Abstract
Climate changes and stresses negatively affected the physiological processes inside plant cells, which led to a clear imbalance in the global diet. In both irrigated and non-irrigated areas, salinity is one of the principal abiotic factors influencing plant growth and production, especially in crop plants. The present experiment was designed to evaluate two types of foliar feeding as anti-salt stress by measuring stress tolerant and antioxidant levels. Foliar feedings, Gluamin Cu, and Ascophyllum nodosum (WeGrow Special) were used as therabutic nutrients and stress raisers on plants irrigated with saline solution (150 mmol) and others irrigated with tap water. After 70 days of plant life, morphological characteristics, plant pigments, osmosis levels, phenols, and antioxidant enzyme activity were measured as indicators of plant recovery from stress damage. Measurements of vegetative growth, photosynthesis, sugars, and protein content decreased significantly in stressed plants. On the contrary, the level of proline, phenol, malondialdehyde, hydrogen peroxide, and sodium (Na +) and the activity of antioxidant enzymes increased compared to non-stressed plants. The beneficial impacts of the foliar feedings (Gluamin Cu and WeGrow Special) have been broadened to increase all growth parameters, photosynthetic pigments, proline, phenol, and enzyme activities, in both unstressed and stressed plants in comparison to control. Interestingly, the harmful impact of salinity on tomato plants was significantly decreased and it can be evident from reduced MDA and H2O2 levels. The results indicated that Gluamin Cu at a concentration of 3 cm L−1 foliar was the best treatment in increasing shoot length by 18.75%, root length by 51.8%, number of leaves by 31.5%, chlorophyll A by 98.9% and chlorophyll B by 47.6%, proline 12.6%, peroxidase by 39.6%, polyphenol oxidase by 14.29%, super oxide dismutase by 16.4%, and catalase by 54.9% in stressed plants compared to the stressed control. These results indicated that the use of any of the foliar nutrients (Gluamin Cu and WeGrow Special) considered to raise the salt stress in the plant and improve its morphological characteristics and metabolic processes inside the cells, and thus it can be used and applied commercially as environmentally friendly anti-salt stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plant growth-stimulating organisms force plants to create chemicals that can boost physiological immunity and boost tolerance to biotic and abiotic obstacles [1,2,3]. Each year, the productivity of crops declines across the globe due to abiotic stressors, which are frequently present in the environment and expose crop plants to adverse circumstances [4]. In either irrigated or non-irrigated areas, salinity constitutes one of the primary abiotic factors impacting plant growth and production, particularly in crop plants, which displayed decreased seed germination, plant growth, and biomass, ionic transport, nutrient uptake, and general enzymatic activity, making salinity a pressing problem in numerous countries around the globe [5, 6]. Because they utilize minimal chemical input and have no negative effects on humans or the environment, salinity resistance techniques that boost plant output are an excellent approach to practicing sustainable agriculture [7]. Bio-fertilizers have been hailed as an intriguing new approach to agricultural production and one of most efficient tools available today to nourish plants with nutrients [8, 9]. Marine microalgae and macroalgae-derived biomass production are generally recognized as a rich source of chemical components with potential in the agricultural fields [10]. Spraying an algae extract on a plant that was being water-stressed led to an increase in chlorophyll synthesis and plant growth [11]. Additionally, water was utilized more effectively when algal extract and other crucial nutrients were present in higher amounts [12]. Spraying with algae extracts reduced the generation of resistant elements such as proline and phenols. Algal extract raises yield of plants while also hastening plant growth [13]. Lately, scientists used algae and their extracts to stimulate and activate biochemical compounds inside plant cells, dubbed them environmentally beneficial inducers [14]. Seaweed releases a variety of chemicals, including sugars, amino acids, organic acids, and pathogen-inhibiting compounds [15]. According to recent scientific research, these important substances extracted from algae disseminate in the soil near plant roots or through leaves and are the most powerful bio-fertilizers [16]. The main benefit of fertilizing with algae and its extracts is that its pH ranges between 6 and 6.5, which assists in lowering alkalinity in a variety of lands, foremost alkaline ones, and creates acids that dissolve existing minerals and facilitate them in the soil, such as transforming insoluble rock phosphate salts into soluble phosphate salts and liberating potassium and other elements linked to agricultural use [17]. Plant tolerant to various stress factors depends on the nutritional status [18]. In comparison to untreated plants, spraying with various organic extracts causes dramatically enhanced yield, protein content, photosynthetic pigments, and nutrient uptake under salinity stress [19]. Attention turned to feeding the plant with compounds that contain copper, as scientific reports have proven the antimicrobial efficiency of copper, as it stimulates the activation of the enzyme Laccases, which is one of the enzymatic analogs of polyphenol oxidase that breaks down and oxidizes phenolic substances [20]. The researchers in the following work used copper on potato plants under biotic stress, and the results showed the ability of copper to enhance physiological responses against stress [21]. It is worth noting that plants treated with copper showed a significant improvement in morphological characteristics and physiological immune responses [22]. Gluconic acid has multidimensional properties to promote plant growth by increasing the absorption of phosphates from the soil [23]. This study’s major goal was to utilize Gluamin Cu to boost tomato plants’ resilience to salinity stress. The major purpose of this study was to explore the potential impacts of Gluamin Cu and Ascophyllum nodosum (WeGrow Special) as fertilizers to minimize the harmful effect of tomato plants under salinity stress by enhancing physiological immunity. Furthermore, the administration of Gluamin Cu on stressed as well as unstressed plants caused a substantial rise in phenol content along with elevated expression of the antioxidant enzymes peroxidase as well as polyphenol oxidase.
