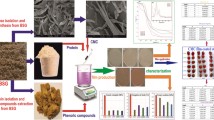
Abstract
Walnut is among the four most consumed dry fruits around the globe. Apart from the edible walnut kernel, walnut fruit consists of a walnut shell (WS) and walnut husk/hull (WH), usually discarded in walnut processing and consumption. These walnut by-products are filled with beneficial compounds that find their use in different fields. This review summarizes recent developments and research on functional aspects of walnut waste (shell and husk/hull) in various fields. WS has many important bioactive compounds, including lignin, cellulose, oleic, and palmitic acids. The creation of WS and carbon-based materials, such as activated carbons and unmodified/modified WS, as adsorbents have been explored. Possible uses for WS-derived by-products include all-natural but powerful adsorbents for eliminating hazardous substances, such as heavy metals, dangerous compounds, and synthetic industrial colors. Similarly, WH also has many beneficial compounds like juglone. WH has antioxidant properties and can be used as textile and protein strainers. These wastes are used in agriculture, laboratory, medical, and food industries, which can be employed as sustainable and environment-friendly alternatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Walnuts are one of the four most consumed dry fruits worldwide and are thus considered an important oil tree, economic and ecological tree species. Walnut fruit comprises three major parts, i.e., walnut kernel, walnut shell (WS), and walnut husk/hull (WH). Even though WSs and husks/hulls are considered processing waste, recent studies have shown many benefits of these by-products showing their potential in the medical, nutrition, agriculture, and industrial field. Mates et al. [1] studied the antioxidant capacity of the walnut septum and reported that the septal hydro-alcohol extracts showed a high antioxidant capacity compared to butylated hydroxyl toluene. The overall composition and application of WS and WH (Fig. 1), while the pictorial representation of WS composition and its application is given in Fig. 2.
The WS is made up of lignin (52.3%), cellulose (25.5%), and hemicellulose (22.2%). The tough, non-toxic, recyclable, and renewable nature of the shell is made possible by its structural makeup. The shell and tissues that have stopped growing are supported by parenchymatous cells. The lignified cells that make up the secondary wall of shells are dense, robust, and hard. WSs can be used to create biological oil rich in organic compounds like phenol, ketone, and ester through pyrolysis. They may be used to develop biological carbon using modified manufacturing techniques which is a key component in creating active carbons with specific surface areas and high porosity [2].
WSs have unique application capabilities in producing supercapacitor electrodes and industrial wastewater treatment. Since WSs are tough and fragile, they can polish delicate equipment and passivate ultra-hard blades after superfine grinding. Additionally, after processing and employing specialized technologies, this shell may be utilized as a composite filler, industrial reductant, material for extracting biomass compounds, and a tool for separating oil from water [3].
The greenish color covering of the walnut surrounding the fruit is known as WH. It is a by-product of the walnut processing business that, if used correctly, can bring many benefits and find its uses in various fields of life [4]. WH not only contains many important polyphenols and phytochemicals but the chemical juglone present in the WH finds its use as a sustainable dyeing agent. Flavonoids are polyphenolic compounds with antioxidant, anti-inflammatory, and anti-carcinogenic properties [5]. Nutraceuticals have a range of benefits, including growth promoters, antibiotics, immunostimulants, antioxidants, flavorings, colorants, and natural substitutes for artificial compounds [6]. Li et al. [7] identified 75 phenolic compounds, including flavonoids, ellagic acid, and gallotanins, in the methanolic extract of the walnut septum. They reported that walnut septum contained 122.78 mg GAE/g dimers and trimers of procyanidins. Walnut septum extracted on human A172 glioblastoma cells. A 70 µg/ml dose significantly reduced the A172 cell viability without altering HFF-1 cell viability [8]. The pictorial representation of WH composition and its application is given in Fig. 3.
This review aims to explore different by-products of walnut produced during processing and identify various biochemicals and components present in the WS and WH and their potential uses. Through the comprehensive research exploration, this review highlights the potential applications of these two by-products in different industries as eco-friendly materials and energy sources for pharmaceutical, nutraceutical, and agriculture applications.
2 Search study
We thoroughly searched the literature in PubMed, Google Scholar, Science Direct, and Scopus for publication addressing the chemical composition and potential application of walnut by-products from 2017- 2023 (January). The keywords used for searching literature were ''walnut'', ''walnut husk'', ''walnut hull'', ''walnut shell'', ''walnut by-products'', ''industrial application of walnut by-products'', "agriculture application of walnut husk", "agriculture application of walnut hull", "agriculture application of walnut shell", "agriculture application of walnut by-product", "medicinal application of walnut husk", "medicinal application of walnut hull", "medicinal application of walnut shell", "medicinal application of walnut by-product", "food application of walnut husk", "food application of walnut hull", "food application of walnut shell", "food application of walnut by-product", ''nutraceutical application of walnut husk", ''nutraceutical application of walnut hull", ''nutraceutical application of walnut shell", ''nutraceutical application of walnut by-product'', "antioxidant profile of walnut by-products'', "antioxidant profile of walnut husk'', "antioxidant profile of walnut hull'', "antioxidant profile of walnut shell", ''nutritional profile of walnut by-products'', ''nutritional profile of walnut husk'', ''nutritional profile of walnut hull", ''nutritional profile of walnut shell" and ''applications of Juglan regia by-products.
3 Important compounds present in the WS
3.1 Investigation of essential oils and fatty acids of WS
Several studies demonstrated that extracts from WSs could be rich in essential oils and fatty acids (FA). The proximate composition of WS is shown in Table 1.
For example, Andrade et al. [12] studied the physicochemical and mechanical attributes of oil and macadamia WSs. The physicochemical and FA profile was examined using NMX-F-083–1986 gas chromatography and mass spectrometry (GC–MS) standards. The FA content in the oil is stearic acid (3.39%), oleic acid (31.74%), palmitic acid (13.61%), linoleic acid (1.21%), and linolenic acid (0.08%). It is shown that the oil has a higher content of oleic acid (31.74%), which is monounsaturated and called omega-9, which involves lipids' metabolism. The Fourier transfer infrared spectroscopy (FT-IR) spectra were used to analyze the sample, and spectra were obtained ranging from 400–4250 cm−1. Two major absorption areas could be seen in the examined sample: the first was between 700–1750 cm−1, and the second was between 2700–3600 cm−1; Hydroxyl (OH) group typical of cellulose could be the source of the signal at 3330 cm−1. The stretching vibration of the carbon-hydrogen bond is what the signal is at 2923 cm−1. A signal associated with the O–H bond of water could be seen between 1630 cm−1 and 1650 cm−1. Another research by Yuan et al. [13] checked the bioactive compounds and FA profile in walnut using GC–MS. The highest to the lowest concentration of the major substances identified in the WS were oleic acid (0.30 mg/g), palmitic acid (0.21 mg/g), sitosterol (0.41 mg/g), and stearic acid (0.06 mg/g).
In another research, the nutritional attributes of the oil from WSs were determined using GC–MS [14]. 26 FA were discovered in the WS, including saturated (13) and unsaturated FAs (13). Saturated FAs included decyclic acid (0.53 mg/kg dry weight (DW), palmitic acid (1778.28 mg/kg DW), arachidic acid (22.7 mg/kg DW), stearic acid (483.7 mg/kg DW). In contrast, unsaturated FA included linoleic acid (7041.5 m/kg DW), oleic acid (2001.71 mg/kg DW), arachidonic acid (1.29 mg/kg DW), and cis-11-eicosenoic acid (15.5 mg/kg DW). Oleic, as well as linoleic acid, was dominant. Linoleic acid is a member of the omega-6 series and is recognized as a necessary compound with good oxidation ability, which lowers the amount of low-density lipoprotein cholesterol and the maintenance of high-density lipoproteins in the blood. These FAs were also called "safety fatty acids." As found by GC and GC–MS, major FAs and oils are present in the WS (Table 2).
