Abstract
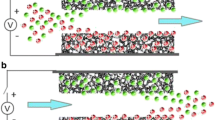
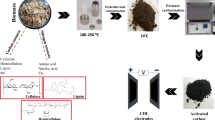
Capacitive deionization (CDI) is a promising water purification technology which works by removing salt ions or charged species from aqueous solutions. Currently, most of the research on CDI focuses on the desalination of water with low or moderate salt concentration due to the low salt adsorption capacity of the electrodes. The electrosorption capacity of CDI relies on the structural and textural characteristics of the electrode materials. The cost of electrode materials, the complicated synthesis methods, and the environmental concerns arising from material synthesis steps hinder the development of large-scale CDI units. By considering the good electrical conductivity, high specific surface area (SSA), porous structure, availability, mass production, and cost, porous carbon derived from biomass materials may be a promising CDI electrode material. This review presents an update on carbon nanomaterials derived from various biomasses for CDI electrodes. It covers different synthesis methods and the electrosorption performance of each material and discusses the impact of the SSA and porous structure of the materials on desalination. This review shows that a variety of biomass materials can be used to synthesize cost-effective CDI electrode materials with different structures and good desalination performance. It also shows that diverse precursors and synthesis routes have significant influences on the properties and performance of the resulting carbon electrodes. Additionally, the performance of CDI does not depend only on BET surface area and pore structure but also on the applied voltage, initial concentration of the feed solution, and mass, as well as the capacitance of the electrodes.






















Similar content being viewed by others
References
Fujioka R, Wang LP, Dodbiba G, Fujita T (2013) Application of progressive freeze-concentration for desalination. Desalination 319:33–37. https://doi.org/10.1016/j.desal.2013.04.005
Ng KC, Thu K, Oh SJ, Ang L, Shahzad MW, Ismail AB (2015) Recent developments in thermally-driven seawater desalination: energy efficiency improvement by hybridization of the MED and AD cycles. Desalination 356:255–270
Greenlee LF, Lawler DF, Freeman BD, Marrot B, Moulin P (2009) Reverse osmosis desalination: water sources, technology, and today's challenges. Water Res 43:2317–2348
Souhaimi MK, Matsuura T (2011) Membrane distillation: principles and applications. Elsevier
Youssef PG, Al-Dadah RK, Mahmoud SM (2014) Comparative analysis of desalination technologies. Energy Procedia 61:2604–2607. https://doi.org/10.1016/j.egypro.2014.12.258
Demirer ON, Naylor RM, Rios Perez CA, Wilkes E, Hidrovo C (2013) Energetic performance optimization of a capacitive deionization system operating with transient cycles and brackish water. Desalination 314:130–138. https://doi.org/10.1016/j.desal.2013.01.014
Desale GR, Vasudevan P, Pothal JK, Zala KS, Bhatti BA, Singh SN, Sen PK (2011) Purification of water using vertical multiple effect distillation unit. J Sci Ind Res 70:634–638
Al-Shammiri M, Safar M (1999) Multi-effect distillation plants: state of the art. Desalination 126:45–59. https://doi.org/10.1016/S0011-9164(99)00154-X
Abdul-Wahab SA, Abdo J (2007) Optimization of multistage flash desalination process by using a two-level factorial design. Appl Therm Eng 27:413–421. https://doi.org/10.1016/j.applthermaleng.2006.07.010
Hamed OA, Al-Sofi MAK, Imam M, Mustafa GM, Ba Mardouf K, Al-Washmi H (2000) Thermal performance of multi-stage flash distillation plants in Saudi Arabia. Desalination 128:281–292. https://doi.org/10.1016/S0011-9164(00)00043-6
Ettouney HE-DH (1999) Single-effect thermal vapor-compression desalination process: thermal analysis. Heat Transfer Eng 20:52–68. https://doi.org/10.1080/014576399271583
Al-Juwayhel F, El-Dessouky H, Ettouney H (1997) Analysis of single-effect evaporator desalination systems combined with vapor compression heat pumps. Desalination 114:253–275. https://doi.org/10.1016/s0011-9164(98)00017-4
Bahar R, Hawlader MNA, Woei LS (2004) Performance evaluation of a mechanical vapor compression desalination system. Desalination 166:123–127. https://doi.org/10.1016/j.desal.2004.06.066
Ettouney H (2006) Design of single-effect mechanical vapor compression. Desalination 190:1–15. https://doi.org/10.1016/j.desal.2005.08.003
Fritzmann C, Löwenberg J, Wintgens T, Melin T (2007) State-of-the-art of reverse osmosis desalination. Desalination 216:1–76. https://doi.org/10.1016/j.desal.2006.12.009
Kaschemekat J, Hilgendorff W, Bőddeker K, Hassan A, Malik A (1983) Two-stage reverse osmosis seawater desalination. Desalination 46:151–156