2 Materials and methods
2.1 Experimental design
The experiment was designed and conducted in the research garden at Al-SALAM International for Development & Agriculture Investment, Egypt. Gluamin Cu and Ascophyllum nodosum (WeGrow Special) as fertilizers were made from the Hydro Fert company, Via dei Fornai, Barletta BT, Italia. The products were obtained from Al-SALAM International for Development & Agriculture Investment, Egypt. Gluamin Cu is a liquid fertilizer that contains a unique formula (an element copper Cu 5%, organic carbon 10%, organic nitrogen 3%, and sulfur 2.5% in the presence of free amino acids 10% and glycolic acid 5%). Also Ascophyllum nodosum (WeGrow Special) is a liquid fertilizer (Ascophyllum nodosum 21%, free amino acids 7.2%, organic carbon 13.5%, organic nitrogen 3%, boron 1.2%, zinc 1.2%, and humic acid 2.5%).
Similar seedlings which are 3 weeks age tomato (Solanum lycopersicum L. var. 023) were planted into plastic pots (40 × 40cm) including a mix of sand and clay (1:3 W/W), total 7 kg, in a plastic greenhouse. After planting, the seedlings are left for 7 days before any treatments with normal irrigation. Next, saline liquid (150 mM NaCl) was applied for three times (one time each 7 days). The fertilizers (Gluamin Cu and WeGrow Special) were applied for three times (one time each week) (in the period before and after flowering). Treatments were performed by foliar shoots spraying (FS) techquies until dropping. Seedlings were planted in 6 groups as follows:
-
1)
Control plants without any treatments referred to as absolute control and irrigated by tap water
-
2)
Plants treated with Gluamin Cu 3 cm/L (foliar spray/shoot) and irrigated with tap water
-
3)
Plants treated with WeGrow Special 3 cm/L (foliar spray/shoot) and irrigated tap water
-
4)
NaCl (150 mM) (foliar spray/shoot): plants irrigated with 150 mM NaCl as saline stressed treaments
-
5)
Plants treated with Gluamin Cu 3 cm/L (foliar spray/shoot) and irrigated with 150 mM NaCl
-
6)
Plants treated with WeGrow Special 3 cm/L (foliar spray/shoot) and irrigated with 150 mM NaCl
2.2 Morphological characters
Morphological characters of were described at 70 days after sowing. Samples include shoot fresh weight and root fresh weight (FW, g) and shoot and root dry weights (DW, g). Morover, plant height (cm), root length (cm), and number of leaves per plant were also noted.
2.3 Photosynthetic measurements
A former procedure mentioned in the study (Vernon and Seely 1966) was used to assess the existence of chlorophyll a (Chl a), chlorophyll b (Chl b), and chlorophyll a + b (Chl a + b) and carotenoid (Lichtenthaler and Buschmann (2001).
2.4 Determination of the content of osmolytes
The soluble sugar content of the dried shoot (0.5 g) was combined with 2.5 mL of 2% phenol and 5 mL of 30% trichloroacetic acid, and filtrated via filter paper to extract the soluble sugars. From the resulting filtrate, 1 mL was combined with 2 mL of anthrone reagent (2 g anthrone/L of 95% H2SO4). At 620 nm, the resulting blue green color was observed [24]. The procedure of Abdelaziz et al. [25] was used to determine the soluble protein content of the dry shoot. The proline content was measured in the dry shoot according to Attia et al. [26]. The 0.5 g of dried shoots was broken down to produce 10 mL (3%) of sulfosalicylic acid. In a boiling water bath for 1 h, 2 mL of filtrate is combined with 2 mL of ninhydrin acid (1.25 g ninhydrin in 30 mL of glacial acetic acid and 20 mL of 6 M phosphoric acid) and 2 mL of glacial acetic acid. This process was then stopped through placing the mixture in an ice bath. We infused the mix with 4 mL of toluene before measuring the absorbance at 520 nm.
2.5 Determination of ascorbic acid and total phenol contents
The technique of [27] was used to estimate the ascorbic acid content of the dry shoot. Total dry shoot phenol content was measured using the [28] procedure.
2.6 Estimation of malondialdehyde and hydrogen peroxide contents
The content of MDA in fresh tomato leaf was measured by adding 2.5 mL of 0.1% trichloroacetic acid (TCA) served to homogenize leaf samples, and then 4 mL of 20% TCA containing 0.5% TBA was added to every milliliter [29]. The resulting mixture was subjected to heat for 30 min in a water bath at 95°C before cooling within an ice bath and centrifuging it for 15 min at 10,000 g. The supernatant’s absorbance was measured at 532 nm. The estimation of the level of MDA utilized the extinction coefficient (155 mM). The H2O2 content of fresh tomato leaf was measured as stated by [30].
2.7 Antioxidant enzymes assay
POD activity was assayed according to that method described by Verduyn et al. [31]. The activity of PPO was calculated by the procedure used by Matta and Dimond [32]. SOD activity was determined by using a method described by Marklund and Marklund [33]. CAT activity was assayed according to the method of Aebi [34]. The activities of POD, PPO, SOD, and CAT were assayed in fresh tomato leaves.
