Abstract
This study is aimed at maximizing the recovery of anthocyanins from Sicana odorifera fruit epicarp by heat- and ultrasound-assisted extraction (HAE and UAE, respectively) and at evaluating the in vitro bioactive properties of the anthocyanin-rich extracts obtained under optimized conditions. The optimization process utilized response surface methodology (RSM) through a central composite rotatory design (CCRD), combining time, ethanol percentage, and temperature or ultrasonic power. Response criteria included extraction yield (determined by gravimetry) and total anthocyanin content (TAC, quantified by HPLC-DAD). Under global optimum conditions, HAE (62 min, 90 °C, 27% ethanol) recovered 281 mg TAC/g extract with a yield of 23% (w/w) extract, while UAE (23 min, 500 W, 40% ethanol) yielded 200 mg TAC/g extract and 26% (w/w) extract, thus confirming the models’ predictive capacity. Furthermore, although HAE has produced extracts with higher anthocyanin concentrations, the cost-benefit associated with the processing conditions of each method must be considered. Both extracts showed antioxidant activity by inhibiting thiobarbituric acid reactive substances and oxidative hemolysis, but did not show anti-inflammatory, cytotoxic, and antimicrobial effects. Overall, this study promotes the upcycling of fruit waste into new ingredients for the food industry and other sectors that use anthocyanins, in line with sustainability and circular economy principles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Food additives are of utmost importance to ensure food quality and safety. Furthermore, some additives are necessary to improve the sensory characteristics of foods and for the development of new processed products [1]. In this sense, food colorants are widely used by the food industry, either to correct or improve the color of a wide range of products that may change during processing or to produce differentiated and more appealing products for better marketing [2, 3]. However, the most used food colorants are of synthetic or artificial origin, which are more efficient, stable, and cheaper than those obtained from natural sources [4, 5]. Nonetheless, in recent decades, the safety of some artificial food colorants has been questioned by different stakeholders [6, 7].
To develop safer colorants than the artificial ones commonly used in foods, dietary supplements, and related products, the search for sustainable natural alternatives has been in the spotlight of many researchers and industry professionals [2, 3]. Natural colorants can be obtained from animal, mineral, microbial, and plant sources. In the specific case of plant-derived colorants, these include chlorophylls, carotenoids, betacyanins, and anthocyanins, among other pigments. In addition to imparting a particular color shade, these natural compounds can exert a wide range of bioactivities and health-promoting effects [5, 8]. For anthocyanins, which are water-soluble pigments with colors ranging from pink-red-purple-blue depending on the pH of the medium, several bioactivities have been reported, such as antioxidant, antidiabetic, antihypertensive, and anti-inflammatory activities, thus contributing to reducing the risk of cardiovascular and neurodegenerative diseases [4, 9, 10].
Although the use and commercialization of natural anthocyanin colorants is allowed by European legislation, obtaining these additives from plant materials requires a comprehensive study, ranging from the selection of raw materials, solvents, and extraction techniques, as well as methods for purification, stabilization, and incorporation into foods [11, 12]. Regarding alternative sources of food additives, fruit by-products such as peels are gaining visibility for obtaining natural colorants, since these plant materials are generally rich in pigments [11,12,13,14]. Therefore, the valorization of by-products can be an asset for obtaining food-grade colorants, in addition to promoting the circularity and sustainable use of these natural resources.
The fruit of Sicana odorifera (Vell.) Naudin, popularly known as kurugua or melão croá in South American countries, is consumed for its succulent and nutritive pulp that can be used in the preparation of beverages, salads, and desserts. This fruit has a rigid inedible epicarp that represents about 13% of the fruit weight [15]. Despite this, previous studies have shown that this by-product has a high concentration of anthocyanins, including cyanindin-3-O-glucoside, cyanindin-3-O-rutinoside, cyanidin hexoside rhamnoside, pelargonidin hexoside rhamnoside, peonidin-3-O-(6″-O-p-coumaroyl)-β-D-glucopyranoside, and malvidin rhamnoside shikimate [16, 17]. Although data on the annual production of this fruit are scarce, an increase in both the popularity and production of this fruit is expected. For instance, the government of Paraguay has embraced the cultivation of this species as a strategy to promote biodiversity conservation [18]. Therefore, the production of by-products from this fruit may also increase.
Although several intrinsic characteristics of the plant material can affect the recovery of target bioactive compounds, the processing conditions, including the extraction solvent and the applied process intensification factors, also interfere with the extraction efficiency [4, 19,20,21]. Conventional solid-liquid extraction consists of mass transfer from the sample to a solvent, a process that can be improved by optimizing the inherent extraction parameters, such as time, temperature, stirring rate, and solvent characteristics, among others [4]. According to the literature, the extraction of anthocyanins from plant tissue increases with the use of polar solvents, such as water, methanol, ethanol, and propanol [8]. Although some studies report the efficiency of anthocyanin recovery using conventional methods [4, 20], these techniques may have techno-economic limitations due to the long extraction time, use of high temperatures, and high consumption of organic solvents. Thus, new extraction systems have been studied to maximize the recovery of target compounds and reduce the costs associated with the process [22]. Ultrasound-assisted extraction (UAE) has been showing advantages mainly in reducing the processing time and solvent volume, since the mass transfer is intensified by the ultrasonic waves that cause cavitation effects capable of disrupting the plant tissue [8, 19, 21].
With the aim of proposing an effective method for extracting anthocyanins from the S. odorifera fruit peel, the present work evaluated and compared two extraction techniques, namely, dynamic maceration or heat-assisted extraction (HAE) and UAE. The effects of three factors inherent to each extraction method, namely, time (t), ethanol percentage (S), and temperature (T) or ultrasonic power (P) for HAE and UAE, respectively, were investigated in a central composite rotatory design (CCRD) with six replicates at the center. The obtained experimental data (extraction yield and anthocyanin content) were modelled by RSM to obtain optimal extraction conditions capable of producing extracts as concentrated as possible in anthocyanin pigments. In addition, the bioactivity of the extracts obtained under optimized conditions was evaluated through in vitro assays for the determination of antioxidant, anti-inflammatory, antimicrobial, and cytotoxic activities.