4 Investigation of polysaccharide content of WS
Walnut oligopeptides which have a dosage ranging from 110–440 mg/kg, have the potential to greatly enhance innate as well as adaptive immunity by the activation of natural killer cells along with improving in vivo immunoglobulin synthesis. Polysaccharides content of the WS includes cellulose, hemicellulose, and lignin, which are 25, 19, and 56%, respectively [16].
Another study was conducted by Domingos et al. [17] to check the chemical composition of the WS by FT-IR. The results represented that WSs contained lignin (35%), cellulose (30.4%), and 24.9% polysaccharides and hemicellulose. The high content of lignin makes WSs suitable for producing adhesive agents.
Another research checked the impact of WS and salinized WS on the thermal and mechanical attributes of polyurethane (PUR) foams. PUR foams' compressive, flexural, and impact strengths made using polyol (WS-based) range 255–310 kPa, 420–458 kPa, and 340–368 kPa, respectively. The PUR foams' thermal conductivity varied from 0.026 W/mK to 0.032 W/mK, increasing with the amount of polyol. WS has hemicellulose (24.4%), lignin (50.3%) as well as alpha-cellulose (23.9%) [18]. Major polysaccharides present in WSs are given in Table 3.
5 Investigation of phenolics of WS
Another research was performed by Kizatova et al. [20] to check the chemical content of walnut and their application in the food industry. Catechin tannins, quercetin, and P-vitamins are flavonoid compounds found in WSs that are particularly beneficial for the heart and safeguard cognitive processes. Having an antibacterial (antimicrobial) function, they promote connective tissue and enhance blood circulation. Quercetin concentrations in WSs ranged from 0.945–1.51 mg, whereas catechin concentrations ranged from 2.46–12.07 mg, and tannin concentrations ranged from 611.32–805.62 mg.
Another study was performed by Simsek & Sufer [21] to check the antioxidant profile of WS-based tea by employing liquid chromatography coupled with MS. Eight different types of compounds were identified, which were salicylic acid, hydroxybenzoic acid, hydroxycinnamic acid, vanillic acid, trans-resveratrol, catechin hydrate, gallic acid and fumaric acid with concentrations of 5.35, 10.76, 184.79, 371.79, 38.13, 46.21, 56.30 and 235.92 µg/L, respectively.
Han et al. [22] checked the impact of ultrasound-assisted extraction on the characteristics of WS phenolic compounds. The extraction yield from an ultrasonic probe was 51.2 mg GAE/g DW, two times more than that of the ultrasonic bath method (20.6 mg GAE/g DW) and the shaking method (25.8 mg GAE/g DW), respectively. The size of the WSs was reduced, which further increased phenolic extraction. The best extraction yield for the particle size (45–100 mesh) was observed, 52.8 mg GAE/g DW.
6 Applications of WS across various fields
6.1 Industrial applications of WS
Many value-added products extracted from WSs and hulls have found uses in the food industry and other areas. For example, Santigo et al. [23] used two distinct extraction sequences for lignin from WSs. Autohydrolysis, delignification, solid cleansing, and precipitation are the processes involved in lignin formation. The soda procedure used sodium hydroxide solution (7.5%, w/v) as a reagent at 121 °C in the same solid/liquid ratio (1:6) as the organosolv technique (solubilize lignin without affecting its original structure), which used water and ethanol (30:70, v/v) at 200 °C in solid to liquid ratio of 1:6. Lignin was used extensively in lignin-based adhesive and WS-derived activated carbon [24].
A crucial step toward creating light, eco-efficient bricks with increased thermal insulating properties is the creation of agriculture waste-doped burned bricks. Research conducted by Barnabas et al. [25] utilized WS waste as an additive in developing fire ceramics bricks in fired clay. The findings demonstrated higher water absorption and specific heat capacity. Higher WS content was associated with increased porosity and water absorption properties. When walnut content increased, linear shrinkage and thermal conductivity decreased at higher temperatures, i.e., 1100˚C and 950˚C, respectively. Fire temperature enhanced the resistance of bricks against salt crystallization. Another study was conducted by [26] to examine the elimination of metolachlor (MET) by biochar (BC) generated from WS (WSBC) as opposed to BC made using corn cobs (CBC) as well as cow dung (DBC). The removal rates of MET were 87.89%, 10.91%, and 52.91% for WSBC, CBC, and DBC, respectively. Sorption capacities of BCs employing the Langmuir isotherm model were 37.88, 96.15, and 11.98 mg/g for DBC, WSBC, and CBC, respectively. Another research conducted by Mohammed et al. [27] used WS as a sand replacer from cement mortar subjects. The outcomes showed that taking grounded walnut lowered every tested property. Initial experiments with a water-to-cement ratio (w/c) of 0.5 and an optimal grounded walnut utilization ratio of 20% produced a lightweight cement mortar appropriate for structural uses before and after subjection to 400 °C.
Yu et al. [28] investigated the impact of WS extract on Haematococcus pulvialis’s concurrent synthesis of astaxanthin and lipids during abiotic extract. When 15% WS extract was added, it increased the number of lipids and astaxanthin by 77.57% and 23.39%, respectively, versus the control. The result showed increased light-oxygen-voltage histidine kinase (LOV-HK), reactive oxygen species (ROS), and glutathione levels, but chlorophyll, protein, and carbohydrate levels decreased. Zhou et al. [29] investigated the impact of WS on pore structural properties when Zhoundong (ZD) coal is sintered with 1200˚C as the working temperature. According to X-ray computed microtomography (XCT) findings, during the whole sintering process, the porosity of samples ranged from 5.46–40.92%. Small pores with a diameter of less than 0.3 mm or the corresponding spherical diameter comprised most of the pore samples' internal structure. The porosity increased from 42.50% to 50.30% when adding 10% Walnut shell ash (WSA). Other studies regarding the industrial application of WS have been mentioned in Table 4.
7 Environmental applications of WS
To eliminate Cu (II) found in groundwater samples, the study was carried out by [30] to create a novel magnetic nanocomposite using WSA, starch, and Fe3O4. Using WSA and WSA/starch/Fe3O4, maximum Cu (II) sorption capacities of 29.0 mg/g and 45.4 mg/g were observed. Depending on the water quality, the composite adsorbent (AC) could be reused thrice with adequate efficiency based on its capacity to extract copper from three groundwater samples. WSBC was also used by [31] to remove Cr (VI) from wastewater. With a pH of 5.5, 2 h of spinning, and 1.1 g/L of WSBC, it was possible to effectively remediate Cr (VI) by removing up to 93% of the maximum Cr (VI) concentration from an aqueous phase. Palaniyappan et al. [32] prepared a walnut/PLA biopolymeric composite, including a hexagonal lattice structure. According to the experiment results, the optimal printing conditions of a 210˚C nozzle temperature, height (0.1 mm), infill density (100%), and printing speed (20 mm/sec) produced a maximum tensile strength of 2.684 MPa. [33] used WS powder to remove lead from an aqueous medium. At optimum values of the parameters mentioned earlier, for starting 100 mg/L content of Pb2+ and using 1 g/100 mL natural WS dose, more than 90% yield was reached in less than 2 min. From the Langmuir equation, the adsorption capacity was calculated as 9.912 mg/g at room temperature. Dovi et al. [34] used a cationic surfactant modified (CTAB) based functionalized WS surfactant for the removal of bisphenol A (BPA) along with Congo red (CR) dye from the solution. For BPA and CR dye, the maximal monolayer adsorption quantities of WS-CTAB have been determined as 38.5 mg/g and 104.4 mg/g, respectively. The adsorption capability of BPA decreased by 66% in the binary system, whereas CR only slightly reduced by 8.0%. This showed that compared to BPA, WS-CTAB exhibited a greater binding affinity for CR with greater selectivity. Dovi et al. [35] used amino-functionalized WS to remove CR from the solution. At 303 K, the Langmuir model determined that WSAC (walnut shell-based adsorbent) had an adsorption capacity of 224.4 mg/g. Due to a stronger driving force for CR adsorption onto WSAC, column masses transfer capacity parameter (β) rose as concentration increased (0.461, 1.59, and 2.47 for 50, 100, and 150 mg/L, respectively), but adsorbate-adsorbent compatibility (β) showed a declining trend. Similar studies have been displayed in Table 4.
