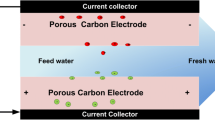
Abstract
Capacitive deionization (CDI), an emerging and promising new method, is being utilized to remove ionic and polarizable species from water. The development of effective electrode materials has been the main driving force behind the increased interest in the CDI system for water purification because it is simple to use, affordable, and environmentally friendly compared to other traditional methods. Carbon electrodes made from biomass are often considered as low-cost, high-performance energy storage materials due to their abundance, simple synthesis methods, and high specific surface area. Herein, we developed materials from biowastes of banana plant leaves (B) and java black (J) that were hydrothermally carbonized. These materials were then chemically treated with KOH at a 1:4 ratio to produce extremely porous activated carbon from the biowastes of banana plant leaves (AC-B) and java black (AC-J). The surface areas of AC-B and AC-J were 84.155 and 295.517 m2 g−1 respectively, with high mesoporous properties. Poly(vinyl alcohol) (PVA) was employed as a binder to bind the synthetic activated carbons like AC-B and AC-J together in order to make electrodes. Despite the fact that PVA is hydrophilic by nature, the hydrophobic glutaraldehyde (GA) was used to cross-link the PVA molecules in order to increase the durability of the carbon material and produced AC-B-PVA-GA and AC-J-PVA-GA electrodes. X-ray diffraction, scanning electron microscopy, Fourier-transform infrared spectroscopy, Brunauer-Emmett-Teller, and cyclic voltammetry were used to characterize the synthesized carbon materials. The newly developed CDI electrodes, which demonstrated a variety of electrosorption capacities at different applied potentials, were used to investigate the effectiveness of the desalination process. The developed electrodes showed good stability under shear conditions, and the CDI process was repeatable and stable after around 10 electrosorption cycles. For example, the specific capacities of the AC-B-PVA-GA and AC-J-PVA-GA electrodes were 31.01 and 40.78 F/g, respectively in a 0.5 M NaCl aqueous solution. At 1.2 V, it was found that the AC-B-PVA-GA and AC-J-PVA-GA electrodes removed 2.41 and 3.64 mg g−1 of salt, respectively. The findings obtained encourage the development of CDI systems for desalination and water treatment and provide essential information on ion electrosorption. It also demonstrates an effective method for environmental cleanup utilizing a basic CDI system.








Similar content being viewed by others
Data availability
Not applicable.
References
Elimelech M, Phillip WA (2011) The future of seawater desalination: energy, technology, and the environment. Science 333(6043):712
Qiu Y, Zhang C, Zhang R, Liu Z, Yang H, Qi S, Hou Y, Wen G, Liu J, Wang D (2023) Integration of pore structure modulation and B, N co-doping for enhanced capacitance deionization of biomass-derived carbon. Green Energy Environ 8(5):1488–1500. https://doi.org/10.1016/j.gee.2023.01.005
Duan S, Zhao Y, Jiang S, Yang Z, Ju Y, Chen C, Huang L, Chen F (2022) Potential of zero charge regulating highly selective removal of nitrate anions through capacitive deionization. Chem Eng J 442:136287. https://doi.org/10.1016/j.cej.2022.136287
Tan G, Wan S, Mei S-C, Gong B, Qian C, Chen J-J (2023) Boosted brackish water desalination and water softening by facilely designed MnO2/hierarchical porous carbon as capacitive deionization electrode. Water Res X 19:100182. https://doi.org/10.1016/j.wroa.2023.100182