2 Materials and method
2.1 Plant material
Ripe fruits of S. odorifera were acquired from Companhia de Entrepostos e Armazéns Gerais de São Paulo (CEAGESP), Brazil. The fruits were washed in tap water, and the epicarp (peel) was removed with a knife, frozen and lyophilized until total dryness, and reduced to a homogeneous powder (~20 mesh) using a domestic grinder (La moulinette 800 W, Moulinex Groupe SEB, France). The sample was stored protected from light at -20 °C until use.
2.2 Experimental design for RSM optimization
For extraction optimization of anthocyanin from S. odorifera fruit epicarp, an RSM-CCRD with three factors at five levels was implemented to determine the experimental points for heat-assisted extraction (HAE) and ultrasound-assisted extraction (UAE). The factors time (t: min), ethanol percentage (S: % (v/v), and temperature (T: °C) or power (P: W), for HAE and UAE, respectively, and their range of values were determined based on previous studies [14]. The natural and coded values of the selected factors are shown in Table 1. As dependent or response variables, the extraction yield (Y1: %, w/w) and total anthocyanin content per gram of extract (Y2: mg TAC/g E) were analyzed.
t time, T temperature, P power, S solvent (ethanol percentage)
2.3 Extraction methods
2.3.1 Heat-assisted extraction (HAE)
The experimental points determined for HAE were performed using a carousel system (Carousel™ 6 plus, Radleys Discovery Technologies, UK) with stirring at 500 rpm. Epicarp powder was mixed with solvent (S, 0–100% ethanol absolute 99.8% (Fisher Scientific) acidified with citric acid until pH 3), and the solid/liquid ratio (S/L) was kept at 50 g/L [23]. After that, the samples were submitted to different extraction conditions, combining time (t, 2–90 min) and temperature (T, 20–90 °C) according to the CCRD matrix. The extract solutions were then centrifuged (2000×g for 10 min at 10 °C), and the liquid phase was collected. The extraction yield (Y1: %, w/w) was determined based on the weight of the dry extract obtained by a gravimetric method that involves drying 5 mL of liquid extract at 105 °C for 24 h [14]. For anthocyanins, a 2 mL aliquot of extract solution was filtered through 0.22-μm filter disks for HPLC analysis.
2.3.2 Ultrasound-assisted extraction (UAE)
An ultrasonic homogenizer equipped with a titanium probe (model CY-500, 20 kHz, Optic Ivymen System, Barcelona, Spain) was used for UAE. Powder samples were placed in a beaker with acidified solvent (S, 0–100% ethanol at pH 3) at 50 g/L and processed under the CCRD conditions of time (t, 2–45 min) and power (P, 5–500 W). The temperature was kept at 20 ± 3 °C during processing. After extraction, the extract solutions were processed as described above for HAE.
2.4 Determination of the total anthocyanin content
The total anthocyanin content (Y2, TAC) in each extract was analyzed by high-performance liquid chromatography (Dionex Ultimate 3000 HPLC, Thermo Scientific) as previously described [24]. In a previous study carried out by the authors [24], cyanidin-O-deoxyhexosyl-hexoside and pelargonidin-O-deoxyhexosyl-hexoside were identified in S. odorifera fruit epicarp. These data were used to identify and quantify the anthocyanins detected in the extracts by using seven-level calibration curves constructed with standards of cyanidin-3-O-glucoside (y = 129688x − 42802, r2 = 0.9969, limit of detection (LOD) = 0.59 μg/mL, and limit of quantification (LOQ) = 1.81 μg/mL) and perlagonidin-3-O-glucoside (y = 28699x + 761.27, r2 = 0.9992, LOD = 0.96 μg/mL, and LOQ = 2.90 μg/mL). The TAC (mg/g E) was determined by the sum of the contents of both anthocyanins (Table S1).
2.5 Definition of mathematical models, response surfaces, and validation procedure
The experimental responses obtained under the CCRD were adjusted through least-squares calculation to a second-order polynomial model using Design-Expert software, version 11 (Stat-Ease, Inc., Minneapolis, USA). Analysis of variance (ANOVA) was used to determine the parametric coefficient, model significance, coefficient of determination (R2), adjusted coefficient of determination (R2 adj), and the adequacy of the models by the statistic lack-of-fit. To build the models, only significant coefficients (p < 0.05) or those required for the hierarchy were considered. The software also was used to engender 2D plots to depict the effect of each factor on the target responses and to create the response surfaces in 3D to visually illustrate the combined effect between factors on the responses. The global HAE and UAE conditions that maximize both the extraction yield and the anthocyanin recovery were determined using numerical optimization criteria, given the same importance to the variables. The best global conditions were experimentally tested in triplicate to assess the predictive accuracy of the theoretical models, which was evaluated (α = 0.05) by the post-analysis verification of the Design-expert software. Furthermore, the mean absolute percentage error (MAPE) was determined according to the following equation [25]:
where A is the experimental value, F is the predicted value, and N denotes the number of fitted points.
2.6 Color and bioactivity assessment of the anthocyanin-rich extracts
2.6.1 Preparation of dry extracts
The anthocyanin-rich extracts obtained under the global optimized conditions established for HAE and UAE were centrifugated at 2000×g for 10 min at 10 °C, and the supernatants were concentrated under reduced pressure until separation of ethanol. Afterwards, the remaining aqueous phases were frozen (−20 °C) and lyophilized to complete dryness for subsequent color measurement and bioactivity assessment.