8 Agricultural application of WS
WS or WSBC is extensively used to remove pesticide residue from the soil. For example, another research by Liu et al. [36] applied WSBC to remove MET by adsorption method from the soil. It modified it with montmorillonite, kaolinite, illite, and the original BC. The results demonstrated that montmorillonite BC (MBC) had the most stability, with illite BC (IBC) and kaolinite BC (KBC) having superior thermal and chemical stability than OBC (original BC). OBC, MBC, IBC, and KBC had Met adsorption rates of 62.15, 92.47, 87.97, and 83.31%, respectively. Adsorption mechanisms and the results of the kinetic fitting demonstrated that adding minerals to BC improved MET's physical adsorption. OBC, MBC, IBC, and KBC each had a maximum MET adsorption capacity of 39.68, 68.49, 65.79, and 65.36 mg/g, respectively.
8.1 Use of WS as a Catalyst of WS
There is a great trend in developing eco-friendly catalysts because metal-based catalysts harm the environment. For example, research performed by Wu et al. [37] used WS as the raw material in their experiment to create nitrogen-doped BC, which is then used as a green catalyst for the chlorination of acetylene using the condensation product of dicyandiamide or dicyandiamide-formaldehyde as a nitrogen source. A large proportion of quaternary nitrogen as the active center is responsible for the 2.5 DF/BC-850 catalyst's unusual catalytic activity, which results in acetylene conversion (94.5%) at 45 h−1 and 220 °C. The metal-free nitrogen-doped BC catalyst's thermal stability is increased by the addition of formaldehyde, which successfully prevents carbon from depositing throughout the catalytic reaction.
Jovičić et al. [38] studied the liquefaction characteristic of distinct WS particle sizes (five) employing liquefaction agent (glycerol) under different reaction parameters. Results showed that WS biomass might be successfully converted into glycerol utilizing sulfuric acid as a catalyst, with liquefaction efficiencies ranging from 89.21–90.98%.
8.2 Food applications of WS
To preserve product quality, Zhang et al. [39] used a procedure involving the pasteurization of in-shell walnuts (15.01% moisture) employing a radio frequency oven (27.12 MHz; 6 kW). During variations in kernel color among radio frequency (RF) samples and control after accelerated storage were insignificant, this RF treatment yielded more than the decrease in Staphylococcus aureus ATCC (4-log). FA levels of both samples were under 0.6% throughout storage. In contrast, the peroxide content of RF treatments rose by more than 1 mEq/kg during storage but did not significantly vary from controls. During accelerated storage, moisture and water activities of WS and kernels of both treatments first declined and then stabilized. S. aureus ATCC 25923's population in RF treatments steadily declined throughout storage to levels below the detection threshold. Other food applications of WSs have been summarized in Table 4.
8.3 Medicinal applications of WS
Copper nanoparticles (CuNPs) attached to cellulose WS material were studied for their antibacterial, antioxidant, and anticancer properties. By raising the concentration to 10%, CuNPs' antioxidant capabilities were markedly enhanced. On K562 cells, CuNPs have a dose-dependent cytotoxic impact. The IC50 of materials against PBMCs (peripheral blood mononuclear cells) was substantially higher than the IC50 of the produced NPs against K562 cancer cells (25.24 5 g/mL) [40].
Moxibustion with WS glasses has been examined to determine its effectiveness and acceptability in treating patients with dry eye disease (DED) and to offer therapy choices. Moxibustion combined with WS eyewear may help DED patients stabilize their clinical symptoms and tear film. However, it is not significantly more effective than sodium hyaluronate eye drops in reducing corneal damage and tear production. Compared to sodium hyaluronate eye drops, moxibustion with WS spectacles might have a superior impact on ocular pain (self-assessment) [41]. Similar medical applications of WSs have been shown in Table 4.
9 Walnut husk/hull
9.1 Investigation of essential oils and fatty acids in WH
Supercritical carbon dioxide extraction of WH shows that WH is more unsaturated than saturated FAs. The most abundant saturated FA present in WH extract was found to be palmitic acid which was 27.16%. Linoleic acid and eicosenoic acid were major unsaturated FAs present in WH, with 33.83% and 21.90%, respectively. Even though supercritical fluid extraction of WH showed an abundance of unsaturated FAs, docosahexaenoic acid (0.31%) was found to present in a minor amount [61].
9.2 Investigation of polysaccharides in WH
Polysaccharides present in walnut green husk/hull (GWH) are found to be beneficial in terms of their role in preventing inflammation, oxidative stress, and obesity, as well as damage to the colon or liver due to a high-fat diet among rats. These polysaccharides positively affect gut microbiota and enhance the number of short-chain FAs [62]. Another study by He et al. [63] focused on isolating and characterizing polysaccharides (water soluble) from the WH. Glucose, Galactose, along with Arabinose containing heteropolysaccharides were observed. Other monosaccharides present in WH included rhamnose, ribose, mannose, and fucose.
9.3 Investigation of polyphenols and phytochemicals in WH
Supercritical carbon dioxide extraction of WH showed the high antioxidant capacity of polyphenols present in it, as shown by 2,2-diphenyl-1-picryl-hydrazine-hydrate (DPPH) and 2,2'-casino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) analyses. 10,750 mg GAE/100 g total polyphenols were measured in the extract using supercritical carbon dioxide extraction, while the water extract of WH contained total polyphenolic content of 3038.32 mg GAE/100 g. The hydroxybenzoic acid, chlorogenic acid, syringic acid, vanillic acid, caffeic acid, o-coumaric acid, ferulic acid as well as juglone was identified in WH extract in large amounts of 103.98 mg/100 g, 27.88, 6.16, 15.34, 631.78, 986.96, 77.03 and 1192.04 mg/100 g, respectively. The chlorogenic (23.37 mg/100 g) and caffeic acid (121.77 mg/100 g) were found to be in greater quantity in methanolic extracts of WH [61].
Ultra-high-performance liquid chromatography coupled to Orbitrap high-resolution mass spectrometry (UPLC-Q_Orbitrap HRMS) and high-performance liquid chromatography (HPLC) test of the WH and a pellicle, was carried out to identify 110 different compounds, including tannins, phenolics, flavonoids, and quinones. WH's total phenolic and flavonoid content ranged from 0.54–1.33 mg gallic acid and 0.34–1.01 mg rutin equivalent/g, respectively. Gallotannins were found in large quantities, while ( +)-catechin was found to be the major falavan-3-ol. Naphthoquinones and flavonoids were majorly identified in the husk compared to the pellicle. WH contained compounds like quercetin pentoxide isomer, prorcatechualdehyde 7-hydroxy-methyl coumarin, and taxifolin [64].