Caldera U, Breyer C (2023) Afforesting arid land with renewable electricity and desalination to mitigate climate change. Nat Sustain 6(5):526–538. https://doi.org/10.1038/s41893-022-01056-7
Son M, Pothanamkandathil V, Yang W, Vrouwenvelder JS, Gorski CA, Logan BE (2020) Improving the thermodynamic energy efficiency of battery electrode deionization using flow-through electrodes. Environ Sci Technol 54(6):3628–3635. https://doi.org/10.1021/acs.est.9b06843
Zhang B, Boretti A, Castelletto S (2022) Mxene pseudocapacitive electrode material for capacitive deionization. Chem Eng J 435:134959. https://doi.org/10.1016/j.cej.2022.134959
Zhang L, Mishra D, Zhang K, Perdicakis B, Pernitsky D, Lu Q (2020) Electrokinetic study of calcium carbonate and magnesium hydroxide particles in lime softening. Water Res 186:116415. https://doi.org/10.1016/j.watres.2020.116415
Chae H-R, Lee J, Lee C-H, Kim I-C, Park P-K (2015) Graphene oxide-embedded thin-film composite reverse osmosis membrane with high flux, anti-biofouling, and chlorine resistance. J Membr Sci 483:128–135. https://doi.org/10.1016/j.memsci.2015.02.045
Chen R, Deng X, Wang C, Du J, Zhao Z, Shi W, Liu J, Cui F (2022) A newly designed graphite-polyaniline composite current collector to enhance the performance of flow electrode capacitive deionization. Chem Eng J 435:134845. https://doi.org/10.1016/j.cej.2022.134845
Chen W, He X, Jiang Z, Li B, Li X-Y, Lin L (2023) A capacitive deionization and electro-oxidation hybrid system for simultaneous removal of heavy metals and organics from wastewater. Chem Eng J 451:139071. https://doi.org/10.1016/j.cej.2022.139071
Xu L, Tang L, Peng S, Mao Y, Wu D (2022) Magnetic array for efficient and stable flow-electrode capacitive deionization. Chem Eng J 446:137415. https://doi.org/10.1016/j.cej.2022.137415
Sufiani O, Tanaka H, Teshima K, Machunda RL, Jande YAC (2020) Enhanced electrosorption capacity of activated carbon electrodes for deionized water production through capacitive deionization. Sep Purif Technol 247:116998. https://doi.org/10.1016/j.seppur.2020.116998
Wang H, Mi X, Li Y, Zhan S (2020) 3D graphene-based macrostructures for water treatment. Adv Mater 32(3):1806843. https://doi.org/10.1002/adma.201806843
Zhang H, Zhang F, Wei Y, Miao Q, Li A, Zhao Y, Yuan Y, Jin N, Li G (2021) Controllable design and preparation of hollow carbon-based nanotubes for asymmetric supercapacitors and capacitive deionization. ACS Appl Mater Interfaces 13(18):21217–21230. https://doi.org/10.1021/acsami.1c01137
Zhao Y, Li K, Sheng B, Chen F, Song Y (2023) Nitrogen-doped core-shell mesoporous carbonaceous nanospheres for effective removal of fluorine in capacitive deionization. Water 15(3):608
Kong W, Ge X, Zhang Q, Wang Y, Wang Y, Lu J, Zhang M, Kong D, Feng Y (2022) Ultrahigh content boron and nitrogen codoped hierarchically porous carbon obtained from biomass byproduct okara for capacitive deionization. ACS Omega 7(51):48282–48290. https://doi.org/10.1021/acsomega.2c06449
Zhang Y, Bu X, Wang Y, Hang Z, Chen Z (2024) Hierarchically porous biochar derived from aerobic granular sludge for high-performance membrane capacitive deionization. Environ Sci Ecotechnology 17:100297. https://doi.org/10.1016/j.ese.2023.100297
Abro NQ, Memon N, Samejo BA, Halepoto MR, Hakro AA (2022) Characterization and capacitive performance assessment of potato peels derived salt-induced porous carbons. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02579-x
Siekierka A, Bryjak M (2019) Novel anion exchange membrane for concentration of lithium salt in hybrid capacitive deionization. Desalination 452:279–289. https://doi.org/10.1016/j.desal.2018.10.009