Lee J-G, Kim W-S (2013) Numerical modeling of the vacuum membrane distillation process. Desalination 331:46–55. https://doi.org/10.1016/j.desal.2013.10.022
Ali MI, Summers EK, Arafat HA, Lienhard JHV (2012) Effects of membrane properties on water production cost in small scale membrane distillation systems. Desalination 306:60–71. https://doi.org/10.1016/j.desal.2012.07.043
Khayet, M, Matsuura, T, (2011) Membrane distillation: principles and applications, access online via Elsevier
Quist-Jensen CA, Macedonio F, Drioli E (2015) Membrane technology for water production in agriculture: desalination and wastewater reuse. Desalination 364:17–32. https://doi.org/10.1016/j.desal.2015.03.001
Al-Karaghouli A, Kazmerski LL (2013) Energy consumption and water production cost of conventional and renewable-energy-powered desalination processes. Renew Sust Energ Rev 24:343–356
Warsinger DM, Swaminathan J, Guillen-Burrieza E, Arafat HA, Lienhard JHV (2015) Scaling and fouling in membrane distillation for desalination applications: a review. Desalination 356:294–313. https://doi.org/10.1016/j.desal.2014.06.031
Moon AS, Lee M (2012) Energy consumption in forward osmosis-desalination compared to other desalination techniques. World Acad Sci Eng Technol 65:537–539
Welgemoed TJ, Schutte CF (2005) Capacitive Deionization Technology™: an alternative desalination solution. Desalination 183:327–340. https://doi.org/10.1016/j.desal.2005.02.054
Gaikwad MS, Balomajumder C (2016) Capacitive deionization for desalination using nanostructured electrodes. Anal Lett 49:1641–1655. https://doi.org/10.1080/00032719.2015.1118485
Anderson MA, Cudero AL, Palma J (2010) Capacitive deionization as an electrochemical means of saving energy and delivering clean water. Comparison to present desalination practices: will it compete? Electrochim Acta 55:3845–3856. https://doi.org/10.1016/j.electacta.2010.02.012
Khayet M (2011) Membranes and theoretical modeling of membrane distillation: a review. Adv Colloid Interf Sci 164:56–88. https://doi.org/10.1016/j.cis.2010.09.005
Cath T, Childress A, Elimelech M (2006) Forward osmosis: principles, applications, and recent developments. J Membr Sci 281:70–87. https://doi.org/10.1016/j.memsci.2006.05.048
Farmer JC, Fix DV, Mack GV, Pekala RW, Poco JF (1996) Capacitive deionization of NaCl and NaNO3 solutions with carbon aerogel electrodes. J Electrochem Soc 143:159–169. https://doi.org/10.1149/1.1836402
Welgemoed, TJ (2005) Capacitive deionization technology™: development and evaluation of an industrial prototype system. MEng (Water utilization), University of Pretoria, Pretoria, South Africa
Jande YAC, Kim WS (2013) Desalination using capacitive deionization at constant current. Desalination 329:29–34. https://doi.org/10.1016/j.desal.2013.08.023
Lee JY, Seo SJ, Yun SH, Moon SH (2011) Preparation of ion exchanger layered electrodes for advanced membrane capacitive deionization (MCDI). Water Res 45:5375–5380. https://doi.org/10.1016/j.watres.2011.06.028
Zhao R, Biesheuvel PM, Van der Wal A (2012) Energy consumption and constant current operation in membrane capacitive deionization. Energy Environ Sci 5:9520. https://doi.org/10.1039/c2ee21737f
Zhao R, Satpradit O, Rijnaarts HH, Biesheuvel PM, van der Wal A (2013) Optimization of salt adsorption rate in membrane capacitive deionization. Water Res 47:1941–1952. https://doi.org/10.1016/j.watres.2013.01.025
Suss ME, Porada S, Sun X, Biesheuvel PM, Yoon J, Presser V (2015) Water desalination via capacitive deionization: what is it and what can we expect from it? Energy Environ Sci 8:2296–2319. https://doi.org/10.1039/c5ee00519a
Liu P, Wang H, Yan T, Zhang J, Shi L, Zhang D (2016) Grafting sulfonic and amine functional groups on 3D graphene for improved capacitive deionization. J Mater Chem 4:5303–5313. https://doi.org/10.1039/c5ta10680j
Yang J, Zou L, Choudhury NR (2013) Ion-selective carbon nanotube electrodes in capacitive deionisation. Electrochim Acta 91:11–19
Yan T, Xu B, Zhang J, Shi L, Zhang D (2018) Ion-selective asymmetric carbon electrodes for enhanced capacitive deionization. RSC Adv 8:2490–2497. https://doi.org/10.1039/c7ra10443j
Lu M, Liu J-Y, Cheng J, Wang S-P, Yang J-M (2014) Functionalized graphene/activated carbon composite electrodes for asymmetric capacitive deionization. Acta Phys -Chim Sin 30:2263–2271
Zou L, Li L, Song H, Morris G (2008) Using mesoporous carbon electrodes for brackish water desalination. Water Res 42:2340–2348. https://doi.org/10.1016/j.watres.2007.12.022
Zhao, R (2013) Theory and operation of capacitive deionization systems. PhD thesis, Wageningen University,