2.6.2 Color assessment
The color parameters (L*, a*, b*) of the anthocyanin-rich extracts were measured using a portable CR-400 colorimeter (Konica Minolta Sensing Inc., Tokyo, Japan) programmed with D65 illuminant (standard defined by the International Commission on Illumination (CIE) as roughly corresponding to the average midday light in Western Europe). The CIELab values were converted to RGB (red, green, blue) using EasyRGB (http://www.easyrgb.com) to create a color model for visual perception.
2.6.3 Antioxidant activity
The antioxidant activity of the anthocyanin-rich extracts was evaluated using two cell-based methodologies previously described [24, 26], namely, the thiobarbituric acid reactive substances (TBARS) formation inhibition assay, which results were expressed as IC50 values (μg/mL) and translate the extract concentration providing 50% of TBARS inhibition, and the oxidative hemolysis inhibition assay (OxHLIA), which results were expressed as IC50 values (μg/mL) for a time interval (Δt) of 60 min and translate to the extract concentration required to keep 50% of the sheep erythrocyte population intact for 60 min. Trolox (0.39–50 μg/mL) was used as a positive control in both assays.
2.6.4 Cytotoxic activity
The cytotoxic activity of the anthocyanin-rich extracts (0.125–8 mg/mL) was assessed by the sulforhodamine B (SRB) assay [24, 27] on human tumor cell lines (NCI-H460 (non-small lung cancer cells)); Caco-2 (colon cancer cells) and MCF-7 (breast cancer cells); and AGS (gastric cancer cells)); and on one non-tumor porcine liver cell line (PLP2). The results were expressed as GI50 values (μg/mL) and translate the extract concentration that inhibits 50% of cell proliferation [28]. Ellipticine (0.38–12.3 μg/mL) was used as a positive.
2.6.5 Anti-inflammatory activity
The anti-inflammatory activity of the anthocyanin-rich extracts (0.125–8 mg/mL) was measured based on the inhibition of nitric oxide (NO) production by lipopolysaccharide-stimulated murine macrophage RAW 264.7 cells. The Griess reagent kit was used for nitrite quantification in the medium. The results were expressed as IC50 values (μg/mL), which translate the extract concentration that causes 50% of NO production inhibition [24]. Dexamethasone (7.65–980 μg/mL) was used as a positive control.
2.6.6 Antimicrobial activity
The antibacterial and antifungal activities of the anthocyanin-rich extracts (0.0078–10 mg/mL) were assessed by serial microdilution methods previously described [24]. Enterobacter cloacae, Escherichia coli, Pseudomonas aeruginosa, Salmonella enterocolitica, Yesinia enterocolitica, Bacillus cereus, Staphylococcus aureus, and Listeria monocytogenes were the tested bacteria, while Aspergillus brasiliensis and Aspergillus fumigatus were the tested fungi. Results were expressed as minimum inhibitory concentration (MIC). Ampicillin (0.078–20 mg/mL) and ketoconazole (0.039–10 mg/mL) were used as positive controls.
2.6.7 Statistical analysis
Optimal responses, color, and bioactivity analyses were evaluated in triplicate and the results are expressed as mean ± standard deviation. Statistical differences between the two samples were assessed by a Student’s t-test (p < 0.05) using R software, version 4.0.3.
3 Results and discussion
3.1 Optimized conditions for anthocyanin extraction
3.1.1 Experimental data
To optimize the extraction of anthocyanins from S. odorifera fruit epicarp by HAE and UAE techniques, the influence of three factors was evaluated on the extraction yield and total anthocyanin content in the obtained extracts. The experimental results for the 20 runs of the design are presented in Table 2. For HAE, the extraction yield (Y1) varied between 17.70 and 25.03% (w/w), with the lowest response observed in the run 14 extract, which combined median-low values of time and temperature with the highest percentage of ethanol, and the highest response was achieved with run 17. For UAE, the Y1 response variation was higher than that observed for HAE, ranging from 10.73 to 29.09% (w/w). As observed for HAE, the lowest UAE yield was achieved with run 14 and the highest with run 12.
Regarding the total anthocyanin content (TAC) in the extracts (Y2), the experimental results obtained with HAE varied from 105.00 to 304 mg/g E. With this extraction system, the highest concentration was achieved with medium-high time and temperature levels, with medium-low ethanol percentages (run 4: 1, 1 −1), and the minimum was observed in run 14, with the highest ethanol percentage (0, 0, 1.68). In turn, a greater variation in the results was observed in the extracts obtained by UAE. The lowest anthocyanin extraction (Y2 = 19.71 mg/g E) was occasioned by the combination of medium-low values for all factors (run 1: −1, −1, −1), while the maximum recovery (Y2 = 212.00 mg/g E) was achieved with run 12 which applied the highest ultrasonic power (0, 1.68, 0).
3.1.2 Fitting of the mathematical models
To describe the effects of the factors and the selected responses, mathematical models were constructed based on the experimental results shown in Table 2. The data were fitted to a second-order polynomial equation using Design-Expert. The significance of the factors and statistical parameters of the models’ fitting procedure was evaluated using an ANOVA (Tables S2–S5 in supplementary material). Only significant terms (at a 95% confidence level) were considered in the mathematical models fitting for HAE and UAE (which significance level and standard deviation of the model regression coefficients are provided in Table S6), which are presented in terms of coded values below:
For HAE:
For UAE:
The mathematical models expressed in terms of natural values are presented in the supplementary material. As shown in Table 2, all models were significant (p-values ≤ 0.0003) and had a non-significant lack-of-fit (p-values ≤ 0.2449). For the R2 and R2 adj coefficients, the models built for UAE (Eqs. 4 and 5) showed higher fitting values (R2 = 0.9296–0.9516 and R2 adj = 0.8971–0.9292) than the HAE models (Eqs. 2 and 3, R2 = 0.7364–0.8564 and R2 adj = 0.6661–0.7901). The adequate precision measures the signal-to-noise ratio, and considering that a good adequate precision must be higher than 4 [23], all models showed acceptable values. Therefore, these predictive models can be used to navigate the design space. In general, the models built for the UAE process showed a better fit than HAE models, which can be seen in Fig. 1, where the model-predicted values for the responses obtained by this extraction method are closer to the experimental values.