2.8 Estimation of sodium (Na+) and potassium (K+) contents
Dry tomato shoot samples (0.1 g) were digested with 80% perchloric acid and concentrated sulfuric acid solution (1:5) for 12 h. The contents of Na+ and K+ in the digested samples were determined by flame photometry using the Williams and Twine [35] method.
2.9 Statistical analysis
One-way analysis of variance (ANOVA) was used to evaluate the pilot data. By using CoStat software and the least significant difference (LSD) test, shown to exist at p 0.05. The results are displayed as mean standard errors (n = 3).
3 Results
3.1 Growth biomarkers
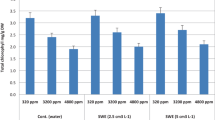
It is evident from Fig. 1 A, B, C that salinity-stressed plants exhibited major reducion in plant height by 40%, root length by 15.78%, and number of leaves by 36.31% compared to control plants. Also, it is obvious from Fig. 2 (A,B,C ), decline of shoot and root fresh weight by (29 % and 53.79%), shoot and root dry weight by (34.9% and 30.32%), unstressed plants treated with foliar feeding (Gluamin Cu or WeGrow Special) presented significant improve in all growth biomarkers compared with control. Moreover, salinity-stressed plants treated with foliar feeding (Gluamin Cu or WeGrow Special) showed promising recovery. Regarding (Glumin Cu and WeGrow Special) application, it was found that Gluamin Cu was the best treatment that effectively improved the loss growth biomarkers followed by WeGrow Special, both on unstressed or stressed plants.
3.2 Physiological traits
It is obvious form Table 1 that decline of Chl a by (54.60 %), Chl b by (42.16%), and carotenoids by (43.2%), reported in salinity-stressed plants, compared with their control plants. However, unstressed plants treated with foliar feeding (Gluamin Cu or WeGrow Special) presented significant enhancement in all photosynthetic pigments, compared with non-treated control plants. Also, salinity-stressed plants treated with foliar feeding (Gluamin Cu or WeGrow Special) showed promising recovery (Table 1). Concerning the effect (Gluamin Cu or WeGrow Special) on the challenged plants with salinity (Table 1), it was found that Gluamin Cu 3 cm L−1 increased Chl a by 98.94 %, Chl b by 47.61 %, and carotenoids by 74.02 %, whereas WeGrow Special 3 cm L−1 recovered the loss of Chl a by 85.09 %, Chl b by 32.38 %, and carotenoids by 62.33 %, compared with stressed plants ones.
3.3 Osmolytes
It is noticeable from Table 2 that decline in the contents of soluble sugar, soluble protein by (7.15% and 34.3%), were reported in stressed plants, compared with their control plants. However, unstressed plants treated with foliar feeding (Gluamin Cu or WeGrow Special) presented a significant boost in contents of soluble sugar and soluble protein, compared with non-treated control plants. Concerning the effect of Gluamin Cu or WeGrow Special on the challenged plants with salinity (Table 2), it was found that Gluamin Cu increased soluble sugar by 19.3 % and soluble protein by 97.93%, whereas WeGrow Special recovered the loss of soluble protein by 87.4 % and soluble protein by 0.18 %, compared with stressed plants ones.
Under salinity stress conditions, tomato plants showed a noticeable increase of proline by 6.75% compared with control plants (Table 2). In contrast, unstressed plants treated with foliar feeding (Gluamin Cu or WeGrow Special) presented significant increase in proline content,compared with non-treated control plants. Regarding the effect of Gluamin Cu or WeGrow Special on the challenged plants with salinity (Table 2), it was found that Gluamin Cu increased proline by 11.11 %, and came next was WeGrow Special by 5.06 % compared with stressed plants ones.
3.4 Total phenol content and ascorbic acid
Tomato plants grown under NaCl stress regimes exhibited significant increases in contents of total phenols and ascorbic acid when compared to control plants (Fig. 3). Moreover, foliar application of (Gluamin Cu or WeGrow Special) resulted in a noticeable increase in the content of total phenols and ascorbic acid contents when compared with untreated salinized plants. The highest increase in contents of total phenols and ascorbic acid were recorded in Gluamin Cu-treated plants (Fig. 3). Under normal conditions, Gluamin Cu- or WeGrow Special-treated tomato plants exhibited higher levels of ascorbic acid and total phenol versus control plants.
3.5 Antioxidant enzymes activity
It is noticeable from the Fig. 4 that increase in (POD, PPO, SOD and CAT) activities related to control plants by (51.4 %,78.72%,103.33% and 218.75%) , reported in stressed plants, compared with their control plants. However, unstressed plants treated with foliar feeding (Gluamin Cu or WeGrow Special) presented a significant boost in POD, PPO, SOD, and CAT activities, compared with non-treated control plants. Regarding the influence of either WeGrow Special or Gluamin Cu on the salinity-challenged plants (Fig. 4), it was discovered that Gluamin Cu increased POD by 39.6%, PPO by 14.29%, SOD by 16.4%, and CAT by 54.9%. With one exception, WeGrow Special increased the activities of antioxidant enzymes (POD by 18.9 %, SOD by 12.31%, and CAT by 66.67%). On the other hand, PPO activity decreased when WeGrow Special was applied.