Zhu Z, Tang S, Yuan J, Qin X, Deng Y, Qu R, Haarberg GM (2016) Effects of various binders on supercapacitor performances. Int J Electrochem Sci 11(10):8270–8279. https://doi.org/10.20964/2016.10.04
Al-Qodah Z, Dweiri R, Khader M, Al-Sabbagh S, Al-Shannag M, Qasrawi S, Al-Halawani M (2023) Processing and characterization of magnetic composites of activated carbon, fly ash, and beach sand as adsorbents for Cr(VI) removal. Case Stud Chem Environ Eng 7:100333. https://doi.org/10.1016/j.cscee.2023.100333
Wang L, Wang X, Jiang C, Yin S, Yang M (2019) Dynamic coordinated active–reactive power optimization for active distribution network with energy storage systems. Appl Sci 9(6):1129
Tang X, Zhou L, Yu H, Dai Y, Ouyang J, Liu Z, Wang Y, Le Z, Adesina AA (2021) Nanoarchitectonics of poly(vinyl alcohol)/graphene oxide composite electrodes for highly efficient electrosorptive removal of U(VI) from aqueous solution. Sep Purif Technol 278:119604. https://doi.org/10.1016/j.seppur.2021.119604
Thom NT, Anh VTK, Trang NTT, Phuong NT, Thanh DTM, Dang LH, Lam TD, Nam PT (2022) Study on the functionalization of activated carbon and the effect of binder toward capacitive deionization application. Green Process Synth 11(1):830–841. https://doi.org/10.1515/gps-2022-0049
Salleh NA, Kheawhom S, Hamid NAA, Rahiman W, Mohamad AA (2023) Electrode polymer binders for supercapacitor applications: a review. J Mater Res Technol 23:3470–3491. https://doi.org/10.1016/j.jmrt.2023.02.013
Wendt H (1988) K. Kinoshita: Carbon, electrochemical and physical properties, John Wiley + Sons, Chichester, New York, Brisbane, Toronto 1988. 533 Seiten, Preis: £ 65. Ber Bunsenges Phys Chem 92(9):1060–1060. https://doi.org/10.1002/bbpc.198800269
Li M, Li W, Zhang X, Wu C, Han X, Chen Y (2021) Polyvinyl alcohol-based monovalent anion selective membranes with excellent permselectivity in selectrodialysis. J Membr Sci 620:118889. https://doi.org/10.1016/j.memsci.2020.118889
McNair R, Szekely G, Dryfe RAW (2021) Ion-exchange materials for membrane capacitive deionization. ACS ES&T Water 1(2):217–239. https://doi.org/10.1021/acsestwater.0c00123
Zhang Z, Liu Y, Lin S, Wang Q (2020) Preparation and properties of glutaraldehyde crosslinked poly(vinyl alcohol) membrane with gradient structure. J Polym Res 27(8):228. https://doi.org/10.1007/s10965-020-02223-0
Zhang Y, Zhu PC, Edgren D (2010) Crosslinking reaction of poly(vinyl alcohol) with glyoxal. J Polym Res 17(5):725–730. https://doi.org/10.1007/s10965-009-9362-z
Nguyen TT, Huynh LTN, Pham TN, Tran TN, Ho TTN, Nguyen TD, Nguyen TTT, Vo TKA, Pham GV, Le VH, Le TT, Nguyen TH, Thai H, Le TL, Tran DL (2021) Enhanced capacitive deionization performance of activated carbon derived from coconut shell electrodes with low content carbon nanotubes–graphene synergistic hybrid additive. Mater Lett 292:129652. https://doi.org/10.1016/j.matlet.2021.129652
Le VH, Huynh LTN, Tran TN, Ho TTN, Hoang MN, Nguyen TH (2021) Comparative desalination performance of activated carbon from coconut shell waste/carbon nanotubes composite in batch mode and single-pass mode. J Appl Electrochem 51(9):1313–1322. https://doi.org/10.1007/s10800-021-01575-9
Samejo BA, Abro NQ, Memon N, Upoma NJ, Habib A (2023) Chicken feather-derived carbon electrodes for capacitive deionization using poly(vinyl alcohol)-glutaraldehyde as the binder. Water Reuse 13(2):180–192. https://doi.org/10.2166/wrd.2023.079
Jain A, Kim J, Owoseni OM, Weathers C, Caña D, Zuo K, Walker WS, Li Q, Verduzco R (2018) Aqueous-processed, high-capacity electrodes for membrane capacitive deionization. Environ Sci Technol 52(10):5859–5867. https://doi.org/10.1021/acs.est.7b05874