10 Applications of WH across various fields
10.1 6.1 Industrial Applications of WH
GWH and compounds like sodium lignosulfonate work synergistically to inhibit the corrosion of materials like cold-rolled steel showing an increased inhibition efficiency (97.2%) [65]. Similarly, the inhibitory impact of WH on the corrosion of mild steel samples in 1 M hydrochloric acid (HCl) electrolyte has also been observed [66], as mentioned in Table 5. Wu et al. [67] also utilized WH extract to inhibit the corrosion of magnesium alloys submerged in sodium chloride solution. The extract was observed to increase inhibition efficiency from 44.7% to 92.5%.
WH may act as a mordant in the dyeing industry. Hosseinnezhad et al. [68] used WH combined with myrobalan as a mordant to dye wool yarn up to 10%. A 7.5:2.5 ratio of WH and myrobalan produced the best results. In another study, color strength and fastness were improved when alginate and WH were used as a mordant to dye viscose rayon fabric using an anthocyanin solution obtained from Hibiscus sabdariffa L. calyces [69]. Wool yarn dyed with WH possessed antimicrobial activity of up to 35%. When the dyed wool yarns were treated with sodium salt NPs, their excellent antimicrobial property was observed, maintaining themselves (91%) after ten repeated washes. A good wash and light fastness properties were also marked [70, 71], and they also attempted to print alpaca wool using WH and iron sulfate. WH-extracted pectin using ultrasound-assisted extraction has been found to contain good emulsifying attributes [72]. A 2.15 times increase in versatile peroxidase production has also been observed by Pleurotus eryngii through solid-state fermentation [73].
10.2 Environmental applications of WH
Methanolic WH extract has the potential to act as a sustainable and environment-friendly replacement of propyl gallate as the antioxidant to improve the oxidation stability index of biodiesels like wasted cooking oil [74]. The comparison of WH extract and propyl gallate through life cycle assessment also proves the ability of WH as an eco-friendly antioxidant for biodiesel fuels.
WH extracts have also been found as environment-friendly absorbents of toxic dyes and metals from the environment. Titan yellow dye also has been removed using WHs up to more than 85.5% when used in a dose of 0.06 g with a dye concentration of 15 ppm [75]. WHs as composites with other compounds have also been administered for the same purposes. Polyaniline and WH nanocomposite have been studied and found cheap, efficient AC of E102 dye [76]. Similarly, nanocomposites of WH extract and silver (Ag) have efficiently removed heavy metals like lead, chromium, and cadmium in wastewater produced during petroleum treatment. 0.75 g of these NPs can remove lead, chromium, and cadmium up to 72.6, 81.3, and 88.1% during 5 h [77]. Other environmental applications of WH have been shown in Table 5.
10.3 Agriculture applications of WH
WH extracts have been found to contain insecticide and nematicide properties showing their potential in agriculture to replace pesticides detrimental to humans and the environment, as shown in Table 5. The nematicidal effect of WH has also been observed against Pratylenchus thornei [78] and Meloidogyne javanica [79]. Insecticide activity against Pieris rapae larvae and Helicoverpa armigera larvae has also been followed by the walnut extract showing mortality in the shape of stomach toxicity up to 50% [80].
10.4 Food applications of WH
WH extract is being utilized in many industries to preserve food items and improve shelf life, as shown in Table 5. Wichita pecans were preserved using a juglone coating from the WH [81]. At the same time, freshly cut apples [82] and rainbow trout [83] also improved shelf life and sensory attributes when WH extract was employed. Dehghani et al. [84] attempted to add WH extract to ketchup to improve its antimicrobial attributes and found an improved quantity of flavonoids in the prepared ketchup. WH extract was also added to sunflower extract to prevent oxidation.
10.5 Nutraceutical/Medicinal Applications of WH
Medicinal properties of WH have also been studied in recent years, and WHs are an effective remedy for many diseases. WH has been found beneficial against cancerous cells. SCG7901 (human gastric cell line) was observed to show inhibition of cancer cell proliferation when treated with WH extract [85]. Twenty-seven naphthoquinones and their derivatives were extracted from GWH and studied for their cytotoxic effect against the human cancer cell line HepG-2 [86] also isolated 13 compounds from husk through recrystallization and column chromatography. They studied their effect on hepatoma cells (HepG-2). Oleanolic acid extracted from WH was found to show an inhibitory effect against human cancer cell lines.
WH is efficient in patients with liver injury. Juglanin has been found to reduce fibrosis and inflammation among mice that suffer from acute liver injury [87]. Similarly, WH extracts protected mice from hepatic steatosis and vascular endothelial dysfunction among fructose-induced mice [88]. WH has been found to improve lipid levels and the gut microbiome of obese rats due to high-fat diets. It improves insulin resistance and glucose metabolism and reduces fat accumulation and adipose tissue hypertrophy among obese rats. The gut microbiota is also changed when WH extract was given to the rats as it reversed the disorders of the gut microbiome by reducing Fuso bacteria, Firmicutes and Peptostreptococcaceae along with increasing Bacteroidetes at the phylum level [89]. Wang et al. [62] also observed inhibition of chronic inflammatory responses and colonic tissue damage among rats fed high-fat diets due to WH extract.
WH extract possesses antimicrobial and antioxidant properties. Hence, it has been found effective against acne vulgaris [90]. In infected rats, candidiasis can also be efficiently treated with WH-based cream [91]. WH has been found to inhibit α-glucosidase, which is a key factor of hyperglycemia, and controlling it can be an effective way of controlling postprandial blood glucose levels [92]. WH extract has been observed to prevent platelet aggregation. Acetone extract from WH has demonstrated protein secretion by 50% along with prevention of aggregation of platelets. The extract activates caspase and suppresses the generation of ROS [93]. WH has also demonstrated wound-healing properties among rats with incisions. [94, 95] studied the potential of WH-extracted juglone to load scaffolds and be used as a potential wound dressing. Other medicinal studies regarding WH are shown in Table 5.
10.6 Cosmetic industry applications of WH
WH found its use in the cosmetic industry and natural and non-irritant hair dye. Beiki et al. [96] developed hair dye using WH as a dye, aloe vera as second, ferrous sulfate as the primary mordant, and ascorbic acid (developer). The hair dye resulted in dark brown color when applied to hair samples. Good color strength, along with hair surface morphology, was observed. Good resistance to daylight fastness and washing was also demonstrated. Irritation tests on rat models were also done, which showed no irritation and maximum antimicrobial attributes when compared with commercial and semi-synthetic dyes. Hair dye developed from an iron mordant and WH extract incorporated in hydrogel hair dyes were also used for dyeing grey unbleached human hairs, which showed good color strength. The irritation potential of plant dyes was also assessed using a reconstructed human epidermis which showed no potential for the developed dye to cause skin irritation, as mentioned in Table 5.
10.7 Laboratory applications of WH
WH extract is employed in some studies as an alternative staining gel, as demonstrated in Table 5. Aqueous extract of the WH can stain proteins in polyacrylamide gels in about ten minutes, with its sensitivity comparable to Coomassie R-250. The bands produced by hull extract are more intense than those produced by Ponceau S and Coomassie. Similarly, WH extract has been used to stain Platyhelminthes [97]. Apart from its use as a staining dye, WH acts as a great catalyst for the synthesis of NPs like phenolic capped silver nanoparticles (AgNPs) that act as colorimetric sensors [98] and benzylpyrazolyl coumarin derivatives [99].