Porada S, Zhao R, van der Wal A, Presser V, Biesheuvel PM (2013) Review on the science and technology of water desalination by capacitive deionization. Prog Mater Sci 58:1388–1442. https://doi.org/10.1016/j.pmatsci.2013.03.005
Yasin AS, Obaid M, Mohamed IM, Yousef A, Barakat NA (2017) ZrO2 nanofibers/activated carbon composite as a novel and effective electrode material for the enhancement of capacitive deionization performance. RSC Adv 7:4616–4626
Srimuk P, Zeiger M, Jäckel N, Tolosa A, Krüner B, Fleischmann S, Grobelsek I, Aslan M, Shvartsev B, Suss ME, Presser V (2017) Enhanced performance stability of carbon/titania hybrid electrodes during capacitive deionization of oxygen saturated saline water. Electrochim Acta 224:314–328. https://doi.org/10.1016/j.electacta.2016.12.060
Gao X, Omosebi A, Holubowitch N, Liu A, Ruh K, Landon J, Liu K (2016) Polymer-coated composite anodes for efficient and stable capacitive deionization. Desalination 399:16–20. https://doi.org/10.1016/j.desal.2016.08.006
Cohen I, Avraham E, Bouhadana Y, Soffer A, Aurbach D (2013) Long term stability of capacitive de-ionization processes for water desalination: the challenge of positive electrodes corrosion. Electrochim Acta 106:91–100
Lee J-H, Bae W-S, Choi J-H (2010) Electrode reactions and adsorption/desorption performance related to the applied potential in a capacitive deionization process. Desalination 258:159–163. https://doi.org/10.1016/j.desal.2010.03.020
He D, Wong CE, Tang W, Kovalsky P, Waite TD (2016) Faradaic reactions in water desalination by batch-mode capacitive deionization. Environ Sci Technol Lett 3:222–226. https://doi.org/10.1021/acs.estlett.6b00124
Gao X, Landon J, Neathery JK, Liu K (2013) Modification of carbon xerogel electrodes for more efficient asymmetric capacitive deionization. J Electrochem Soc 160:E106–E112. https://doi.org/10.1149/2.111309jes
Gao X, Omosebi A, Landon J, Liu K (2015) Surface charge enhanced carbon electrodes for stable and efficient capacitive deionization using inverted adsorption–desorption behavior. Energy Environ Sci 8:897–909. https://doi.org/10.1039/c4ee03172e
Omosebi A, Gao X, Landon J, Liu K (2014) Asymmetric electrode configuration for enhanced membrane capacitive deionization. ACS Appl Mater Interfaces 6:12640–12649. https://doi.org/10.1021/am5026209
Lee J, Kim S, Kim C, Yoon J (2014) Hybrid capacitive deionization to enhance the desalination performance of capacitive techniques. Energy Environ Sci 7:3683–3689. https://doi.org/10.1039/C4EE02378A
Srimuk P, Lee J, Fleischmann S, Choudhury S, Jäckel N, Zeiger M, Kim C, Aslan M, Presser V (2017) Faradaic deionization of brackish and sea water via pseudocapacitive cation and anion intercalation into few-layered molybdenum disulfide. J Mater Chem 5:15640–15649. https://doi.org/10.1039/c7ta03120c
Porada S, Shrivastava A, Bukowska P, Biesheuvel PM, Smith KC (2017) Nickel hexacyanoferrate electrodes for continuous cation intercalation desalination of brackish water. Electrochim Acta 255:369–378. https://doi.org/10.1016/j.electacta.2017.09.137
Srimuk P, Kaasik F, Kruner B, Tolosa A, Fleischmann S, Jackel N, Tekeli MC, Aslan M, Suss ME, Presser V (2016) MXene as a novel intercalation-type pseudocapacitive cathode and anode for capacitive deionization. J Mater Chem 4:18265–18271. https://doi.org/10.1039/C6TA07833H
Xing F, Li T, Li J, Zhu H, Wang N, Cao X (2017) Chemically exfoliated MoS2 for capacitive deionization of saline water. Nano Energy 31:590–595. https://doi.org/10.1016/j.nanoen.2016.12.012
Singh K, Porada S, de Gier HD, Biesheuvel PM, de Smet LCPM (2019) Timeline on the application of intercalation materials in capacitive deionization. Desalination 455:115–134. https://doi.org/10.1016/j.desal.2018.12.015
Cooper, JL (1969) Activated carbon used as electrodes in electrochemical demineralization of saline water. PhD thesis, The University of Oklahoma,
John W B, George W M, Electrochemical demineralization of water with porous electrodes of large surface area, in: Advances in chemistry, American Chemical Society, 1960, 206–223
Johnson AM, Venolia AW, Wilbourne RG, Newman J, Nuys V, Wong C, Gillam WS, Johnson S, Horowitz RH (1970) The electrosorb process for desalting water
Johnson AM, Newman J (1971) Desalting by means of porous carbon electrodes. J Electrochem Soc 118:510–517
Biesheuvel PM, Bazant MZ (2010) Nonlinear dynamics of capacitive charging and desalination by porous electrodes. Phys Rev E Stat Nonlinear Soft Matter Phys 81:031502
Biesheuvel PM, Fu Y, Bazant MZ (2011) Diffuse charge and Faradaic reactions in porous electrodes. Phys Rev E Stat Nonlinear Soft Matter Phys 83:061507. https://doi.org/10.1103/PhysRevE.83.061507