Li H, Pan L, Nie C, Liu Y, Sun Z (2012) Reduced graphene oxide and activated carbon composites for capacitive deionization. J Mater Chem 22(31):15556–15561. https://doi.org/10.1039/c2jm32207b
Liu X, Chen T, Qiao W-C, Wang Z, Yu L (2017) Fabrication of graphene/activated carbon nanofiber composites for high performance capacitive deionization. J Taiwan Inst Chem Eng 72:213–219. https://doi.org/10.1016/j.jtice.2017.01.013
Chong LG, Chen PA, Huang JY, Huang HL, Wang HP (2018) Capacitive deionization of a RO brackish water by AC/graphene composite electrodes. Chemosphere 191:296–301. https://doi.org/10.1016/j.chemosphere.2017.10.064
Hussain F, Shah SZ, Ahmad H, Abubshait SA, Abubshait HA, Laref A, Manikandan A, Kusuma HS, Iqbal M (2021) Microalgae an ecofriendly and sustainable wastewater treatment option: biomass application in biofuel and bio-fertilizer production. A review. Renew Sustain Energy Rev 137:110603. https://doi.org/10.1016/j.rser.2020.110603
Oliveira CYB, Oliveira CDL, Prasad R, Ong HC, Araujo ES, Shabnam N, Gálvez AO (2021) A multidisciplinary review of Tetradesmus obliquus: a microalga suitable for large-scale biomass production and emerging environmental applications. Rev Aquac 13(3):1594–1618. https://doi.org/10.1111/raq.12536
Kyaw HH, Al-Mashaikhi SM, Myint MTZ, Al-Harthi S, El-Shafey E-SI, Al-Abri M (2021) Activated carbon derived from the date palm leaflets as multifunctional electrodes in capacitive deionization system. Chem Eng Process Process Intensif 161:108311. https://doi.org/10.1016/j.cep.2021.108311
Misran E, Bani O, Situmeang EM, Purba AS (2022) Banana stem based activated carbon as a low-cost adsorbent for methylene blue removal: isotherm, kinetics, and reusability. Alex Eng J 61(3):1946–1955. https://doi.org/10.1016/j.aej.2021.07.022
Yanti I, Sationo PP, Winata WF, Anugrahwati M, Anas AK, Swasono YA (2023) Effectiveness of activated carbon magnetic composite from banana peel (Musa acuminata) for recovering iron metal ions. Case Stud Chem Environ Eng 8:100378. https://doi.org/10.1016/j.cscee.2023.100378
Ramutshatsha-Makhwedzha D, Munyengabe A, Mavhungu ML, Mbaya R, Baloyi J (2023) Breakthrough studies for the sorption of methylene blue dye from wastewater samples using activated carbon derived from waste banana peels. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-023-04329-z
Van Thuan T, Quynh BTP, Nguyen TD, Ho VTT, Bach LG (2017) Response surface methodology approach for optimization of Cu2+, Ni2+ and Pb2+ adsorption using KOH-activated carbon from banana peel. Surf Interfaces 6:209–217. https://doi.org/10.1016/j.surfin.2016.10.007
Kabadayi Catalkopru A, Kantarli IC, Yanik J (2017) Effects of spent liquor recirculation in hydrothermal carbonization. Bioresour Technol 226:89–93. https://doi.org/10.1016/j.biortech.2016.12.015
Evans SF, Ivancevic MR, Wilson DJ, Hood ZD, Adhikari SP, Naskar AK, Tsouris C, Paranthaman MP (2019) Carbon polyaniline capacitive deionization electrodes with stable cycle life. Desalination 464:25–32. https://doi.org/10.1016/j.desal.2019.04.002
Park B-H, Kim Y-J, Park J-S, Choi J (2011) Capacitive deionization using a carbon electrode prepared with water-soluble poly(vinyl alcohol) binder. J Ind Eng Chem 17(4):717–722. https://doi.org/10.1016/j.jiec.2011.05.015
Nadew TT, Keana M, Sisay T, Getye B (2023) Synthesis of activated carbon from banana peels for dye removal of an aqueous solution in textile industries: optimization, kinetics, and isotherm aspects. Water Pract Technol 18(4):947–966. https://doi.org/10.2166/wpt.2023.042