According to Eq. 2, which translates the HAE trends, all variables showed significant effects; time and temperature contributed positively to the extraction with linear effects, while the ethanol percentage mainly caused a negative quadratic effect. Due to this quadratic term, the non-significant (p-value > 0.05) linear term of this factor was kept in the model to ensure its hierarchy. For UAE, Eq. 4 includes the linear effects of the three factors. Among them, the variation in ultrasonic power had a greater impact on the response due to the value of its parametric coefficient (b2 = 3.75) being greater than the others (b1 = 0.83 and b3 = 1.71). The ethanol percentage also showed highly impacting negative linear and quadratic effects on Y1.
For the total anthocyanin content (Y2), according to the HAE mathematical model (Eq. 3), the response depended on all factors, and time and temperature had positive linear effects. However, the time showed a significant negative quadratic effect, while both linear and quadratic effects of solvent were negative (thus, lower ethanol percentages were favorable). Furthermore, the negative effect of the interaction between temperature and solvent (b23 = −26.09) had a high weight in the response. For UAE, the mathematical model (Eq. 5) that describes the influence of the extraction variables on Y2 is composed of linear, quadratic and interaction effects. Linear effects were significant for all variables (Table S6), but ultrasonic power (b2 = 39.61) had by far the greatest impact on the response. Furthermore, its interaction with time (b12 = −9.14) and solvent (b23 = −26.10) negatively influenced the recovery of anthocyanins.
3.1.3 Optimal conditions for extraction yield
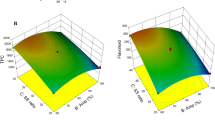
From the models described above, the 2D and 3D plots embedded in Figs. 2 and 3, respectively, were built to visually illustrate the individual and combined effects of the factors on the target responses. From these plots, it is possible to visualize the extraction patterns that lead to the best response values. When analyzing the HAE plots, it was concluded that there was an increase in the extraction yield when the time and temperature values tended to be higher, and the maximum response was achieved by combining these variables at their tested maximum (Fig. 2). High temperatures can benefit the extraction of compounds from plant cells in several ways. Firstly, increasing the temperature reduces the viscosity of the extraction solvent, which facilitates its penetration into plant cells. Furthermore, an increase in temperature can destabilize cell membranes, promoting more effective release of solutes. High temperature also increases the solubility of target compounds, thereby making the extraction process more efficient [29]. Regarding ethanol percentage, low extraction yields were obtained at the two extreme proportions of ethanol (0% and 100%), and medium percentages (25–75% ethanol) were suitable for better results due to the negative quadratic effect of this variable. The use of solvents with different polarities extracts a greater variety of compounds, which may justify the observed trends. For example, water is more effective in the extraction of glycosides, while aglycones can be better recovered with ethanol [30].
Individual effect of the process factors represented in 2D response graphs for extraction yield (Y1) and total anthocyanin content (Y2) obtained by heat-assisted extraction (HAE) and ultrasound-assisted (UAE). In each graph, the red cross indicates the optimum point and the unplotted factors were kept constant at their optimum
For UAE, Y1 benefited from an increase in processing time up to approximately 30 min, after which there was a slight decrease in the response caused by the negative quadratic effect of this variable. The increase in power led to a better response, while a medium ethanol percentage was required for such an increase. The interaction of these variables (Fig. 3) promoted the UAE by combining power > 129 W and solvent < 75% ethanol. For UAE, the response surface combining time and power showed that high extract yields (Y1) occur with power > 376 W and time > 13 min, and higher yields can be reached with extraction time greater than 24 min of sonication at 500 W. These findings agree with previously documented effects for UAE of phenolic compounds, in which higher power settings potentiated higher concentrations of these secondary metabolites. This outcome can be attributed mainly to the impact of cavitation waves, which convey energy capable of inducing cell rupture, enabling greater mass transfer to the solvent [31]. The model (Eqs. 2 and 4) predicted extraction conditions that lead to an absolute maximum yield are presented below:
-
For HAE: t = 90 min, T = 90 °C, and S = 53% ethanol (v/v), yielding 26 ± 1% (w/w) of extract.
-
For UAE: t = 30 min, P = 500 W, and S = 42% ethanol (v/v), yielding of 29 ± 1% (w/w) of extract.
The UAE allowed a higher extraction yield than HAE with a shorter time and ethanol percentage. Other studies on obtaining anthocyanins from fruits or their waste also found that the UAE has better efficiency than HAE. For example, from Myrciaria jaboticaba (Vell.) Berg. epicarp, the maximum yield obtained by UAE was 59.0% and by HAE was 56.6% [14]; from Arbutus unedo L. fruit, UAE provided a yield of 85% against 75% for HAE [27]. The better efficiency of UAE may be a consequence of the disruption of plant cell walls caused by ultrasonic cavitation, thus allowing a better mass transfer and higher yields [8]. It is noteworthy that UAE exhibited lower selectivity for anthocyanins than HAE since the extract obtained by UAE contained molecules other than anthocyanins in its composition (possibly simple sugars and fibers), which were not investigated in this work.