Brogioli D, Zhao R, Biesheuvel PM (2011) A prototype cell for extracting energy from a water salinity difference by means of double layer expansion in nanoporous carbon electrodes. Energy Environ Sci 4:772. https://doi.org/10.1039/c0ee00524j
Biesheuvel PM (2009) Thermodynamic cycle analysis for capacitive deionization. J Colloid Interface Sci 332:258–264. https://doi.org/10.1016/j.jcis.2008.12.018
Biesheuvel PM, van Limpt B, van der Wal A (2009) Dynamic adsorption/desorption process model for capacitive deionization. J Phys Chem 113:5636–5640
Kim T, Dykstra JE, Porada S, van der Wal A, Yoon J, Biesheuvel PM (2015) Enhanced charge efficiency and reduced energy use in capacitive deionization by increasing the discharge voltage. J Colloid Interface Sci 446:317–326. https://doi.org/10.1016/j.jcis.2014.08.041
Enpar (2015) Enpar Technologies Inc. www.enpar-tech.com/products_esd.php. Accessed on June 16, 2015
Voltea (2015) Voltea’s water deionization technology. http://www.voltea.com Accessed on June 18, 2015
Idropan-dell'Orto (2015) Idropan dell'Orto Depuratori S.r.l. www.idropan.com/en/. Accessed on June 16, 2015
Jande YAC, Asif M, Shim SM, Kim WS (2014) Energy minimization in monoethanolamine-based CO2 capture using capacitive deionization. Int J Energy Res 38:1531–1540. https://doi.org/10.1002/er.3168
Paz-Garcia JM, Dykstra JE, Biesheuvel PM, Hamelers HV (2015) Energy from CO2 using capacitive electrodes—a model for energy extraction cycles. J Colloid Interface Sci 442:103–109. https://doi.org/10.1016/j.jcis.2014.11.045
Paz-Garcia JM, Schaetzle O, Biesheuvel PM, Hamelers HVM (2014) Energy from CO2 using capacitive electrodes—theoretical outline and calculation of open circuit voltage. J Colloid Interface Sci 418:200–207. https://doi.org/10.1016/j.jcis.2013.11.081
Jung S-M, Choi J-H, Kim J-H (2012) Application of capacitive deionization (CDI) technology to insulin purification process. Sep Purif Technol 98:31–35. https://doi.org/10.1016/j.seppur.2012.06.005
Janssen AJH, Schrader GA, Verbeek PHJ, (2013) Method and system for enhancing oil recovery (EOR) by injecting treated water into an oil bearing formation, in, Shell Oil Company, Houston, pp. 1–7
Huang WEI, Zhang Y, Bao S, Song S (2013) Desalination by capacitive deionization with carbon-based materials as electrode: a review. Surf Rev Lett 20:1330003. https://doi.org/10.1142/s0218625x13300050
Wang L, Wang M, Huang Z-H, Cui T, Gui X, Kang F, Wang K, Wu D (2011) Capacitive deionization of NaCl solutions using carbon nanotube sponge electrodes. J Mater Chem 21:18295. https://doi.org/10.1039/c1jm13105b
Wang S, Wang D, Ji L, Gong Q, Zhu Y, Liang J (2007) Equilibrium and kinetic studies on the removal of NaCl from aqueous solutions by electrosorption on carbon nanotube electrodes. Sep Purif Technol 58:12–16
Zou L, Morris G, Qi D (2008) Using activated carbon electrode in electrosorptive deionisation of brackish water. Desalination 225:329–340
Oda H, Nakagawa Y (2003) Removal of ionic substances from dilute solution using activated carbon electrodes. Carbon 41:1037–1047
El-Deen AG, Barakat NA, Khalil KA, Kim HY (2014) Hollow carbon nanofibers as an effective electrode for brackish water desalination using the capacitive deionization process. New J Chem 38:198–205
Xu P, Drewes JE, Heil D, Wang G (2008) Treatment of brackish produced water using carbon aerogel-based capacitive deionization technology. Water Res 42:2605–2617. https://doi.org/10.1016/j.watres.2008.01.011
Gabelich CJ, Tran TD, Suffet IHM (2002) Electrosorption of inorganic salts from aqueous solution using carbon aerogels. Environ Sci Technol 36:3010–3019
Li H, Lu T, Pan L, Zhang Y, Sun Z (2009) Electrosorption behavior of graphene in NaCl solutions. J Mater Chem 19:6773. https://doi.org/10.1039/b907703k
Li H, Zou L, Pan L, Sun Z (2010) Novel graphene-like electrodes for capacitive deionization. Environ Sci Technol 44:8692–8697
Liu P, Yan T, Shi L, Park HS, Chen X, Zhao Z, Zhang D (2017) Graphene-based materials for capacitive deionization. J Mater Chem 5:13907–13943. https://doi.org/10.1039/c7ta02653f
Liu Y, Nie C, Liu X, Xu X, Sun Z, Pan L (2015) Review on carbon-based composite materials for capacitive deionization. RSC Adv 5:15205–15225. https://doi.org/10.1039/c4ra14447c
Chen Y, Zhu Y, Wang Z, Li Y, Wang L, Ding L, Gao X, Ma Y, Guo Y (2011) Application studies of activated carbon derived from rice husks produced by chemical-thermal process—a review. Adv Colloid Interf Sci 163:39–52
Oren Y (2008) Capacitive deionization (CDI) for desalination and water treatment—past, present and future (a review). Desalination 228:10–29. https://doi.org/10.1016/j.desal.2007.08.005
Al-Marzooqi FA, Al-Ghaferi AA, Saadat I, Hilal N (2014) Application of capacitive deionisation in water desalination: a review. Desalination 342:3–15. https://doi.org/10.1016/j.desal.2014.02.031