Muniandy L, Adam F, Mohamed AR, Ng E-P (2014) The synthesis and characterization of high purity mixed microporous/mesoporous activated carbon from rice husk using chemical activation with NaOH and KOH. Microporous Mesoporous Mater 197:316–323. https://doi.org/10.1016/j.micromeso.2014.06.020
Tadesse MG, Kasaw E, Lübben JF (2023) Valorization of banana peel using carbonization: potential use in the sustainable manufacturing of flexible supercapacitors. Micromachines 14(2):330
Razzaq Z, Hamayun M, Murtaza S, Kausar S, Altaf AA, Khan RU, Javaid T (2023) Removal of As(V) and Cr(VI) with low-cost novel virgin and iron-impregnated banana peduncle-activated carbons. ACS Omega 8(2):2098–2111. https://doi.org/10.1021/acsomega.2c05957
Sari SN, Melati A (2019) Facile preparation of carbon nanofiber from banana peel waste. Mater Today Proc 13:165–168. https://doi.org/10.1016/j.matpr.2019.03.208
Njewa JB, Vunain E, Biswick T (2022) Synthesis and characterization of activated carbons prepared from agro-wastes by chemical activation. J Chem 2022:9975444. https://doi.org/10.1155/2022/9975444
Giri DD, Jha JM, Tiwari AK, Srivastava N, Hashem A, Alqarawi AA, Abd_Allah EF, Pal DB (2021) Java plum and amaltash seed biomass based bio-adsorbents for synthetic wastewater treatment. Environ Pollut 280:116890. https://doi.org/10.1016/j.envpol.2021.116890
Priya DS, Kennedy LJ, Anand GT (2023) Effective conversion of waste banana bract into porous carbon electrode for supercapacitor energy storage applications. Results Surf Interfaces 10:100096. https://doi.org/10.1016/j.rsurfi.2023.100096
Tian D, Liu Y, Sun B (2023) Preparation of a highly functionalized activated carbon from waste third-monomer pressure filter liquid for removal of methylene blue in aqueous solution. RSC Adv 13(28):19403–19411. https://doi.org/10.1039/d3ra02216a
Moralı U, Demiral H, Şensöz S (2018) Optimization of activated carbon production from sunflower seed extracted meal: Taguchi design of experiment approach and analysis of variance. J Clean Prod 189:602–611. https://doi.org/10.1016/j.jclepro.2018.04.084
Yaseen M, Khattak S, Ullah S, Subhan F, Ahmad W, Shakir M, Tong Z (2022) Oxidative desulfurization of model and real petroleum distillates using Cu or Ni impregnated banana peels derived activated carbon–NaClO catalyst–oxidant system. Chem Eng Res Des 179:107–118. https://doi.org/10.1016/j.cherd.2022.01.018
Devi B, Goswami M, Rabha S, Kalita S, Sarma HP, Devi A (2023) Efficacious sorption capacities for Pb(II) from contaminated water: a comparative study using biowaste and its activated carbon as potential adsorbents. ACS Omega 8(17):15141–15151. https://doi.org/10.1021/acsomega.3c00142
Nguyen TN, Le PA, Phung VBT (2020) Facile green synthesis of carbon quantum dots and biomass-derived activated carbon from banana peels: synthesis and investigation. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-020-00839-2
Hussain OA, Hathout AS, Abdel-Mobdy YE, Rashed MM, Abdel Rahim EA, Fouzy ASM (2023) Preparation and characterization of activated carbon from agricultural wastes and their ability to remove chlorpyrifos from water. Toxicol Rep 10:146–154. https://doi.org/10.1016/j.toxrep.2023.01.011
Silva AP, Argondizo A, Juchen PT, Ruotolo LAM (2021) Ultrafast capacitive deionization using rice husk activated carbon electrodes. Sep Purif Technol 271:118872. https://doi.org/10.1016/j.seppur.2021.118872
Giri DD, Jha JM, Srivastava N, Shah M, Almalki AH, Alkhanani MF, Pal DB (2022) Waste seeds of Mangifera indica, Artocarpus heterophyllus, and Schizizium commune as biochar for heavy metal removal from simulated wastewater. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-02078-5