3.1.4 Optimal conditions for total anthocyanin content
The effects of the process factors on anthocyanin recovery are graphically shown in Figs. 2 and 3. For HAE, the TAC yield was promoted by the increase in time up to ≈ 68 min and decreased after this point possibly due to the degradation of these compounds. The increase in temperature had a positive effect on the process when combined with lower ethanol percentages, while when combined with more than 25% ethanol, the yield was very low (Fig. 3). Although anthocyanins are generally considered thermosensitive compounds, the optimal conditions determined for HAE suggest that higher temperatures can enhance the recovery of these pigments. This result agrees with previous findings on the extraction of anthocyanins from other sources [29, 32,33,34,35]. Elevated temperatures boost the mass transfer rate and can also lead to the inactivation of enzymes, such as polyphenol oxidase and peroxidase, naturally present in plant tissues, which could otherwise degrade anthocyanins. Therefore, striking the right extraction temperature may be crucial for the successful recovery of anthocyanins [30, 33, 36]. In turn, it is important to highlight that higher concentrations of ethanol can reduce the extraction solvent polarity, resulting in a reduced solubility of anthocyanins. Furthermore, ethanol can potentially lead to protein denaturation, hindering the solvent's ability to penetrate plant cells [36]. As previously discussed, a mix of water and an organic solvent may offer greater efficiency in extracting various compounds. Regarding anthocyanin recovery, various studies have demonstrated that a mixture of ethanol and water ranging from 20% to 70% can significantly enhance the extraction process for these compounds [30, 36]. For UAE, although the variable time by itself did not have a significant impact (p-value > 0.05) on the response, the performance was better when sonicating the mixture at high power for short periods, while longer processing at high power caused a decrease in TAC. On the other hand, for low ultrasonic power, it was necessary to apply more time to promote pigment extraction. Furthermore, to obtain the maximum response, this condition had to be associated with less than 50% ethanol. Considering these circumstances, the optimal conditions and their respective response values predicted by the model Eqs. 3 and 5 are presented below:
-
For HAE: t = 63 min, T = 90 °C, and S = 20% ethanol (v/v), yielding 315 ± 27 mg anthocyanins/g of extract; and
-
For UAE: t = 11 min, P = 446 W, and S = 20% ethanol (v/v), yielding 239 ± 11 mg anthocyanins/g of extract.
In general, the HAE of anthocyanins from S. odorifera fruit epicarp originated an extract with a higher anthocyanin concentration, thus being more selective for the target anthocyanins (Table S1). On the other hand, a higher extract yield was reached using UAE (~ 10% more). Additionally, the lower anthocyanin recovery by UAE could be related to the utilization of high ultrasound power. While this factor enhanced the extract yield, it also poses a risk of potential degradation of anthocyanins. This degradation primarily arises from the production of OH radicals through the cavitation effect, which can initiate oxidation mechanisms. Moreover, the high energy generated by cavitation waves may result in the cleavage of covalent bonds, causing ring openings and the formation of chalcones [31, 37]. Sun et al. [38] and Yao et al. [39] investigated the degradation rate of pelargonidin-3-glucoside (P3G) and cyanidin-3-O-glucoside (C3G) in strawberry and blueberry juice, respectively, subjected to ultrasound treatments. With 200 W treatment, the half-life (t ½) of C3G was relatively longer compared to that of P3G (t ½ = 51.727 and 41.014, respectively). The half-life of both anthocyanins decreased significantly with increasing ultrasonic power. For instance, at 300 W, the t ½ of C3G was 19.308 min, while for P3G it was 16.1950 min. These values further decreased at 500 W, with C3G having a t ½ of 11.768 min and P3G having a t ½ of 10.3147 min. The authors concluded that in the ultrasound process, cavitation waves play a major role in the degradation of anthocyanins. These waves generate high temperature and pressure, leading to the pyrolysis of water, which requires a significant amount of energy and results in the formation of free radicals. These free radicals, due to their high reactivity, cause an oxidizing effect and contribute to the reduction of anthocyanins [38, 39].
3.1.5 Global optimal condition
Global optimal conditions were determined to obtain the settings that make it possible to simultaneously have a high extraction yield and TAC. In this way, both mathematical models were combined by maximizing the two response variables as far as possible. The global optimal conditions obtained for HAE and UAE are presented below:
-
For HAE: t = 62 min; T = 90 °C, and S = 27% ethanol (v/v), yielding 24 ± 1% (w/w) of extract (Y1) and 305 ± 27 mg TAC/g E (Y2).
-
For UAE: t = 23 min, P = 500 W, and S = 40% ethanol (v/v), yielding 28 ± 1% (w/w) of extract (Y1) and 234 ± 11 mg TAC/g E (Y2).
In general, a higher amount of extract can be obtained from S. odorifera fruit epicarp using the global UAE conditions. This extraction technology has demonstrated certain advantages over HAE for the extraction of intracellular solutes, including faster processing times, less solvent consumption, and improved mass transfer. These UAE requirements result from its capacity to disrupt plant tissue and cell barriers with ultrasonic waves, enabling solvent penetration and subsequent solute recovery. Nonetheless, it can be concluded that HAE was able to recover approximately 74 mg anthocyanins/dry epicarp. In contrast, UAE enabled the recovery of about 65.5 mg/g dry epicarp using only one-sixth the time required compared to HAE. Therefore, HAE may be suitable for obtaining an extract with greater anthocyanin purity, while UAE may be more indicated for producing a greater extract weight. In any case, the cost-benefits must be considered.
3.2 Experimental validation of the mathematical models
Additional extractions were conducted under the optimal global conditions established for HAE and UAE in order to verify the predictive accuracy of theoretical models. With HAE, it was possible to achieve an extraction yield of 23 ± 1% (w/w) and 281±2 mg TAC/g E, while the UAE system yielded 26 ± 2% (w/w) and 200 ± 3 mg TAC/g E. According to the post-analysis performed in the Design-expert software at a 95% confidence interval, these values are within the range of predicted responses of the models. Its predictive ability was thus validated. Regarding the error between predicted and experimental values, for HAE, the MAPE values obtained for the Y1 and Y2 models were equal to 5.45% and 8.48%, respectively. For the UAE models, the values were higher, specifically 6.15% and 13.51% for Y1 and Y2, respectively. This indicates that the models fitted to describe the anthocyanin extraction behavior for UAE may be less accurate than the HAE models.