11 Conclusion and future recommendations
WS and WH are the by-products of walnut processing and consumption and comprise many beneficial bioactive compounds. WS and husk/hull are extensively used in agriculture, industrial, medicinal, cosmetics, and environmental fields. These by-products can act as sustainable, environment-friendly, low-cost alternatives to many expensive toxic chemicals. The thorough review of the literature also reveals the gaps in the current understanding and points to the need for additional research to (I) predict the performance of the adsorption process for the removal of different adsorbates by WS from real effluents under a wide range of operating conditions, (II) look into the removal of adsorbates from mixed wastewater, (III) better understand the adsorption mechanism of various hazardous materials on WS, (IV) confirm the viability of various chemically modified WS types on an industrial scale. Moreover, there is a need for more research regarding the potential health effect of the bioactive compounds present in these waste products on humans and studies regarding their safe and effective dosage and the side effects of their overdose so that they can be employed as nutraceuticals. Even though the positive uses of these by-products are many, there is a need for the systematic implication of these by-products at a large scale, which needs proper communication between researchers and respective stakeholders.
Data availability
Not applicable.
References
Mateș L, Rusu ME, Popa D-S (2023) Phytochemicals and Biological Activities of Walnut Septum: A Systematic Review. J Antioxidants 12(3):604
Yuan X, Zhu X, Zhang L, Luo Z, Zhu X (2020) Fundamental Insights into Walnut Shell Bio-Oil Electrochemical Conversion: Reaction Mechanism and Product Properties. BioEnergy Res 14(1):322–332
Zhou S, Wei Y, Li B, Wang H (2019) Cleaner recycling of iron from waste copper slag by using walnut shell char as green reductant. J Clean Prod 217:423–431
Barczewski M, Sałasińska K, Szulc J (2019) Application of sunflower husk, hazelnut shell and walnut shell as waste agricultural fillers for epoxy-based composites: A study into mechanical behavior related to structural and rheological properties. Polym Testing 75:1–11
Tahir Z, Khan MI, Ashraf U, Mubarik UJIJAB (2023) Industrial Application of Orange Peel Waste; A Review. Int J Agric Biosci 12(2):71–76
Sultanayeva L, Balji Y, Korotkiy V, Shantyz A, Issabekova S, Borovskiy A, Maier Y, Abakanova GJIJVS (2023) Characterisation of phenolics in fruit septum of Juglans regia Linn. by ultra performance liquid chromatography coupled with Orbitrap mass spectrometer. Int J Vet Sci 12(1):114–119
Liu P, Li L, Song L, Sun X, Yan S, Huang W (2019) Characterisation of phenolics in fruit septum of Juglans regia Linn. by ultra-performance liquid chromatography coupled with Orbitrap mass spectrometer. J Food Chem 286:669–677
Genovese C, Cambria MT, D’angeli F, Addamo AP, Malfa GA, Siracusa L, Pulvirenti L, Anfuso CD, Lupo G, Salmeri M (2020) The double effect of walnut septum extract (Juglans regia L.) counteracts A172 glioblastoma cell survival and bacterial growth. Int J Oncol 57(5):1129–1144
El Hamdouni Y, El Hajjaji S, Szabó T, Trif L, Felhősi I, Abbi K, Labjar N, Harmouche L, Shaban A (2022) Biomass valorization of walnut shell into biochar as a resource for electrochemical simultaneous detection of heavy metal ions in water and soil samples: Preparation, characterization, and applications. Arab J Chem 15(11):10452.
Naderi M, Vesali-Naseh MJBC (2021) Biorefinery, Hydrochar-derived fuels from waste walnut shell through hydrothermal carbonization: characterization and effect of processing parameters. Biomass Conversion and Biorefinery 11(5):1443–1451
Li K, Jiang Q, Chen G, Gao L, Peng J, Chen Q, Koppala S, Omran M, Chen J (2021) Kinetics characteristics and microwave reduction behavior of walnut shell-pyrolusite blends. Bioresour Technol 319:124172
Andrade JEC, Garibay AS, Solís II, Cruz UG, Reynoso ANM (2017) Study of the physicochemical and mechanical properties of oil and macadamia walnut shell. J Mech Civil Eng 14(5):8–12
Yuan X, Zhu X, Zhang L, Luo Z, Zhu XJBR (2021) Fundamental Insights into Walnut Shell Bio-Oil Electrochemical Conversion: Reaction Mechanism and Product Properties. Bio Energy Res 14(1):322–332
Hu Q, Liu J, Li J, Liu H, Dong N, Geng YY, Lu Y, Wang Y (2020) Phenolic composition and nutritional attributes of diaphragma juglandis fructus and shell of walnut (Juglans regia L.). Food Sci Biotechnol 29(2):187–196
Herrera R, Hemming J, Smeds A, Gordobil O, Willfor S, Labidi J (2020) Recovery of bioactive compounds from hazelnuts and walnuts shells: quantitative-qualitative analysis and chromatographic purification. Biomolecules 10(10):1363
Rodriguez-Padron D, Zhao D, Garín Ortega RN, Len C, Balu AM, García A, Luque R (2020) Characterization and antioxidant activity of microwave-extracted phenolic compounds from biomass residues. J ACS Sustain Chem Eng 8(3):1513–1519
Domingos I, Ferreira J, Cruz-Lopes LP, Esteves B (2022) Liquefaction and chemical composition of walnut shells. J Open Agriculture 7(1):249–256
Członka S, Strąkowska A, Kairytė A (2020) Effect of walnut shells and silanized walnut shells on the mechanical and thermal properties of rigid polyurethane foams. Polymer Testing 87:106534
Queirós CSGP, Cardoso S, Lourenço A, Ferreira J, Miranda I, Lourenço MJV, Pereira H (2019) Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Biomass Conversion Biorefinery 10(1):175–188
Kizatova M, Sultanova M, Baikenov A, Saduakas A, Akzhanov N (2022) Revealing the features of the composition of the walnut shell from the point of view of the possibility of its use in the food industry. Eastern-Eur J Enterprise Technol 1(11(115)):49–55
Simsek M, Süfer Ö (2021) Infusion of walnut (Juglans regia L.) shell tea: multi-response optimization and antioxidant potential. J Appl Res Med Aromatic Plants 20:100278
Han H, Wang S, Rakita M, Wang Y, Han Q, Xu Q (2018) Effect of Ultrasound-Assisted Extraction of Phenolic Compounds on the Characteristics of Walnut Shells. Food Nutr Sci 09(08):1034–1045
Santiago B, Martelo Smalbach N, González Alriols M, Feijoo G, Labidi J, González-García S (2022) Environmental assessment of different integrated biorefinery scenarios using walnut shells as a source for lignin production. Publishing ECI DIGITAL Web. Archives https://dc.engconfintl.org/lca_waste_3/31. Accessed 10 Jun 2022