Jia B, Zhang W (2016) Preparation and application of electrodes in capacitive deionization (CDI): a state-of-art review. Nanoscale Res Lett 11:64. https://doi.org/10.1186/s11671-016-1284-1
Thamilselvan A, Nesaraj AS, Noel M (2016) Review on carbon-based electrode materials for application in capacitive deionization process. Int J Environ Sci Technol 13:2961–2976. https://doi.org/10.1007/s13762-016-1061-9
Ratajczak P, Suss ME, Kaasik F, Béguin F (2019) Carbon electrodes for capacitive technologies. Energy Storage Mater 16:126–145. https://doi.org/10.1016/j.ensm.2018.04.031
Choi J, Dorji P, Shon HK, Hong S (2019) Applications of capacitive deionization: desalination, softening, selective removal, and energy efficiency. Desalination 449:118–130. https://doi.org/10.1016/j.desal.2018.10.013
Han B, Cheng G, Wang Y, Wang X (2019) Structure and functionality design of novel carbon and faradaic electrode materials for high-performance capacitive deionization. Chem Eng J 360:364–384. https://doi.org/10.1016/j.cej.2018.11.236
Chew JJ, Doshi V (2011) Recent advances in biomass pretreatment—torrefaction fundamentals and technology. Renew Sust Energ Rev 15:4212–4222. https://doi.org/10.1016/j.rser.2011.09.017
Marsh, H, Reinoso, FR, (2006) Activated carbon, Elsevier
Rufford TE, Hulicova-Jurcakova D, Zhu Z, Lu GQ (2008) Nanoporous carbon electrode from waste coffee beans for high performance supercapacitors. Electrochem Commun 10:1594–1597
Taer, E, Taslim, R, Aini, Z, Hartati, S, Mustika, W (2017) Activated carbon electrode from banana-peel waste for supercapacitor applications. AIP Conference Proceedings AIP Publishing, 1801. 040004
Wang P, Wang Q, Zhang G, Jiao H, Deng X, Liu L (2016) Promising activated carbons derived from cabbage leaves and their application in high-performance supercapacitors electrodes. J Solid State Electrochem 20:319–325
Zhao S, Yan T, Wang Z, Zhang J, Shi L, Zhang D (2017) Removal of NaCl from saltwater solutions using micro/mesoporous carbon sheets derived from watermelon peel via deionization capacitors. RSC Adv 7:4297–4305
Lado JJ, Zornitta RL, Calvi FA, Tejedor-Tejedor MI, Anderson MA, Ruotolo LAM (2016) Study of sugar cane bagasse fly ash as electrode material for capacitive deionization. J Anal Appl Pyrolysis 120:389–398. https://doi.org/10.1016/j.jaap.2016.06.009
Li G-X, Hou P-X, Zhao S-Y, Liu C, Cheng H-M (2016) A flexible cotton-derived carbon sponge for high-performance capacitive deionization. Carbon 101:1–8. https://doi.org/10.1016/j.carbon.2015.12.095
Yeh C-L, Hsi H-C, Li K-C, Hou C-H (2015) Improved performance in capacitive deionization of activated carbon electrodes with a tunable mesopore and micropore ratio. Desalination 367:60–68
Guo P-Z, Ji Q-Q, Zhang L-L, Zhao S-Y, Zhao X-S (2011) Preparation and characterization of peanut shell-based microporous carbons as electrode materials for supercapacitors. Acta Phys -Chim Sin 27:2836–2840
Quan G, Chu L, Han X, Ding C, Chen T, Yan J (2016) Facile synthesis of novel hierarchically porous carbon derived from nature biomass for enhanced removal of NaCl. Water Sci Technol 74:1821–1831
Enock TK, King’ondu CK, Pogrebnoi A, Jande YAC (2017) Biogas-slurry derived mesoporous carbon for supercapacitor applications. Mater Today Energy 5:126–137
Enock TK, King’ondu CK, Pogrbnoi A, Jande YAC (2017) Status of biomass derived carbon materials for supercapacitor application. Int J Electrochem 2017:1–14
Zhang D, Yan T, Shi L, Peng Z, Wen X, Zhang J (2012) Enhanced capacitive deionization performance of graphene/carbon nanotube composites. J Mater Chem 22:14696. https://doi.org/10.1039/c2jm31393f
Elisadiki J, Jande YAC, Machunda RL, Kibona TE (2019) Porous carbon derived from Artocarpus heterophyllus peels for capacitive deionization electrodes. Carbon 147:582–593. https://doi.org/10.1016/j.carbon.2019.03.036
Feng C, Chen YA, Yu CP, Hou CH (2018) Highly porous activated carbon with multi-channeled structure derived from loofa sponge as a capacitive electrode material for the deionization of brackish water. Chemosphere 208:285–293. https://doi.org/10.1016/j.chemosphere.2018.05.174
Liu M, Xu M, Xue Y, Ni W, Huo S, Wu L, Yang Z, Yan Y (2018) Efficient capacitive deionization using natural basswood derived, free standing, hierarchically porous carbon electrodes. ACS Appl Mater Interfaces 10:31260–31270. https://doi.org/10.1021/acsami.8b08232
Qian M, Xuan XY, Pan LK, Gong SQ (2019) Porous carbon electrodes from activated wasted coffee grounds for capacitive deionization. Ionics 25:3443–3452. https://doi.org/10.1007/s11581-019-02887-9
Manocha S, Manocha LM, Joshi P, Patel B, Dangi G, Verma N (2013) Activated carbon from biomass. AIP conference 1538. 120–123