By comparing the experimental results obtained with the two extraction methods, it was concluded that UAE produced an apparently superior value for extract yield. However, the statistical analysis revealed the absence of a statistically significant variation (p-value = 0.1666) between the results obtained by the two methods. On the other hand, for the TAC quantified in the extracts, with HAE, it was possible to obtain a significantly enriched extract (p-value = 0.0006). Nonetheless, when the extract yield and TAC values achieved by each method are converted into milligrams of anthocyanins per gram of dry epicarp, it was possible to recover 64.1±4.5 mg/g using HAE, while UAE yielded 52.6±4.5 mg/g. Still, both methods demonstrate comparable efficiency (p-value = 0.1294) for extracting the target pigments.
Under optimized conditions, both extraction methods allowed obtaining a higher anthocyanin content from S. odorifera fruit epicarp than others described in the literature. For example, a dynamic maceration conducted at room temperature with 80% ethanol allowed the recovery of 111 mg TAC/g extract [24], while a UAE (40 kHz) of 15 min with 60% ethanol resulted in 68 mg cyanidin equivalents/g extract [16].
3.3 Color of the anthocyanin-rich extracts
Table 3 presents the color parameters and the RGB color of the extracts obtained under the global condition specified for HAE and UAE. The HAE extract exhibited a darker shade (lower L* value) compared to the UAE extract and it also had a more yellowish tone (higher b* value). However, both extracts displayed the same redness, as indicated by the non-significant (p > 0.05) difference in a* values. The RGB representation confirmed that both anthocyanin-rich extracts shared a similar color towards black. This coloration could be attributed to the high concentration of cyanidin-O-deoxyhexosyl-hexoside, a compound known for contributing to the black color observed in some flowers, fruits, vegetables, and grains [40].
3.4 Bioactivity of the anthocyanin-rich extracts
The anthocyanin-rich extracts obtained under the global conditions determined for HAE and UAE were tested for their in vitro bioactive properties and the results are shown in Table S7. Regarding the antioxidant activity assessed by the thiobarbituric acid reactive substances (TBARS) formation inhibition assay, both extracts showed a similar capacity to inhibit lipid peroxidation, which was slightly higher activity than that (48.2 ± 0.2 μg/mL) determined in a previous study [24]. Regarding the OxHLIA bioassay, the HAE extract concentration required to inhibit 50% of oxidative hemolysis (IC50 value) for a 60 min Δt was higher than the UAE extract concentration. Thus, a significantly better antihemolytic activity was presented by the extract obtained by UAE, which was comparator to that (IC50 value = 27±1 μg/mL) of an extract previously obtained by maceration in 80% ethanol at room temperature [24]. Therefore, it can be concluded that the extract with the highest concentration of anthocyanins does not necessarily have better antioxidant activity. Previously, Jaramillo et al. [17] evaluated the antioxidant activity of compounds isolated from S. odorifera fruit epicarp through the TEAC and ABTS chemical methods and found that quercetin derivatives were the constituents with the greatest antioxidant effect of this plant material.
Regarding the other tested bioactivities, the extracts did not show anti-inflammatory, cytotoxic, or antimicrobial activity within the tested concentrations (Table S7). These results are consistent with a previous study [23], except for the antibacterial activity, as the authors showed that the S. odorifera fruit epicarp extract obtained with 80% ethanol had the capacity to inhibit the growth (MIC = 1.1 mg/mL) and kill the inoculum (MBC = 2.2 mg/mL) of E. cloacae, E. coli, B. cereus, L. monocytogenes, and S. aureus. Therefore, the increase in the concentration of anthocyanins in the extract of S. odorifera fruit epicarp does not seem to be related to the bioactivities evaluated in this work (except for the antioxidant activity), thus corroborating the previous results of Jaramillo et al. [17].
4 Conclusion
The HAE and UAE processes were successfully optimized to extract anthocyanins from S. odorifera fruit epicarp. HAE offered an extract with higher anthocyanin content but required a longer processing time than UAE (62 vs. 23 min, respectively). On the other hand, it was possible to reach a slightly higher extract yield with UAE than with HAE (28 vs. 25% (w/w), respectively). As a process intensification factor, HAE needed 90 °C and UAE 500 W of ultrasonic power. Regarding the solvent, both methods required medium-low ethanol percentages; HAE required a lower percentage than UAE (27 vs. 40% (v/v), respectively) to simultaneously maximize extract weight and total anthocyanin content. The color of the lyophilized anthocyanin-rich extracts was similar, but that obtained by HAE was darker and yellowish. Although this extract also had a higher anthocyanin content, the UAE-obtained extract showed better in vitro antioxidant activity. However, none of the extracts showed antimicrobial, anti-inflammatory, and cytotoxic effects, evidencing the lack of toxicity for the tested normal cells.
Overall, both extraction methods produce extracts with high-anthocyanin concentrations. Although HAE appears to be more effective, the cost-benefit associated with the processing conditions of each method must be considered when aiming its exploitation by the industry. Further studies will be important to analyze the cost-benefits of the studied methodologies and to explore the impact of additional factors such as particle size, stirring rate, and pH, among others. The investigation of alternative extraction methodologies like pressurized liquids, supercritical fluids, pulsed electric fields, or other non-thermal disruption technologies would also be interesting, as well as the implementation of purification methods to obtain extracts with higher anthocyanin content.
This study also showed that the fruit epicarp of S. odorifera has the potential to be used as a renewable source of anthocyanins. Therefore, the exploitation of its coloring extracts by the food and cosmetic industries, among others that use anthocyanins in their products, could be a future-oriented strategy to promote the sustainable use of this underexploited by-product.