Bayuo J, Rwiza MJ, Mtei KM (2023) Non-competitive and competitive detoxification of As(III) ions from single and binary biosorption systems and biosorbent regeneration. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-03734-0
Gurten II, Ozmak M, Yagmur E, Aktas ZJB, Bioenergy, (2012) Preparation and characterisation of activated carbon from waste tea using K2CO3. J Biomass Bioenergy 37:73–81
Neolaka YAB, Lawa Y, Naat J, Riwu AAP, Darmokoesoemo H, Widyaningrum BA, Iqbal M, Kusuma HS (2021) Indonesian Kesambi wood (Schleichera oleosa) activated with pyrolysis and H2SO4 combination methods to produce mesoporous activated carbon for Pb(II) adsorption from aqueous solution. Environ Technol Innov 24:101997. https://doi.org/10.1016/j.eti.2021.101997
Fedoseeva YV, Okotrub AV, Koroteev VO, Borzdov YM, Palyanov YN, Shubin YV, Maksimovskiy EA, Makarova AA, Münchgesang W, Bulusheva LG, Vyalikh A (2019) Graphitization of 13C enriched fine-grained graphitic material under high-pressure annealing. Carbon 141:323–330. https://doi.org/10.1016/j.carbon.2018.09.065
Bakhsh EM, Bilal M, Ali M, Ali J, Wahab A, Akhtar K, Fagieh TM, Danish EY, Asiri AM, Khan SB (2022) Synthesis of activated carbon from Trachycarpus fortunei seeds for the removal of cationic and anionic dyes. Materials 15(6):1986
Hou C-H, Huang C-Y (2013) A comparative study of electrosorption selectivity of ions by activated carbon electrodes in capacitive deionization. Desalination 314:124–129. https://doi.org/10.1016/j.desal.2012.12.029
Hou C-H, Huang C-YJD (2013) A comparative study of electrosorption selectivity of ions by activated carbon electrodes in capacitive deionization. J Desalination 314:124–129
Kyaw HH, Myint MTZ, Al-Harthi S, Al-Abri M (2020) Removal of heavy metal ions by capacitive deionization: effect of surface modification on ions adsorption. J Hazard Mater 385:121565. https://doi.org/10.1016/j.jhazmat.2019.121565
Hamsan MH, Aziz SB, Nofal MM, Brza MA, Abdulwahid RT, Hadi JM, Karim WO, Kadir MFZ (2020) Characteristics of EDLC device fabricated from plasticized chitosan:MgCl2 based polymer electrolyte. J Market Res 9(5):10635–10646. https://doi.org/10.1016/j.jmrt.2020.07.096
Moronshing M, Subramaniam C (2017) Scalable approach to highly efficient and rapid capacitive deionization with CNT-thread as electrodes. J ACS Appl Mater Interfaces 9(46):39907–39915. https://doi.org/10.1021/acsami.7b11866
Yin H, Zhao S, Wan J, Tang H, Chang L, He L, Zhao H, Gao Y, Tang Z (2013) Three-dimensional graphene/metal oxide nanoparticle hybrids for high-performance capacitive deionization of saline water. J Adv Mater 25(43):6270–6276. https://doi.org/10.1002/adma.201302223
Duan H, Yan T, An Z, Zhang J, Shi L, Zhang D (2017) Rapid construction of 3D foam-like carbon nanoarchitectures via a simple photochemical strategy for capacitive deionization. RSC Adv 7(62):39372–39382. https://doi.org/10.1039/c7ra04613h
Lester Y, Shaulsky E, Epsztein R, Zucker I (2020) Capacitive deionization for simultaneous removal of salt and uncharged organic contaminants from water. Sep Purif Technol 237:116388. https://doi.org/10.1016/j.seppur.2019.116388
Kim Y-J, Choi J-H (2010) Improvement of desalination efficiency in capacitive deionization using a carbon electrode coated with an ion-exchange polymer. Water Res 44(3):990–996. https://doi.org/10.1016/j.watres.2009.10.017
Tian G, Liu L, Meng Q, Cao B (2014) Preparation and characterization of cross-linked quaternised polyvinyl alcohol membrane/activated carbon composite electrode for membrane capacitive deionization. Desalination 354:107–115. https://doi.org/10.1016/j.desal.2014.09.024