Bansal RC, Goyal M (2005) Activated carbon adsorption. CRC, Boca Raton
Hui TS, Zaini MAA (2015) Potassium hydroxide activation of activated carbon: a commentary. Carbon Lett 16:275–280
Dehkhoda, AM (2016) Development and characterization of activated biochar as electrode material for capacitive deionization. PhD thesis, The University of British Columbia
Wang T, Zhai Y, Zhu Y, Li C, Zeng G (2018) A review of the hydrothermal carbonization of biomass waste for hydrochar formation: process conditions, fundamentals, and physicochemical properties. Renew Sust Energ Rev 90:223–247. https://doi.org/10.1016/j.rser.2018.03.071
Jain A, Balasubramanian R, Srinivasan MP (2016) Hydrothermal conversion of biomass waste to activated carbon with high porosity: a review. Chem Eng J 283:789–805. https://doi.org/10.1016/j.cej.2015.08.014
Xie Z, Shang X, Yan J, Hussain T, Nie P, Liu J (2018) Biomass-derived porous carbon anode for high-performance capacitive deionization. Electrochim Acta 290:666–675. https://doi.org/10.1016/j.electacta.2018.09.104
Titirici M-M, Funke A, Kruse A, (2015) Hydrothermal carbonization of biomass. In: Recent advances in thermochemical conversion of biomass, 325–352
Li J, Wang X, Wang H, Wang S, Hayat T, Alsaedi A, Wang X (2017) Functionalization of biomass carbonaceous aerogels and their application as electrode materials for electro-enhanced recovery of metal ions. Environ Sci: Nano 4:1114–1123. https://doi.org/10.1039/c7en00019g
Yan J, Zhang H, Xie Z, Liu J (2017) Preparation of the Lentinus edodes-based porous biomass carbon by hydrothermal method for capacitive desalination. AIP Conf Proc 1864:020218
Zhang XF, Wang B, Yu J, Wu XN, Zang YH, Gao HC, Su PC, Hao SQ (2018) Three-dimensional honeycomb-like porous carbon derived from corncob for the removal of heavy metals from water by capacitive deionization. RSC Adv 8:1159–1167. https://doi.org/10.1039/c7ra10689k
Zhang S, Tian K, Cheng B-H, Jiang H (2017) Preparation of N-doped supercapacitor materials by integrated salt templating and silicon hard templating by pyrolysis of biomass wastes. ACS Sustain Chem Eng 5:6682–6691. https://doi.org/10.1021/acssuschemeng.7b00920
Fechler N, Fellinger TP, Antonietti M (2013) “Salt templating”: a simple and sustainable pathway toward highly porous functional carbons from ionic liquids. Adv Mater 25:75–79. https://doi.org/10.1002/adma.201203422
Deng X, Zhao B, Zhu L, Shao Z (2015) Molten salt synthesis of nitrogen-doped carbon with hierarchical pore structures for use as high-performance electrodes in supercapacitors. Carbon 93:48–58. https://doi.org/10.1016/j.carbon.2015.05.031
Liu Y, Chen T, Lu T, Sun Z, Chua DHC, Pan L (2015) Nitrogen-doped porous carbon spheres for highly efficient capacitive deionization. Electrochim Acta 158:403–409. https://doi.org/10.1016/j.electacta.2015.01.179
Liu Y, Xu X, Wang M, Lu T, Sun Z, Pan L (2015) Nitrogen-doped carbon nanorods with excellent capacitive deionization ability. J Mater Chem 3:17304–17311. https://doi.org/10.1039/c5ta03663a
Porada S, Schipper F, Aslan M, Antonietti M, Presser V, Fellinger TP (2015) Capacitive deionization using biomass-based microporous salt-templated heteroatom-doped carbons. ChemSusChem 8:1867–1874
Zhao C, Liu G, Sun N, Zhang X, Wang G, Zhang Y, Zhang H, Zhao H (2018) Biomass-derived N-doped porous carbon as electrode materials for Zn-air battery powered capacitive deionization. Chem Eng J 334:1270–1280. https://doi.org/10.1016/j.cej.2017.11.069
Chang L, Hang Hu Y (2019) 3D channel-structured graphene as efficient electrodes for capacitive deionization. J Colloid Interface Sci 538:420–425. https://doi.org/10.1016/j.jcis.2018.11.105
Zhang J, Fang J, Han J, Yan T, Shi L, Zhang D (2018) N, P, S co-doped hollow carbon polyhedra derived from MOF-based core–shell nanocomposites for capacitive deionization. J Mater Chem 6:15245–15252. https://doi.org/10.1039/c8ta04813d
Yan T, Liu J, Lei H, Shi L, An Z, Park HS, Zhang D (2018) Capacitive deionization of saline water using sandwich-like nitrogen-doped graphene composites via a self-assembling strategy. Environ Sci: Nano 5:2722–2730. https://doi.org/10.1039/c8en00629f
Khan ZU, Yan T, Shi L, Zhang D (2018) Improved capacitive deionization by using 3D intercalated graphene sheet–sphere nanocomposite architectures. Environ Sci: Nano 5:980–991. https://doi.org/10.1039/c7en01246b
Han J, Shi L, Yan T, Zhang J, Zhang D (2018) Removal of ions from saline water using N, P co-doped 3D hierarchical carbon architectures via capacitive deionization. Environ Sci: Nano 5:2337–2345. https://doi.org/10.1039/c8en00652k