Wang L, Feng X, Li X, Ma H, Wu J, Chen Y, Zhou JJD, Materials R (2022) Valorization of lignin: application of lignin-derived activated carbon in capacitors and investigation of its textural properties and electrochemical performance. Diam Relat Mater 122:108791
Barnabas AA, Balogun OA, Akinwande AA, Ogbodo JF, Ademati AO, Dongo EI, Romanovski V (2022) Reuse of walnut shell waste in the development of fired ceramic bricks. Environ Sci Pollut Res Int 30(5):11823–1837
Liu L, Dai Y (2021) Strong adsorption of metolachlor by biochar prepared from walnut shells in water. Environ Sci Pollut Res Int 28(35):48379–48391
Mohammed AS, Hilal NN, Mohammed Ali TK, Sor NH (2021) An investigation of the effect of walnut shell as sand replacement on the performance of cement mortar subjected to elevated temperatures. J Phys: Conf Ser 1973(1):012034
Yu C, Li X, Han B, Zhao Y, Geng S, Ning D, Ma T, Yu X (2021) Simultaneous improvement of astaxanthin and lipid production of Haematococcus pluvialis by using walnut shell extracts. Algal Research 54:10217
Zhou H, Luo J, Wang Z, Ji M, Zhang M (2021) Effect of walnut shell ash on pore structure characteristics during Zhundong coal sintering. Fuel Processing Technology 221:106923
Foroutan R, Peighambardoust SJ, Mohammadi R, Peighambardoust SH, Ramavandi B (2022) Development of new magnetic adsorbent of walnut shell ash/starch/Fe(3)O(4) for effective copper ions removal: Treatment of groundwater samples. Chemosphere 296:133978
Kokab T, Ashraf HS, Shakoor MB, Jilani A, Ahmad SR, Majid M, Ali S, Farid N, Alghamdi RA, Al-Quwaie DAH, Hakeem KR (2021) Effective removal of Cr(VI) from wastewater using biochar derived from walnut shell. Int J Environ Res Public Health 18(18):9670
Palaniyappan S, Veeman D, Sivakumar NK, Natrayan L (2022) Development and optimization of lattice structure on the walnut shell reinforced PLA composite for the tensile strength and dimensional error properties. Structures 45:163–178
Çelebi H, Gök O (2017) Evaluation of Lead Adsorption Kinetics and Isotherms from Aqueous Solution Using Natural Walnut Shell. Int J Environ Res 11(1):83–90
Dovi E, Aryee AA, Kani AN, Mpatani FM, Li J, Li Z, Qu L, Han R (2021) Functionalization of walnut shell by grafting amine groups to enhance the adsorption of Congo red from water in batch and fixed-bed column modes. J Environ Chem Eng 9(5):106301
Dovi E, Kani AN, Aryee AA, Jie M, Li J, Li Z, Qu L, Han R (2021) Decontamination of bisphenol A and Congo red dye from solution by using CTAB functionalised walnut shell. Environ Sci Pollut Res Int 28(22):28732–28749
Liu L, Li X, Wang X, Wang Y, Shao Z, Liu X, Shan D, Liu Z, Dai Y (2022) Metolachlor adsorption using walnut shell biochar modified by soil minerals. J Environmental Pollution 308:119610
Wu S, Jiang A, Zhou X, Liu Y, Cao S (2022) Environmentally friendly high-efficient metal-free catalyst for acetylene hydrochlorination derived from walnut shell-based N-doped biochar. Molecular Catalysis 532:112719
Jovičić N, Antonović A, Matin A, Antolović S, Kalambura S, Krička T (2022) Biomass Valorization of Walnut Shell for Liquefaction Efficiency. Energies 15(2):495
Zhang L, Lyng JG, Xu R, Zhang S, Zhou X, Wang S (2019) Influence of radio frequency treatment on in-shell walnut quality and Staphylococcus aureus ATCC 25923 survival. Food Control 102:197–205
Mehdizadeh T, Zamani A, AbtahiFroushani SM (2020) Preparation of Cu nanoparticles fixed on cellulosic walnut shell material and investigation of its antibacterial, antioxidant and anticancer effects. Heliyon 6(3):e03528
Zhang G, Fu W, Xu J, Hu P, Zhang Y, Sang Z, Wu W, Zheng K, Wu L, Liu Z (2022) Moxibustion with Walnut Shell Spectacles Could Improve the Objective Symptoms and Tear Film Stability of Patients with Dry Eye Disease: A Randomized Controlled Trial. Evid Based Complement Alternat Med 2022:1773444
Cebin AV, Ralet MC, Vigouroux J, Karaca S, Martinic A, Komes D, Bonnin E (2021) Valorisation of walnut shell and pea pod as novel sources for the production of xylooligosaccharides. Carbohydr Polym 263:117932
Villasante J, Martin-Lujano A, Almajano MP (2020) Characterization and Application of Gelatin Films with Pecan Walnut and Shell Extract (Carya illinoiensis). Polymers (Basel) 12(6):1424
Ali A, Ali S, Yu L, Liu H, Khalid S, Hussain A, Qayum MMN, Ying C (2019) Preparation and characterization of starch-based composite films reinforced by apricot and walnut shells. J Appl Polymer Sci 136(38):47978
Foroutan R, Peighambardoust SJ, Mohammadi R, Peighambardoust SH, Ramavandi B (2022) Application of walnut shell ash/ZnO/K2CO3 as a new composite catalyst for biodiesel generation from Moringa oleifera oil. Fuel 311:122624
Yabalak E, Erdogan Eliuz EA (2022) Green synthesis of walnut shell hydrochar, its antimicrobial activity and mechanism on some pathogens as a natural sanitizer. Food Chem 366:130608
Zou X, Xu K, Chang W, Qu Y, Li Y (2021) A novel microalgal biofilm reactor using walnut shell as substratum for microalgae biofilm cultivation and lipid accumulation. Renewable Energy 175:676–685
Hekimoğlu G, Sarı A, Kar T, Keleş S, Kaygusuz K, Tyagi V, Sharma R, Al-Ahmed A, Al-Sulaiman FA, Saleh TAJJOES (2021) Walnut shell derived bio-carbon/methyl palmitate as novel composite phase change material with enhanced thermal energy storage properties. J Energy Storage 35:102288
Zhao H, Yu Q, Li M, Sun S (2020) Preparation and water vapor adsorption of “green” walnut-shell activated carbon by CO2 physical activation. Adsorpt Sci Technol 38(1–2):60–76
Hasanzadeh R, AbbasiSouraki B, Pendashteh A, Khayati G, Ahmadun FR (2020) Application of isolated halophilic microorganisms suspended and immobilized on walnut shell as biocarrier for treatment of oilfield produced water. J Hazard Mater 400:123197
Uslu OS, Babur E, Alma MH, Solaiman ZM (2020) Walnut Shell Biochar Increases Seed Germination and Early Growth of Seedlings of Fodder Crops. Agriculture 10(10):427
Xiong X, Wang Y, Liao X, Lai S, Zhang H (2019) Preparation of walnut shell powders-supported Cu nanoparticles and its application in ynones synthesis. J Chem Industry Forest Products 39(2):103–108
Halysh V, Sevastyanova O, Riazanova AV, Pasalskiy B, Budnyak T, Lindström ME, Кartel M (2018) Walnut shells as a potential low-cost lignocellulosic sorbent for dyes and metal ions. Cellulose 25(8):4729–4742
Zamani A, PoursattarMarjani A, Nikoo A, Heidarpour M, Dehghan A (2018) Synthesis and characterization of copper nanoparticles on walnut shell for catalytic reduction and C-C coupling reaction. Inorganic Nano-Metal Chem 48(3):176–181
Hemmati F, Jafari SM, Kashaninejad M, BaraniMotlagh M (2018) Synthesis and characterization of cellulose nanocrystals derived from walnut shell agricultural residues. Int J Biol Macromol 120(Pt A):1216–1224