References
Amorim A, Silva VL, Paulo José do Amaral Sobral (2023) Food processing: an overview on links between safety, security, supply chains, and NOVA classification. CLCB 5:100047. https://doi.org/10.1016/J.CLCB.2023.100047
Ghosh S, Sarkar T, Chakraborty R et al (2022) Nature’s palette: an emerging frontier for coloring dairy products. Crit Rev Food Sci Nutr 10:2117785
Jurić S, Jurić M, Król-Kilińska Ż et al (2020) Sources, stability, encapsulation and application of natural pigments in foods. Food Rev Int 38:1735–1790. https://doi.org/10.1080/87559129.2020.1837862
Rodríguez-Mena A, Ochoa-Martínez LA, González-Herrera SM et al (2023) Natural pigments of plant origin: classification, extraction and application in foods. Food Chem 398:133908. https://doi.org/10.1016/J.FOODCHEM.2022.133908
Dey S, Nagababu BH (2022) Applications of food color and bio-preservatives in the food and its effect on the human health. Food Chem Adv 1:100019. https://doi.org/10.1016/J.FOCHA.2022.100019
Oplatowska-Stachowiak M, Elliott CT (2016) Food colors: existing and emerging food safety concerns. Crit Rev Food Sci Nutr 57:524–548. https://doi.org/10.1080/10408398.2014.889652
Amchova P, Kotolova H, Ruda-Kucerova J (2015) Health safety issues of synthetic food colorants. Regul Toxicol Pharmacol 73:914–922. https://doi.org/10.1016/j.yrtph.2015.09.026
Guo Y, Zhang H, Shao S et al (2022) Anthocyanin: a review of plant sources, extraction, stability, content determination and modifications. Int J Food Sci Technol 57:7573–7591. https://doi.org/10.1111/IJFS.16132
Câmara JS, Locatelli M, Pereira JAM et al (2022) Behind the scenes of anthocyanins - from the health benefits to potential applications in food, pharmaceutical and cosmetic fields. Nutrients 14:5133. https://doi.org/10.3390/NU14235133
Qi Q, Chu M, Yu X et al (2023) Anthocyanins and proanthocyanidins: chemical structures, food sources, bioactivities, and product development. Food Rev Int 39:4581–4609. https://doi.org/10.1080/87559129.2022.2029479
Tan J, Han Y, Han B et al (2022) Extraction and purification of anthocyanins: a review. J Agric Food Res 8:100306. https://doi.org/10.1016/J.JAFR.2022.100306
Benucci I, Lombardelli C, Mazzocchi C, Esti M (2022) Natural colorants from vegetable food waste: recovery, regulatory aspects, and stability—a review. Compr Rev Food Sci Food Saf 21:2715–2737. https://doi.org/10.1111/1541-4337.12951
de la Luz Cádiz-Gurrea M, del Carmen Villegas-Aguilar M, Leyva-Jiménez FJ et al (2020) Revalorization of bioactive compounds from tropical fruit by-products and industrial applications by means of sustainable approaches. Food Res Int 138:109786. https://doi.org/10.1016/J.FOODRES.2020.109786
Albuquerque BR, Pinela J, Barros L et al (2020) Anthocyanin-rich extract of jabuticaba epicarp as a natural colorant: optimization of heat- and ultrasound-assisted extractions and application in a bakery product. Food Chem 316:126364. https://doi.org/10.1016/j.foodchem.2020.126364
De Paula Filho GX, Barreira TF, Pinheiro SS et al (2015) “Melão croá” (Sicana sphaerica Vell.) and “maracujina” (Sicana odorifera Naud.): chemical composition, carotenoids, vitamins and minerals in native fruits from the Brazilian Atlantic forest. Fruits 70:341–349. https://doi.org/10.1051/FRUITS/2015035
Mereles L, Caballero S, Burgos-Edwards A et al (2021) Extraction of total anthocyanins from Sicana odorifera black peel fruits growing in Paraguay for food applications. Appl Sci 11:6026. https://doi.org/10.3390/APP11136026/S1
Jaramillo K, Dawid C, Hofmann T et al (2011) Identification of antioxidative flavonols and anthocyanins in Sicana odorifera fruit peel. J Agric Food Chem 59:975–983. https://doi.org/10.1021/JF103151N/ASSET/IMAGES/LARGE/JF-2010-03151N_0002.JPEG
Correa L, Gamarra M, Sanabria K et al (2022) Obtaining integral kurugua flour with antioxidant potential as ingredient foodstuffs. In: IV Conference Ia ValSe-Food CYTED and VII Symposium Chia-Link. MDPI, p 228
Shirsath SR, Sonawane SH, Gogate PR (2012) Intensification of extraction of natural products using ultrasonic irradiations—a review of current status. Chem Eng Process 53:10–23. https://doi.org/10.1016/J.CEP.2012.01.003
Tena N, Asuero AG (2022) Up-to-date analysis of the extraction methods for anthocyanins: principles of the techniques, optimization, technical progress, and industrial application. Antioxidants 11:286. https://doi.org/10.3390/ANTIOX11020286
Das P, Nayak PK, Kesavan R krishnan (2022) Ultrasound assisted extraction of food colorants: Principle, mechanism, extraction technique and applications: a review on recent progress. Food Chem Adv 1:100144. https://doi.org/10.1016/J.FOCHA.2022.100144
Nguyen TL, Ora A, Häkkinen ST et al (2023) (2023) Innovative extraction technologies of bioactive compounds from plant by-products for textile colorants and antimicrobial agents. Biomass Convers Biorefin 1:1–30. https://doi.org/10.1007/S13399-023-04726-4
Pantuzza Silva GF, Pereira E, Melgar B et al (2021) Eggplant fruit (Solanum melongena L.) and bio-residues as a source of nutrients, bioactive compounds, and food colorants, using innovative food technologies. Appl Sci 11:151. https://doi.org/10.3390/APP11010151
Albuquerque BR, Dias MI, Pereira C et al (2021) Valorization of Sicana odorifera (Vell.) Naudin epicarp as a source of bioactive compounds: chemical characterization and evaluation of its bioactive properties. Foods 10:700. https://doi.org/10.3390/FOODS10040700