3.6 Stress biomarkers
Tomato plants grown under salinity stress had higher levels of MDA and H2O2 compared to non-stressed controls (Fig. 5). Application of Gluamin Cu 3 cm L−1 or WeGrow Special 3 cm L−1 to salinity-stressed plants played a pivotal role in minimizing the level of MDA and H2O2 compared with the plants exposed to salinity stress only (Fig. 5). Under normal conditions, tomato plants sprayed with Gluamin Cu 3 cm L−1 or WeGrow Special 3 cm L−1 showed a decrease in the level of MDA and H2O2, respectively, as compared with that of untreated control.
3.7 Minerals contents
Under salinity stress conditions, tomato plants showed significant increases in sodium content. However, potassium content was significantly decreased in tomato shoots when compared to control plants (Table 3). Moreover, application of Gluamin Cu 3 cm L−1 or WeGrow Special 3 cm L−1 resulted in a remarkable decrease in the sodium content, while potassium content was significantly decreased when compared with untreated salinity stress tomato plants (Table 3). The greatest boost in potassium content was noticed in Gluamuin Cu-treated plants.
4 Discussion
Salinity stress can cause oxidative damage to plant cells, which can lead to reduced growth, physiological damage, and mineral nutrient imbalances. The study aims to investigate whether the two products can mitigate the negative effects of salinity stress on tomato plants. One of the most harmful stresses on the plant is considered salt stress, as its effect is deadly and more toxic to the cells and destructive to the quantity and yield of the crop. Serious thinking of exploiting the efficiency of many fertilizer and nutrient technologies to reduce the harmful effects of salt stress by stimulating the formation of antioxidants within the cells to avoid the effects of toxic ions in the soil solution or to clean the cells from free radicals. In this regard, resistance or stress tolerance depends on the nutritional status of the plant. In this experiment, foliar nutrients containing a mixture of organic matter and algae extracts (Gluamin Cu and WeGrow Special) were tested to evaluate their effectiveness in reducing salt stress damage and improving the vital processes of tomato plants. It is evident from the present study that stressed plants exhibited major reductions in plant height by 40%, root length by 15.78%, shoot and root fresh weight by 29 % and 53.79%, shoot and root dry mass by 34.9% and 30.32%, and number of leaves by 36.31%, in comparison to control plants. There were significant decreases. These results are consistent with many studies [36], where NaCl stress can cause harmful effect on main metabolic and absorptive processes such as the absorption, transport, and assimilation of mineral elements, which causes an imbalance in growth hormones [37]. The dangerous deficit in the morphological parameters as a result of the salinity stress may be also described by the oxidative burst in the cells and increase of reactive oxygen species (ROS), causing growth hormone disorders [38]. On the other hand, the efficiency of Gluamin Cu or WeGrow Special has been exploited to reduce the harmful effects either through the ability to inhibit water salinity and ion toxicities or to induce physiological immunity. Growth measurements were significantly affected by Gluamin Cu or WeGrow Special. In this context, Gluamin Cu and WeGrow Special are promising foliar feedings that are anti-stress rich vital contents known for their ability to carry out the vital processes. The levels of Chl a, Chl b, and carotenoids in salinity-stressed plants were dramatically lowered by 54.60 %, 42.16%, and 43.2%, respectively, compared to the control. Reduced photosynthesis is caused by the plants’ diminished capacity to absorb sunlight as a result of the drop in chlorophyll levels [39]. The deficiency in the synthesis of pigments leads to an imbalance in energy production and the disruption of many necessary cellular functions [40]. However, as compared to salinity-stressed control plants, the application of foliar feedings (Gluamin Cu or WeGrow Special) dramatically boosted the levels of Chl a, Chl b, and carotenoids in stressed plants. Increased synthesis of photosynthetic pigments due to the application of Gluamin Cu was explained by presence of Cu, organic carbon [41], organic nitrogen [42], sulfur [43], and free amino acids [44]. The improved photosynthetic pigments due to application of WeGrow Special may be due to the presence of A. nodosum extract [45]. A. nodosum extract produces substances which endure stress conditions and reduce oxidative blast in cells [46, 47]. Algae produce compounds that inhibit the stress such as phenols [48].
Several previous studies reported a significant increase in osmolite levels and interpreted this increase as playing a vital role in collecting reactive oxygen species (ROS) [49]. In this work, salt-stressed plants showed decreases in contents of soluble sugar and soluble protein. Our results are in harmony with other heavy previous researchers [50]. In the present study, foliar application of Gluamin Cu or WeGrow Special enhanced the contents of tested osmolytes in salinity-stressed plants when compared to salinity untreated plants [51]. These results are in harmony with [52]. Preventing photothynthetic pigments damage caused by reactive oxygen species [53]. In this study, salinity stress increased the contents of total phenols and ascorbic acid in shoots of tomato plants. Foliar application of Gluamin Cu or WeGrow Special resulted in a noticeable increase in the content of total phenols and ascorbic acid contents when compared with untreated salinized plants. The highest increase in contents of total phenols and ascorbic acid was recorded in Gluamin Cu-treated plants. The aforementioned increases in the contents of total phenols and ascorbic acid are in correlation with a reduction in the contents of malondialdehyde (MDA) and hydrogen peroxide (H2O2) [54, 55]. The accumulation of phenolic compounds and ascorbic acid serves as an adaptive strategy to salinity stress [56]. The obtained results are in line with [57]. It was observed that the activity of antioxidant enzymes like POD, PPO, SOD, and CAT was significantly higher due to salinity stress [58]. The previous reports showed that antioxidant enzyme activity increased in plants exposed to salt stress [59]. Our results revealed that salinity-stressed plants gave high significant increases in antioxidant enzyme activities related to control plants (unstressed). Plants under stress are forced to try to increase the activity of antioxidant enzymes to keep the level of reactive oxygen at a lower level in the cell. SOD converts O2− to H2O2, which plays an important anti-oxidative stress role, while CAT and POD help convert H2O2 to H2O [60, 61]. Our results of the present study revealed that Gluamin Cu increased POD by 39.6 %, PPO by 14.29%, SOD by 16.4%, and CAT by 54.9%. It was reported that the activity of antioxidant enzymes increased in tomato plants under salt stress conditions as a result of treatment with copper [62], organic carbon [51], organic nitrogen, free amino acids [63], and A. nodosum extract [64]. The Gluamin Cu constitution includes sulfur, which is known as one of the most important nutrients needed in plants [65]. Most of the sulfur absorbed by plants is metabolized after reduction to cysteine and methionine, both of which are important in plant growth and anti-stress proteins [66]. WeGrow Special contains natural elements and hormones and seaweed extract; it may work in successive reactions such as producing hormones and expressing antioxidant enzymes that raise the efficiency of cells against salt stress [67].