Bordbar M, Mortazavimanesh N (2017) Green synthesis of Pd/walnut shell nanocomposite using Equisetum arvense L. leaf extract and its application for the reduction of 4-nitrophenol and organic dyes in a very short time. Environ Sci Pollut Res Int 24(4):4093–4104
Kamal I, Sherwani A, Ali A, Khalid A, Saadi I, Harbi AJJCER (2017) Walnut shell for partial replacement of fine aggregate in concrete: modeling and optimization. J Civil Eng Res 4:109
Zhang L, Ma H, Wang SJL (2021) Pasteurization mechanism of S. aureus ATCC 25923 in walnut shells using radio frequency energy at lab level. LWT-Food Sci Technol 143:111129
Arama ZA (2021) Experimental and numerical study to investigate the usability of walnut shell waste as an additive for foundations of highway embankments. Arab J Geosci 14(18):1–17
Lee HJ, Ohk SH (2021) Technology, physiological study of the extract of junglans nigra shells for the cosmeceutical application. J Korean Appl Sci Technol 38(1):29–36
Romano R, Aiello A, Meca G, De Luca L, Pizzolongo F, Masi P (2021) Recovery of bioactive compounds from walnut (Juglans regiaL.) green husk by supercritical carbon dioxide extraction. Int J Food Sci Technol 56(9):4658–4668
Wang G, Yang X, Wang J, Zhong D, Zhang R, Zhang Y, Feng L, Zhang Y (2021) Walnut green husk polysaccharides prevent obesity, chronic inflammatory responses, nonalcoholic fatty liver disease, and colonic tissue damage in high-fat diet-fed rats. Int J Biol Macromol 182:879–898
He N, Zhai X, Zhang X, Zhang X, Wang X (2020) Extraction, purification and characterization of water-soluble polysaccharides from green walnut husk with anti-oxidant and anti-proliferative capacities. Process Biochem 88:170–179
Sheng F, Hu B, Jin Q, Wang J, Wu C, Luo Z (2021) The Analysis of Phenolic Compounds in Walnut Husk and Pellicle by UPLC-Q-Orbitrap HRMS and HPLC. Molecules 26(10):3013
Li X, Deng S, Du G, Xie X (2020) Synergistic inhibition effect of walnut green husk extract and sodium lignosulfonate on the corrosion of cold rolled steel in phosphoric acid solution. J Taiwan Inst Chem Eng 114:263–283
Shahmoradi AR, Ranjbarghanei M, Javidparvar AA, Guo L, Berdimurodov E, Ramezanzadeh B (2021) Theoretical and surface/electrochemical investigations of walnut fruit green husk extract as effective inhibitor for mild-steel corrosion in 1M HCl electrolyte. J Mol Liquids 338:116550
Wu Y, Zhang Y, Jiang Y, Li N, Zhang Y, Wang L, Zhang J (2021) Exploration of walnut green husk extract as a renewable biomass source to develop highly effective corrosion inhibitors for magnesium alloys in sodium chloride solution: Integrated experimental and theoretical studies. Colloids Surf A: Physicochem Eng Asp 626:126969
Hosseinnezhad M, Gharanjig K, Iamin H, Rouhani S, Adeel S (2022) Environmentally dyeing of wool yarns using of combination of myrobalan and walnut husk as bio-mordants, J Progress in Color, Colorants Coatings 16(2):197–205
ShahmoradiGhaheh F, KamaliMoghaddam M, Tehrani M (2022) Eco-Friendly Dyeing of Viscose Rayon Fabrics Using Anthocyanin from Hibiscus sabdariffa Linn. J Nat Fibers 19:1–11
Sadeghi-Kiakhani M, Tehrani-Bagha AR, Gharanjig K, Hashemi E (2019) Use of pomegranate peels and walnut green husks as the green antimicrobial agents to reduce the consumption of inorganic nanoparticles on wool yarns. J Clean Prod 231:1463–1473
Glogar M, Tancik J, Brlek I, Sutlovic A, Tkalec M (2020) Optimisation of process parameters of Alpaca wool printing withJuglans regianatural dye. Coloration Technol 136(2):188–201
Asgari K, Labbafi M, Khodaiyan F, Kazemi M, Hosseini SS (2020) High-methylated pectin from walnut processing wastes as a potential resource: Ultrasound assisted extraction and physicochemical, structural and functional analysis. Int J Biol Macromol 152:1274–1282
Lü C, Luo X, Dong X, Peng J, Cao F (2019) Enhanced versatile peroxidase production by Pleurotus eryngii using saccharide-and fatty acid-rich agro-industrial waste in solid-state fermentation. J Biobased Mater Bioenergy 13(1):134–139
Khounani Z, Hosseinzadeh-Bandbafha H, Nizami A-S, Sulaiman A, Goli SAH, Tavassoli-Kafrani E, Ghaffari A, Rajaeifar MA, Kim K-H, Talebi AF, Aghbashlo M, Tabatabaei M (2020) Unlocking the potential of walnut husk extract in the production of waste cooking oil-based biodiesel. Renew Sustain Energy Rev 119:109588
Ibrahim HK, Allah MAAH, Muneer A (2021) Adsorption of Titan Yellow Using Walnut Husks: Thermodynamics Kinetics and Isotherm Studies. J Ann Rom Soc Cell Biol 25(6):12576–12587
Kadhium SS, Al-Lamei AJ, Majed N (2021) Removal of E102 dye from aqueous solution by adsorption on the surface of polyaniline walnut husks nanocomposite. J Egyptian J Chem 64(9):4783–4790
Ituen E, Yuanhua L, Verma C, Alfantazi A, Akaranta O, Ebenso EE (2021) Synthesis and characterization of walnut husk extract-silver nanocomposites for removal of heavy metals from petroleum wastewater and its consequences on pipework steel corrosion. J Mol Liquids 335:116132
Esteves I, Maleita C, Fonseca L, Braga ME, Abrantes I, De Sousa HC (2017) In vitro nematicidal activity of naphthoquinones against the root-lesion nematode Pratylenchus thornei. J Phytopathologia Mediterranea 56(1):127–132
Alikarami M, Charehgani H, Abdollahi M (2018) Nematicidal activity of some plant extracts on root-knot nematode on tomato (Solanum lycopersicum) in vitro and in vivo conditions. Iranian J Plant Protection Sci 48(2):317–326
Hu W, Du W, Bai S, Lv S, Chen G (2018) Phenoloxidase, an effective bioactivity target for botanical insecticide screening from green walnut husks. Nat Prod Res 32(23):2848–2851
Li W, Wang C, Ye J (2022) Decolorizing shellac incorporated with natural antibacterial juglone from walnut green husk extract for preserving the postharvest quality of Wichita pecans (Carya illinoinensis [Wangenh.] K. Koch) during storage. Scientia Horticulturae 304:111313
Jiang L, Wang F, Xie X, Xie C, Li A, Xia N, Gong X, Zhang H (2022) Development and characterization of chitosan/guar gum active packaging containing walnut green husk extract and its application on fresh-cut apple preservation. Int J Biol Macromol 209(Pt A):1307–1318
Mozaffari P, Pashangeh S, Berizi E, Majlesi M, Hosseinzadeh S, Salehi SO, Derakhshan Z, Giannakis S (2022) Potential of nanochitosan coating combined with walnut green husk to improve the preservation of rainbow trout (Oncorhynchus mykiss) during refrigerated storage. Environ Res 214(Pt 3):114019
Dehghani S, Nouri M, Baghi M (2019) The effect of adding walnut green husk extract on antioxidant and antimicrobial properties of ketchup. J Food Bioprocess Eng 2(2):93–100