Wang Z, Yan T, Shi L, Zhang D (2017) In situ expanding pores of dodecahedron-like carbon frameworks derived from MOFs for enhanced capacitive deionization. ACS Appl Mater Interfaces 9:15068–15078. https://doi.org/10.1021/acsami.7b02712
Wang Z, Yan T, Chen G, Shi L, Zhang D (2017) High salt removal capacity of metal–organic gel derived porous carbon for capacitive deionization. ACS Sustain Chem Eng 5:11637–11644. https://doi.org/10.1021/acssuschemeng.7b03015
Wang H, Yan T, Shi L, Chen G, Zhang J, Zhang D (2017) Creating nitrogen-doped hollow multiyolk@shell carbon as high performance electrodes for flow-through deionization capacitors. ACS Sustain Chem Eng 5:3329–3338. https://doi.org/10.1021/acssuschemeng.6b03183
Duan H, Yan T, Chen G, Zhang J, Shi L, Zhang D (2017) A facile strategy for the fast construction of porous graphene frameworks and their enhanced electrosorption performance. Chem Commun (Camb) 53:7465–7468. https://doi.org/10.1039/c7cc03424e
Yin H, Zhao S, Wan J, Tang H, Chang L, He L, Zhao H, Gao Y, Tang Z (2013) Three-dimensional graphene/metal oxide nanoparticle hybrids for high-performance capacitive deionization of saline water. Adv Mater 25:6270–6276. https://doi.org/10.1002/adma.201302223
Shi W, Li H, Cao X, Leong ZY, Zhang J, Chen T, Zhang H, Yang HY (2016) Ultrahigh performance of novel capacitive deionization electrodes based on a three-dimensional graphene architecture with nanopores. Sci Rep 6:18966. https://doi.org/10.1038/srep18966
Shi W, Ye C, Xu X, Liu X, Ding M, Liu W, Cao X, Shen J, Yang HY, Gao C (2018) High-performance membrane capacitive deionization based on metal–organic framework-derived hierarchical carbon structures. ACS Omega 3:8506–8513. https://doi.org/10.1021/acsomega.8b01356
Xu D, Tong Y, Yan T, Shi L, Zhang D (2017) N,P-codoped meso-/microporous carbon derived from biomass materials via a dual-activation strategy as high-performance electrodes for deionization capacitors. ACS Sustain Chem Eng 5:5810–5819. https://doi.org/10.1021/acssuschemeng.7b00551
Wang G, Qian B, Dong Q, Yang J, Zhao Z, Qiu J (2013) Highly mesoporous activated carbon electrode for capacitive deionization. Sep Purif Technol 103:216–221
Endarko, Fadilah N, Anggoro D, Rupiasih NN, Suharta WG, Suyanto H (2016) Carbon electrode for desalination purpose in capacitive deionization. AIP Conf Proc AIP Publ 1719:030026
Dehkhoda AM, Ellis N, Gyenge E (2016) Effect of activated biochar porous structure on the capacitive deionization of NaCl and ZnCl2 solutions. Microporous Mesoporous Mater 224:217–228. https://doi.org/10.1016/j.micromeso.2015.11.041
Sriramulu D, Vafakhah S, Yang HY (2019) Activated Luffa derived biowaste carbon for enhanced desalination performance in brackish water. RSC Adv 9:14884–14892. https://doi.org/10.1039/c9ra01872g
Quan G, Wang H, Zhou F, Yan J (2018) Porous biomass carbon coated with SiO2 as high performance electrodes for capacitive deionization. BioResources 13:437–449
Gaikwad MS, Balomajumder C (2017) Tea waste biomass activated carbon electrode for simultaneous removal of Cr(VI) and fluoride by capacitive deionization. Chemosphere 184:1141–1149. https://doi.org/10.1016/j.chemosphere.2017.06.074
Alfredy, T, Jande, YAC, Pogrebnaya, T (2019) Removal of lead ions from water by capacitive deionization electrode materials derived from chicken feathers. J Water Reuse Desal https://doi.org/10.2166/wrd.2019.074
Gaikwad MS, Balomajumder C (2018) Removal of Cr(VI) and fluoride by membrane capacitive deionization with nanoporous and microporous Limonia acidissima (wood apple) shell activated carbon electrode. Sep Purif Technol 195:305–313. https://doi.org/10.1016/j.seppur.2017.12.006
Chen PA, Cheng HC, Wang HP (2018) Activated carbon recycled from bitter-tea and palm shell wastes for capacitive desalination of salt water. J Clean Prod 174:927–932. https://doi.org/10.1016/j.jclepro.2017.11.034
El-Deen AG, Choi J-H, Khalil KA, Almajid AA, Barakat NA (2014) A TiO2 nanofiber/activated carbon composite as a novel effective electrode material for capacitive deionization of brackish water. RSC Adv 4:64634–64642
Porada S, Borchardt L, Oschatz M, Bryjak M, Atchison J, Keesman K, Kaskel S, Biesheuvel P, Presser V (2013) Direct prediction of the desalination performance of porous carbon electrodes for capacitive deionization. Energy Environ Sci 6:3700–3712
Li H, Liang S, Li J, He L (2013) The capacitive deionization behaviour of a carbon nanotube and reduced graphene oxide composite. J Mater Chem 1:6335. https://doi.org/10.1039/c3ta10681k
Sun Z, Chai L, Liu M, Shu Y, Li Q, Wang Y, Qiu D (2018) Effect of the electronegativity on the electrosorption selectivity of anions during capacitive deionization. Chemosphere 195:282–290. https://doi.org/10.1016/j.chemosphere.2017.12.031