5 Conclusion
Based on the findings, it is possible to derive that Gluamin Cu and Ascophyllum nodosum (WeGrow Special) significantly improved tomato plant growth traits, leaf pigments, soluble sugars, protein contents, and antioxidant enzymes during the current study by reducing the negative effects of salinity issues caused by NaCl irrigation. Additionally, employing Gluamin Cu and Ascophyllum nodosum (WeGrow Special) decreased the levels of proline, malondialdehyde, and hydrogen peroxide while raising the levels of total phenol and asocrbic acid in tomato plants as a mitigating strategy for the adverse effects caused by salt stress. Therefore, the present work recommends that the primary option for promoting crop growth under both normal and salinity stress environments is Gluamin Cu at 3 cm /L (foliar spray/shoot).
Data availability
All data and materials available.
References
Attia MS, Abdelaziz AM, Al-Askar AA, Arishi AA, Abdelhakim AM, Hashem AH (2022) Plant growth-promoting fungi as biocontrol tool against fusarium wilt disease of tomato plant. J Fungus 8(8):775. https://doi.org/10.3390/jof8080775
Abdelaziz AM, Hashem AH, El-Sayyad GS, El-Wakil DA, Selim S, Alkhalifah DH, Attia MS (2023) Biocontrol of soil borne diseases by plant growth promoting rhizobacteria. Trop Plant Pathol 48(2):105–127. https://doi.org/10.1007/s40858-022-00544-7
Abdelaziz AM, Attia MS, Salem MS, Refaay DA, Alhoqail WA, Senousy HH (2022) Cyanobacteria-mediated immune responses in pepper plants against fusarium wilt. Plants 11(15):2049. https://doi.org/10.3390/plants11152049
Fadiji AE, Babalola OO, Santoyo G, Perazzolli M (2022) The potential role of microbial biostimulants in the amelioration of climate change-associated abiotic stresses on crops. Front Microbiol 12:829099. https://doi.org/10.3389/fmicb.2021.829099
Safdar H, Amin A, Shafiq Y, Ali A, Yasin R, Shoukat A et al (2019) A review: impact of salinity on plant growth. Nat Sci 17(1):34–40. https://doi.org/10.7537/marsnsj170119.06
Badawy AA, Alotaibi MO, Abdelaziz AM, Osman MS, Khalil AM, Saleh AM et al (2021) Enhancement of seawater stress tolerance in barley by the endophytic fungus Aspergillus ochraceus. Metabolites 11(7):428. https://doi.org/10.3390/metabo11070428
Edwards CA (2020) The importance of integration in sustainable agricultural systems. In: Sustainable agricultural systems. CRC Press, pp 249–264
Yadav M (2018) Biotechnology for sustainable agriculture and livelihood security: a preliminary review. Int J Environ Sci 7(2):68–71 ISSN: 2277-1948
Attia MS, Salem MS, Abdelaziz AM (2022) Endophytic fungi Aspergillus spp. reduce fusarial wilt disease severity, enhance growth, metabolism and stimulate the plant defense system in pepper plants. Biomass Convers Biorefin:1–11. https://doi.org/10.1007/s13399-022-03607-6
Khattab AM, Abo-Taleb HA, Abdelaziz AM, El-Tabakh MA, El-Feky MM, Abu-Elghait M (2022) Daphnia magna and Gammarus pulex, novel promising agents for biomedical and agricultural applications. Sci Rep 12(1):13690. https://doi.org/10.1038/s41598-022-17790-z
De Vasconcelos ACF, Chaves LHG (2019) Biostimulants and their role in improving plant growth under abiotic stresses. Biostimulants in plant science, pp 3–16
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Factories 17(1):1–21. https://doi.org/10.1186/s12934-018-0879-x
Almaroai YA, Eissa MA (2020) Role of marine algae extracts in water stress resistance of onion under semiarid conditions. J Soil Sci Plant Nutr 20:1092–1101. https://doi.org/10.1007/s42729-020-00195-0
Razzak SA, Lucky RA, Hossain MM, deLasa H (2022) Valorization of microalgae biomass to biofuel production: a review. Energy Nexus 100139. https://doi.org/10.1016/j.nexus.2022.100139
Attia MS, Elsayed SM, Abdelaziz AM, Ali MM (2023) Potential impacts of Ascophyllum nodosum, Arthrospira platensis extracts and calcium phosphite as therapeutic nutrients for enhancing immune response in pepper plant against Fusarium wilt disease. Biomass Convers Biorefin 1-10. https://doi.org/10.1007/s13399-023-03949-9
Itelima, J. U., Bang, W. J., Onyimba, I. A., Sila, M. D., & Egbere, O. J. (2018). Bio-fertilizers as key player in enhancing soil fertility and crop productivity: a review. http://hdl.handle.net/123456789/1999.