Silva AR, Ayuso M, Oludemi T et al (2024) Response surface methodology and artificial neural network modeling as predictive tools for phenolic compounds recovery from olive pomace. Sep Purific Technol 330:125351. https://doi.org/10.1016/j.seppur.2023.125351
Takebayashi J, Iwahashi N, Ishimi Y, Tai A (2012) Development of a simple 96-well plate method for evaluation of antioxidant activity based on the oxidative haemolysis inhibition assay (OxHLIA). Food Chem 134:606–610. https://doi.org/10.1016/j.foodchem.2012.02.086
Skehan P, Storeng R, Scudiero D et al (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–1112. https://doi.org/10.1093/JNCI/82.13.1107
Lockowandt L, Pinela J, Roriz CL et al (2019) Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: the blue flowers and the unexplored non-edible part. Ind Crops Prod 128:496–503. https://doi.org/10.1016/j.indcrop.2018.11.059
Antony A, Farid M (2022) Effect of temperatures on polyphenols during extraction. Appl Sci 12:2107. https://doi.org/10.3390/app12042107
Boateng ID, Mustapha A, Kuehnel L et al (2023) From purple corn waste (pericarp) to polyphenol-rich extract with higher bioactive contents and superior product qualities using two-step optimization techniques. Ind Crops Prod 200:116871. https://doi.org/10.1016/j.indcrop.2023.116871
Dzah CS, Duan Y, Zhang H et al (2020) The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: a review. Food Biosci 35:100547. https://doi.org/10.1016/j.fbio.2020.100547
López CJ, Caleja C, Prieto MA et al (2018) Optimization and comparison of heat and ultrasound assisted extraction techniques to obtain anthocyanin compounds from Arbutus unedo L. fruits. Food Chem 264:81–91. https://doi.org/10.1016/j.foodchem.2018.04.103
Ijod G, Musa FN, Anwar F et al (2022) Thermal and nonthermal pretreatment methods for the extraction of anthocyanins: a review. J. Food Process Pres 46. https://doi.org/10.1111/jfpp.17255
Dranca F, Oroian M (2016) Optimization of ultrasound-assisted extraction of total monomeric anthocyanin (TMA) and total phenolic content (TPC) from eggplant (Solanum melongena L.) peel. Ultrason Sonochem 31:637–646. https://doi.org/10.1016/j.ultsonch.2015.11.008
Fan G, Han Y, Gu Z, Chen D (2008) Optimizing conditions for anthocyanins extraction from purple sweet potato using response surface methodology (RSM). LWT 41:155–160. https://doi.org/10.1016/j.lwt.2007.01.019
Lianza M, Marincich L, Antognoni F (2022) The greening of anthocyanins: Eco-friendly techniques for their recovery from agri-food by-products. Antioxidants 11:2169. https://doi.org/10.3390/antiox11112169
Boateng ID, Kumar R, Daubert CR et al (2023) Sonoprocessing improves phenolics profile, antioxidant capacity, structure, and product qualities of purple corn pericarp extract. Ultrason Sonochem 95:106418. https://doi.org/10.1016/j.ultsonch.2023.106418
Sun J, Mei Z, Tang Y et al (2016) Stability, antioxidant capacity and degradation kinetics of pelargonidin-3-glucoside exposed to ultrasound power at low temperature. Molecules 21:1109. https://doi.org/10.3390/MOLECULES21091109
Yao GL, Ma XH, Cao XY, Chen J (2016) Effects of power ultrasound on stability of cyanidin-3-glucoside obtained from blueberry. Molecules 21:1564. https://doi.org/10.3390/MOLECULES21111564
Ahmad S, Chen J, Chen G et al (2022) Why black flowers? An extreme environment and molecular perspective of black color accumulation in the ornamental and food crops, 885176. Front Plant Sci 13. https://doi.org/10.3389/FPLS.2022.885176/BIBTEX
Availability of data and materials
The article presents all data relevant to this study.
Funding
Open access funding provided by FCT|FCCN (b-on). National funding was provided by the Foundation for Science and Technology (FCT) (PI), through the scientific employment program-contract for the contracts of J Pinela (CEECIND/01011/2018), C Pereira (CEEC Institutional), RC Calhelha (CEEC Institutional), S Heleno (CEEC Institutional), and L Barros (CEEC Institutional), and the research grants of BR Albuquerque (SFRH/BD/136370/2018 and COVID/BD/152908/2022) and I Oliveira (SFRH/BD/06017/2020). FCT also provided financial support through national funds FCT/MCTES (PIDDAC) to CIMO (UIDB/00690/2020 and UIDP/00690/2020) and SusTEC (LA/P/0007/2020).
Author information
Authors and Affiliations
Contributions
BRA: methodology, investigation, formal analysis, experimental design, data analysis, and writing—original draft. JP: experimental design, methodology, data analysis, validation, and writing—review and editing. CP: methodology and data analysis. RCC: formal analysis and data analysis. IO: formal analysis. SH: methodology, data analysis, and validation. MBPPO: conceptualization, supervision, and writing—review and editing. LB: conceptualization, supervision, project administration, and writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
All authors agreed on the publication of this research work.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 40 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Albuquerque, B.R., Pinela, J., Pereira, C. et al. Optimization and comparison of heat- and ultrasound-assisted extraction methods for anthocyanin recovery from Sicana odorifera fruit epicarp. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-05097-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-05097-6