Zhang J, Zhang J, Zhao C, Sui H, Li CF, Zhong L, Zhou Q, Bai Y, An S, Du X, Wei X, Liu L (2022) Green walnut husk extracts Proliferation and Migration in Gastric Cancer. J Cancer 13(4):1130–1144
Fulin Y, Tiantian YJMP (2019) Chemical Constituents and Antitumor Activities of Walnut Green Husk. Medicinal plant 10(2):17–24
Dong ZW, Yuan YF (2018) Juglanin suppresses fibrosis and inflammation response caused by LPS in acute lung injury. Int J Mol Med 41(6):3353–3365
Wang G, Zhang Y, Zhang R, Pan J, Qi D, Wang J, Yang X (2020) The protective effects of walnut green husk polysaccharide on liver injury, vascular endothelial dysfunction and disorder of gut microbiota in high fructose-induced mice. Int J Biol Macromol 162:92–106
Wang X, Chen D, Li Y, Zhao S, Chen C, Ning D (2019) Alleviating effects of walnut green husk extract on disorders of lipid levels and gut bacteria flora in high fat diet-induced obesity rats. J Funct Foods 52:576–586
Kilic S, Okullu SO, Kurt O, Sevinc H, Dundar C, Altinordu F, Turkoglu M (2019) Efficacy of two plant extracts against acne vulgaris: Initial results of microbiological tests and cell culture studies. J Cosmet Dermatol 18(4):1061–1065
Abedi P, Yaralizadeh M, Fatahinia M, Namjoyan F, Nezamivand-Chegini S, Yaralizadeh M (2017) Comparison of the effects of Juglans nigra Green Husk and Clotrimazole on Candida albicans in rats. Jundishapur J Microbiol 11(2):e58151
Shen H, Wang J, Ao J, Cai Y, Xi M, Hou Y, Li M, Luo A (2022) Inhibitory kinetics and mechanism of active compounds in green walnut husk against α-glucosidase: Spectroscopy and molecular docking analyses. Lwt 172:114179
Meshkini A, Tahmasbi M (2017) Antiplatelet Aggregation Activity of Walnut Hull Extract via Suppression of Reactive Oxygen Species Generation and Caspase Activation. J Acupunct Meridian Stud 10(3):193–203
Taheri A, Mirghazanfari SM, Dadpay M (2020) Wound healing effects of Persian walnut (Juglans regia L.) green husk on the incision wound model in rats. J Eur J Transl Myol 30(1):210–218
Altan E, Karacelebi Y, Saatcioglu E, Ulag S, Sahin A, Aksu B, Croitoru AM, Codrea CI, Ficai D, Gunduz O, Ficai A (2022) Fabrication of Electrospun Juglans regia (Juglone) Loaded Poly(lactic acid) Scaffolds as a Potential Wound Dressing Material. Polymers (Basel) 14(10):1971
Beiki T, Najafpour GD, Hosseini M (2017) Evaluation of antimicrobial and dyeing properties of walnut ( Juglans regia L.) green husk extract for cosmetics. Coloration Technology 134(1):71–81
Marhaba Z, Haniloo A (2018) Staining of parasitic helminths by extracts of Allium cepa, Juglans regia, and Rubia tinctorum: An approach to herbal dyes. Iranian J Parasitol 13(2):293
Mohammadzadeh SE, Faghiri F, Ghorbani F (2022) Green synthesis of phenolic capping Ag NPs by green walnut husk extract and its application for colorimetric detection of Cd2+ and Ni2+ ions in environmental samples. J Microchem J 179:107475
Hamzehniya M, Mobinikhaledi A, Ahadi N, Sameri F (2022) Zn complexed on CaO coated with walnut husk extract as an efficient and reusable catalyst for the green synthesis of benzylpyrazolyl coumarin derivatives. J Reac Kinet Mech Cat 135(2):897–914
Liu X, Wu Y, Lu Y, Liu X, Liu J, Ren J, Wu W, Wang Y, Li J (2022) Enhanced effects of walnut green husk solution on the phytoextraction of soil Cd and Zn and corresponding microbial responses. Chemosphere 289:133136
Ibrahim HK, Albo Hay Allah MA, Al-Da’amy MA, Kareem ET, Abdulridha AA (2020) Adsorption of basic dye using environmental friendly adsorbent. IOP Conference Series: Mater Sci Eng 871(1):012027
Han MM, Wang CY, Wang F, Pang MX, Qu C, Qi JH (2018) Extraction and Dyeing Properties of Tannin from Walnut Green Husk. IOP Conference Series: Earth Environ Sci 199:042007
Izadiyan Z, Shameli K, Hara H, MohdTaib SH (2018) Cytotoxicity assay of biosynthesis gold nanoparticles mediated by walnut (Juglans regia) green husk extract. J Mol Struct 1151:97–105
Soto-Maldonado C, Vergara-Castro M, Jara-Quezada J, Caballero-Valdés E, Müller-Pavez A, Zúñiga-Hansen ME, Altamirano C (2019) Polyphenolic extracts of walnut (Juglans regia) green husk containing juglone inhibit the growth of HL-60 cells and induce apoptosis. Electron J Biotechnol 39:1–7
Habibie A, Yazdani N, Saba MK, Vahdati K (2019) Ascorbic acid incorporated with walnut green husk extract for preserving the postharvest quality of cold storage fresh walnut kernels. Sci Hortic 245:193–199
Hu W, Du W, Bai S, Lv S, Chen G (2018) Phenoloxidase, an effective bioactivity target for botanical insecticide screening from green walnut husks. J Nat Prod Res 32(23):2848–2851
Sedaghat N, Mohsenzadeh M (2022) Effect of Edible Chicken Feet Gelatin Green Walnut Husk Extracted Coating on Chemical, Physical, Sensory Properties and Storage Time of Refrigerated Temperature Rainbow Trout Fillets (50±10° C). J Food Sci Technol 19(128):37–56
Hamzehniya M, Mobinikhaledi A, Ahadi N, Sameri F (2022) Zn complexed on CaO coated with walnut husk extract as an efficient and reusable catalyst for the green synthesis of benzylpyrazolyl coumarin derivatives. React Kinet Mech Catal 135(2):897–914
Ayadi Hassan S, Ghadam P, Abdi Ali A (2022) One step green synthesis of Cu nanoparticles by the aqueous extract of Juglans regia green husk: assessing its physicochemical, environmental and biological activities. J Bioprocess Biosyst Eng 45(3):605–618
Mushtaq U, Bhat SS, Parray AA, Nabi S, Wani AH, Qurashi AH, Siddiqui KS, Khanday FA (2021) A simple, economical and environmental-friendly method for staining protein gels using an extract from walnut-husk. Chem Biol Interact 333:109310
Acknowledgements
The authors thank the support of the National Institute of Food Science and Technology, University of Agriculture Faisalabad, Pakistan.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
S. Fordos and N. Abid: Collected the literature and prepared and wrote the original manuscript draft. M. Gulzar and A. Shahid: Finalized Figures and Tables, review writing and editing. I. Pasha, M.K.I. Khan, F. Oz, and R.M. Aadil: corrected, review writing and editing and I. Pasha, A. Mousavi Khaneghah & R.M. Aadil: Supervision, review writing and editing.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fordos, S., Abid, N., Gulzar, M. et al. Recent development in the application of walnut processing by-products (walnut shell and walnut husk). Biomass Conv. Bioref. 13, 14389–14411 (2023). https://doi.org/10.1007/s13399-023-04778-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-04778-6