Halford KJ (2002) Design of a wastewater management system for the reduction of contaminants from an agricultural chemical plant. Incitec Ltd. Gibson Island Works. Charles Darwin University, Australia. https://doi.org/10.25913/5e856e8b6ef5a
Eslamian F (2020) Evaluation and development of lime-based products to reduce phosphorus loss from agricultural soils. McGill University, Canada, p 30452524
Merwad ARM (2020) Mitigation of salinity stress effects on growth, yield and nutrient uptake of wheat by application of organic extracts. Commun Soil Sci Plant Anal 51(9):1150–1160. https://doi.org/10.1080/00103624.2020.1751188
Quideau S, Deffieux D, Douat-Casassus C, Pouységu L (2011) Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed 50(3):586–621. https://doi.org/10.1002/anie.201000044
Gaber SE, Hashem AH, El-Sayyad GS, Attia MS (2023) Antifungal activity of myco-synthesized bimetallic ZnO-CuO nanoparticles against fungal plant pathogen Fusarium oxysporum. Biomass Convers Biorefin 1-15. https://doi.org/10.1007/s13399-023-04550-w
Gohari G, Farhadi H, Panahirad S, Zareei E, Labib P, Jafari H et al (2023) Mitigation of salinity impact in spearmint plants through the application of engineered chitosan-melatonin nanoparticles. Int J Biol Macromol 224:893–907. https://doi.org/10.1016/j.ijbiomac.2022.10.175
Stephen J, Jisha MS (2011) Gluconic acid production as the principal mechanism of mineral phosphate solubilization by Burkholderia sp.(MTCC 8369). J Trop Agric 49:99–103
Attia MS, Hashem AH, Badawy AA, Abdelaziz AM (2022) Biocontrol of early blight disease of eggplant using endophytic Aspergillus terreus: improving plant immunological, physiological and antifungal activities. Bot Stud 63(1):26. https://doi.org/10.1186/s40529-022-00357-6
Abdelaziz AM, Elshaer MA, Abd-Elraheem MA, Ali OMOM, Haggag MI, El-Sayyad GS, Attia MS (2023) Ziziphus spina-christi extract-stabilized novel silver nanoparticle synthesis for combating Fusarium oxysporum-causing pepper wilt disease: In vitro and in vivo studies. Arch Microbiol 205(2):69. https://doi.org/10.1007/s00203-023-03400-7
Attia MS, Abdelaziz AM, Hassanin MM, Al-Askar AA, Marey SA, Abdelgawad H, Hashem AH (2023) Eco-friendly preparation of thyme essential oil nano emulsion: characterization, antifungal activity and resistance of Fusarium wilt disease of Foeniculum vulgare. Not Bot Horti Agrobot Cluj Napoca 51(3):13312–13312. https://doi.org/10.15835/nbha51313312
Jagota SK, Dani HM (1982) A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal Biochem 127(1):178–182. https://doi.org/10.1016/0003-2697(82)90162-2
Attia MS, Sharaf MH, Hashem AH, Mahfouz AY, Daigham GE, Al-Askar AA et al (2023) Application of Rhizopus microsporus and Aspergillus oryzae to enhance the defense capacity of eggplant seedlings against Meloidogyne incognita. Not Bot Horti Agrobot Cluj Napoca 51(3):13300–13300. https://doi.org/10.15835/nbha51313300
El-Batal AI, Al-shammari BM, El-Sayyad GS, Rizk SH, Abdelaziz AM, Nofel MM, Attia MS (2023) Gum Arabic-assisted biomass synthesis of bimetallic ZnO-CuO nanoparticles using gamma rays for controlling potato post-harvest tuber rots-causing Alternaria solani: towards improving food safety. Biomass Convers Biorefin 1-18. https://doi.org/10.1007/s13399-023-04836-z
El-Batal AI, El-Sayyad GS, Al-Shammari BM, Abdelaziz AM, Nofel MM, Gobara M et al (2023) Protective role of iron oxide nanocomposites on disease index, and biochemical resistance indicators against Fusarium oxysporum induced-cucumber wilt disease: In vitro, and in vivo studies. Microb Pathog 180:106131. https://doi.org/10.1016/j.micpath.2023.106131
Verduyn C, van Dijken JP, Scheffers WA (1984) Colorimetric alcohol assays with alcohol oxidase. J Microbiol Methods 2(1):15–25. https://doi.org/10.1016/0167-7012(84)90027-7
Matta A, Dimond AE (1963) Symptoms of Fusarium wilt in relation to quantity of fungus and enzyme activity in tomato stems. Phytopathology 53(5):574
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47(3):469–474
Aebi H (1984) [13] Catalase in vitro. In Methods in enzymology (Vol. 105, pp. 121-126). Academic press. https://doi.org/10.1016/S0076-6879(84)05016-3
Williams V, Twine S (1960) Flame photometric method for sodium, potassium and calcium. In: Modern methods of plant analysis, pp 3–5
Bello AS, Ben-Hamadou R, Hamdi H, Saadaoui I, Ahmed T (2021) Application of cyanobacteria (roholtiella sp.) liquid extract for the alleviation of salt stress in bell pepper (capsicum annuum L.) plants grown in a soilless system. Plants 11(1):104. https://doi.org/10.3390/plants11010104
