1007/s11947-013-1130-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-013-1130-8