Leong ZY, Lu G, Yang HY (2019) Three-dimensional graphene oxide and polyvinyl alcohol composites as structured activated carbons for capacitive desalination. Desalination 451:172–181. https://doi.org/10.1016/j.desal.2017.07.018
Kumar AS, Ghime D, Ghosh P (2020) Poly (vinyl alcohol)-bonded carbon electrodes for desalination of brackish water using capacitive deionization. J Inst Eng (India): Ser E 101(2):125–131. https://doi.org/10.1007/s40034-020-00165-2
Fan L, Mei X, Zhang J, Zhu J, Wang X, Li Y, Cai W (2021) Effect of multi-walled carbon nanotube on thermal decomposition, mechanical and heat-induced shape memory properties of crosslinked poly(vinyl alcohol). Smart Mater Struct 30(11):115017. https://doi.org/10.1088/1361-665x/ac27c1
Bharath G, Hai A, Rambabu K, Savariraj D, Ibrahim Y, Banat F (2020) The fabrication of activated carbon and metal-carbide 2D framework-based asymmetric electrodes for the capacitive deionization of Cr(vi) ions toward industrial wastewater remediation. Environ Sci: Water Res Technol 6(2):351–361. https://doi.org/10.1039/c9ew00805e
Ma J, Wang L, Yu F, Dai X (2019) Mesoporous amorphous FePO4 nanosphere@ graphene as a faradic electrode in capacitive deionization for high-capacity and fast removal of NaCl from water. Chem Eng J 370:938
Jaoude MA, Alhseinat E, Polychronopoulou K, Bharath G, Darawsheh IFF, Anwer S, Baker MA, Hinder SJ, Banat F (2020) Morphology-dependent electrochemical performance of MnO2 nanostructures on graphene towards efficient capacitive deionization. Electrochim Acta 330:135202. https://doi.org/10.1016/j.electacta.2019.135202
Chang L, Hang Hu Y (2019) 3D channel-structured graphene as efficient electrodes for capacitive deionization. J Colloid Interface Sci 538:420–425. https://doi.org/10.1016/j.jcis.2018.11.105
Alfredy T, Elisadiki J, Jande YAC (2021) Capacitive deionization for the removal of paraquat herbicide from aqueous solution. Adsorpt Sci Technol 2021:9601012. https://doi.org/10.1155/2021/9601012
Chen B, Feng A, Deng R, Liu K, Yu Y, Song L (2020) MXene as a cation-selective cathode material for asymmetric capacitive deionization. ACS Appl Mater Interfaces 12(12):13750–13758. https://doi.org/10.1021/acsami.9b19684
Liu P, Yan T, Zhang J, Shi L, Zhang D (2017) Separation and recovery of heavy metal ions and salt ions from wastewater by 3D graphene-based asymmetric electrodes via capacitive deionization. J Mater Chem A 5(28):14748–14757. https://doi.org/10.1039/c7ta03515b
Funding
The work was carried under the institutional fund of the National Centre of Excellence in Analytical Chemistry, University of Sindh, Jamshoro, Pakistan.
Author information
Authors and Affiliations
Contributions
Bakhtiar Samejo: formal analysis, data curation, experimental results analysis, draft—review and editing. Naveed Qasim Abro: formal analysis, data curation. Najma Memon: conceptualization, methodology, investigation, writing—original draft, writing—review and editing, final review and editing, fund acquisition, supervision. Sandeep Poddar: conceptualization, editing. Ahsan Habib: conceptualization, writing—review and editing, final review and editing.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Samejo, B.A., Abro, N.Q., Memon, N. et al. Waste-derived stable carbon electrodes for capacitive deionization using poly (vinyl alcohol)-glutaraldehyde as binder. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04982-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04982-4