Alam SM (1999) Nutrient uptake by plants under stress conditions. In: Handbook of plant and crop stress, vol 2, pp 285–313
Hasanuzzaman M, Raihan MRH, Masud AAC, Rahman K, Nowroz F, Rahman M et al (2021) Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int J Mol Sci 22(17):9326. https://doi.org/10.3390/ijms22179326
Gohari G, Mohammadi A, Akbari A, Panahirad S, Dadpour MR, Fotopoulos V, Kimura S (2020) Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci Rep 10(1):912. https://doi.org/10.1038/s41598-020-57794-1
Sharma A, Kumar V, Shahzad B, Ramakrishnan M, Singh Sidhu GP, Bali AS et al (2020) Photosynthetic response of plants under different abiotic stresses: a review. J Plant Growth Regul 39:509–531. https://doi.org/10.1007/s00344-019-10018-x
Chandra R, Rohit MV, Swamy YV, Mohan SV (2014) Regulatory function of organic carbon supplementation on biodiesel production during growth and nutrient stress phases of mixotrophic microalgae cultivation. Bioresour Technol 165:279–287. https://doi.org/10.1016/j.biortech.2014.02.102
Yadav M, Gupta P, Seth CS (2022) Foliar application of α-lipoic acid attenuates cadmium toxicity on photosynthetic pigments and nitrogen metabolism in Solanum lycopersicum L. Acta Physiol Plant 44(11):112. https://doi.org/10.1007/s11738-022-03445-z
Siddiqui MH, Alamri S, Alsubaie QD, Ali HM, Khan MN, Al-Ghamdi A et al (2020) Exogenous nitric oxide alleviates sulfur deficiency-induced oxidative damage in tomato seedlings. Nitric Oxide 94:95–107. https://doi.org/10.1016/j.niox.2019.11.002
Sh Sadak M, Abdelhamid MT, Schmidhalter U (2015) Effect of foliar application of aminoacids on plant yield and some physiological parameters in bean plants irrigated with seawater. Acta biológica colombiana 20(1):141–152. https://doi.org/10.15446/abc.v20n1.42865
Campobenedetto C, Agliassa C, Mannino G, Vigliante I, Contartese V, Secchi F, Bertea CM (2021) A biostimulant based on seaweed (Ascophyllum nodosum and Laminaria digitata) and yeast extracts mitigates water stress effects on tomato (Solanum lycopersicum L.). Agriculture 11(6):557. https://doi.org/10.3390/agriculture11060557
Elnahal AS, El-Saadony MT, Saad AM, Desoky ESM, El-Tahan AM, Rady MM et al (2022) The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: a review. Eur J Plant Pathol 162(4):759–792. https://doi.org/10.1007/s10658-021-02393-7
Jain A, Sirisha VL (2020) Algal carotenoids: understanding their structure, distribution and potential applications in human health. Encyclopedia Mar Biotechnol 1:33–64. https://doi.org/10.1002/9781119143802.ch2
Zerrifi SEA, El Khalloufi F, Oudra B, Vasconcelos V (2018) Seaweed bioactive compounds against pathogens and microalgae: potential uses on pharmacology and harmful algae bloom control. Mar Drugs 16(2):55. https://doi.org/10.3390/md16020055
Arif Y, Singh P, Siddiqui H, Bajguz A, Hayat S (2020) Salinity induced physiological and biochemical changes in plants: an omic approach towards salt stress tolerance. Plant Physiol Biochem 156:64–77. https://doi.org/10.1016/j.plaphy.2020.08.042
Amini F, Ehsanpour AA (2005) Soluble proteins, proline, carbohydrates and Na+/K+ changes in two tomato (Lycopersicon esculentum Mill.) cultivars under in vitro salt stress. Am J Biochem Biotechnol 1(4):212–216
Al-Taey DK, Majid ZZ (2018) Study effect of kinetin, bio-fertilizers and organic matter application in lettuce under salt stress. J Glob Pharma Technol 10(1):148–164
El-Badawy HEM (2017) Partial substitution of Valencia orange chemical fertilization by bioorganic fertilization conjoint with algae extract foliar spray. Middle East J Appl Sci 7(4):1016–1030
Verma K, Mehta SK, Shekhawat GS (2013) Nitric oxide (NO) counteracts cadmium induced cytotoxic processes mediated by reactive oxygen species (ROS) in Brassica juncea: cross-talk between ROS, NO and antioxidant responses. Biometals 26:255–269. https://doi.org/10.1007/s10534-013-9608-4
Osman MS, Badawy AA, Osman AI, Abdel Latef AAH (2021) Ameliorative impact of an extract of the halophyte Arthrocnemum macrostachyum on growth and biochemical parameters of soybean under salinity stress. J Plant Growth Regul 40:1245–1256
Chattha MU, Hassan MUU, Khan I, Nawaz M, Shah AN, Sattar A et al (2022) Hydrogen peroxide priming alleviates salinity induced toxic effect in maize by improving antioxidant defense system, ionic homeostasis, photosynthetic efficiency and hormonal crosstalk. Mol Biol Rep 49(6):5611–5624. https://doi.org/10.1007/s11033-022-07535-6