Wang G, Dong Q, Ling Z, Pan C, Yu C, Qiu J (2012) Hierarchical activated carbon nanofiber webs with tuned structure fabricated by electrospinning for capacitive deionization. J Mater Chem 22:21819–21823
Zdravkov B, Čermák J, Šefara M, Janků J (2007) Pore classification in the characterization of porous materials: a perspective. Cent Eur J Chem 5:385–395. https://doi.org/10.2478/s11532-007-0017-9
Zornitta RL, Lado JJ, Anderson MA, Ruotolo LAM (2016) Effect of electrode properties and operational parameters on capacitive deionization using low-cost commercial carbons. Sep Purif Technol 158:39–52. https://doi.org/10.1016/j.seppur.2015.11.043
Tsouris C, Mayes R, Kiggans J, Sharma K, Yiacoumi S, DePaoli D, Dai S (2011) Mesoporous carbon for capacitive deionization of saline water. Environ Sci Technol 45:10243–10249. https://doi.org/10.1021/es201551e
Yang K-L, Ying T-Y, Yiacoumi S, Tsouris C, Vittoratos ES (2001) Electrosorption of ions from aqueous solutions by carbon aerogel: an electrical double-layer model. Langmuir 17:1961–1969
Ying TY, Yang KL, Yiacoumi S, Tsouris C (2002) Electrosorption of ions from aqueous solutions by nanostructured carbon aerogel. J Colloid Interface Sci 250:18–27. https://doi.org/10.1006/jcis.2002.8314
Park B-H, Choi J-H (2010) Improvement in the capacitance of a carbon electrode prepared using water-soluble polymer binder for a capacitive deionization application. Electrochim Acta 55:2888–2893. https://doi.org/10.1016/j.electacta.2009.12.084
Lee J-B, Park K-K, Yoon S-W, Park K-I, Leeb C-W (2009) Desalination performance of a carbon-based composite electrode. Desalination 237:155–161. https://doi.org/10.1016/j.desal.2007.1
Alencherry T, Naveen AR, Somnath Ghosh J, Daniel RV (2017) Effect of increasing electrical conductivity and hydrophilicity on the electrosorption capacity of activated carbon electrodes for capacitive deionization. Desalination 415:14–19. https://doi.org/10.1016/j.desal.2017.04.001
Seo SJ, Jeon H, Lee JK, Kim GY, Park D, Nojima H, Lee J, Moon SH (2010) Investigation on removal of hardness ions by capacitive deionization (CDI) for water softening applications. Water Res 44:2267–2275. https://doi.org/10.1016/j.watres.2009.10.020
Jin HK (2015) Fabrication and characterization of nano carbonbased electrochemical double-layer capacitors. Doctor of philosophy in mechanical engineering (nanotechnology) thesis, University of Waterloo, Ontario, Canada
Liu N-L, Sun S-H, Hou C-H (2019) Studying the electrosorption performance of activated carbon electrodes in batch-mode and single-pass capacitive deionization. Sep Purif Technol 215:403–409. https://doi.org/10.1016/j.seppur.2019.01.029
Frackowiak E, Beguin F (2001) Carbon materials for the electrochemical storage of energy in capacitors. Carbon 39:937–950
Mei B-A, Munteshari O, Lau J, Dunn B, Pilon L (2017) Physical interpretations of Nyquist plots for EDLC electrodes and devices. Phys Chem C 122:194–206. https://doi.org/10.1021/acs.jpcc.7b10582
Prabaharan SRS, Vimala R, Zainal Z (2006) Nanostructured mesoporous carbon as electrodes for supercapacitors. J Power Sources 161:730–736. https://doi.org/10.1016/j.jpowsour.2006.03.074
Zhang D, Wen X, Shi L, Yan T, Zhang J (2012) Enhanced capacitive deionization of graphene/mesoporous carbon composites. Nanoscale 4:5440–5446. https://doi.org/10.1039/c2nr31154b
Saleem MW, Kim W-S (2018) Parameter-based performance evaluation and optimization of a capacitive deionization desalination process. Desalination 437:133–143. https://doi.org/10.1016/j.desal.2018.02.023
Mossad M, Zou L (2012) A study of the capacitive deionisation performance under various operational conditions. J Hazard Mater 213-214:491–497. https://doi.org/10.1016/j.jhazmat.2012.02.036
Wang C, Song H, Zhang Q, Wang B, Li A (2015) Parameter optimization based on capacitive deionization for highly efficient desalination of domestic wastewater biotreated effluent and the fouled electrode regeneration. Desalination 365:407–415. https://doi.org/10.1016/j.desal.2015.03.025
Mossad M, Zhang W, Zou L (2013) Using capacitive deionisation for inland brackish groundwater desalination in a remote location. Desalination 308:154–160. https://doi.org/10.1016/j.desal.2012.05.021
Jande YAC, Kim WS (2014) Modeling the capacitive deionization batch mode operation for desalination. J Ind Eng Chem 20:3356–3360. https://doi.org/10.1016/j.jiec.2013.12.020
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elisadiki, J., Kibona, T.E., Machunda, R.L. et al. Biomass-based carbon electrode materials for capacitive deionization: a review. Biomass Conv. Bioref. 10, 1327–1356 (2020). https://doi.org/10.1007/s13399-019-00463-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00463-9