Azeem M, Pirjan K, Qasim M, Mahmood A, Javed T, Muhammad H et al (2023) Salinity stress improves antioxidant potential by modulating physio-biochemical responses in Moringa oleifera Lam. Sci Rep 13(1):2895. https://doi.org/10.1038/s41598-023-29954-6
Ali MM, Jeddi K, Attia MS, Elsayed SM, Yusuf M, Osman MS et al (2021) Wuxal amino (Bio stimulant) improved growth and physiological performance of tomato plants under salinity stress through adaptive mechanisms and antioxidant potential. Saudi J Biol Sci 28(6):3204–3213. https://doi.org/10.1016/j.sjbs.2021.04.040
Abd Elhamid EM, Sadak MS, Tawfik MM (2014) Alleviation of adverse effects of salt stress in wheat cultivars by foliar treatment with antioxidant 2—changes in some biochemical aspects, lipid peroxidation, antioxidant enzymes and amino acid contents. Agric Sci 5(13):1269 http://creativecommons.org/licenses/by/4.0/
Bybordi A (2012) Effect of ascorbic acid and silicium on photosynthesis, antioxidant enzyme activity, and fatty acid contents in canola exposure to salt stress. J Integr Agric 11(10):1610–1620. https://doi.org/10.1016/S2095-3119(12)60164-6
Zhang M, Fang Y, Ji Y, Jiang Z, Wang L (2013) Effects of salt stress on ion content, antioxidant enzymes and protein profile in different tissues of Broussonetia papyrifera. S Afr J Bot 85:1–9. https://doi.org/10.1016/j.sajb.2012.11.005
Vighi IL, Benitez LC, Amaral MN, Moraes GP, Auler PA, Rodrigues GS et al (2017) Functional characterization of the antioxidant enzymes in rice plants exposed to salinity stress. Biol Plant 61(3):540–550. https://doi.org/10.1007/s10535-017-0727-6
Pérez-Labrada F, López-Vargas ER, Ortega-Ortiz H, Cadenas-Pliego G, Benavides-Mendoza A, Juárez-Maldonado A (2019) Responses of tomato plants under saline stress to foliar application of copper nanoparticles. Plants 8(6):151. https://doi.org/10.3390/plants8060151
Ghasemi S, Khoshgoftarmanesh AH, Afyuni M, Hadadzadeh H (2014) Iron (II)–amino acid chelates alleviate salt-stress induced oxidative damages on tomato grown in nutrient solution culture. Sci Hortic 165:91–98. https://doi.org/10.1016/j.scienta.2013.10.037
Hernández-Herrera RM, Sánchez-Hernández CV, Palmeros-Suárez PA, Ocampo-Alvarez H, Santacruz-Ruvalcaba F, Meza-Canales ID, Becerril-Espinosa A (2022) Seaweed extract improves growth and productivity of tomato plants under salinity stress. Agronomy 12(10):2495. https://doi.org/10.3390/agronomy12102495
Forieri I, Wirtz M, Hell R (2013) Toward new perspectives on the interaction of iron and sulfur metabolism in plants. Front Plant Sci 4:357. https://doi.org/10.3389/fpls.2013.00357
Genisel M, Erdal S, Kizilkaya M (2015) The mitigating effect of cysteine on growth inhibition in salt-stressed barley seeds is related to its own reducing capacity rather than its effects on antioxidant system. Plant Growth Regul 75:187–197. https://doi.org/10.1007/s10725-014-9943-7
Rouphael Y, Colla G (2020) Biostimulants in agriculture. Front Plant Sci 11:40. https://doi.org/10.3389/fpls.2020.00040
Acknowledgements
The authors are grateful to Eng. Mahmud M. Elsayed, for his help during the study. The authors are also grateful to Al-SALAM International for Development & Agriculture Investment, Egypt, for the financial and technical support offered during this work. The authors are also grateful to Hydro Fert company, Via dei Fornai, Barletta BT, Italia. The authors would like to thank the Botany and Microbiology Department, Faculty of Science, Al-Azhar University for promoting this research.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization, M.S.A., A.M.A., S.M.E, M.M.A., and M.S.O. Methodology, M.S.A., A.M.A., and S.M.E. Software, M.S.A., A.M.A., S.M.E, M.M.A., and M.S.O.; formal analysis, M.S.A., A.M.A., S.M.E, M.M.A., and M.S.O. Investigation, M.S.A., A.M.A., M.M.A., and M.S.O. Resources, M.S.A., A.M.A., S.M.E, and M.S.O.; data curation, M.S.A.,A.M.A., S.M.E, and M.S.O.; writing original draft preparation, M.S.A., A.M.A., M.M.A., and M.S.O.; writing review and editing, M.S.A., A.M.A., M.M.A., and M.S.O; supervision, M.S.A., A.M.A., S.M.E., and M.S.O. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All authors approved.
Consent for publication
All authors agree for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Attia, M.S., Abdelaziz, A.M., Elsayed, S.M. et al. Protective role of Ascophyllum nodosum seaweed biomass conjugated organic minerals as therapeutic nutrients to enhance tomato plant grown under salinity stress. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-05103-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-05103-x