Abstract
The gasification of sewage sludge (SS) and digestate was investigated in a pilot-scale fluidized bed gasifier with an output of 100 kWt. The treatment of these by-products is an ongoing challenge for sustainable development. SS and digestate are most commonly used as fertilizers. However, regulations restrict their use, mainly because of the content of heavy metals, pathogens and bacteria. Gasification of these by-products instead of application to agricultural land seems to be more efficient, as the syngas can subsequently be used for combined heat and power (CHP) generation. A series of measurements were carried out to get a better understanding of the gasification process of these fuels and to study the effects of gasifying agent on the syngas composition, particulate matter (PM) and tar. The produced syngas and tar were analyzed using a gas chromatograph with mass spectrometry (GC–MS). The results showed that no ash slagging was observed and therefore it is feasible to operate digestate and SS gasification at 750°C. The lower heating value (LHV) of the syngas from digestate and SS with air as the gasifying agent is comparable, 4.06 MJ·Nm−3 for digestate and 4.11 MJ·Nm−3 for SS. The addition of steam had a positive effect on the amount of tar and the tar dew point, which was below 150°C. Tar reduction in digestate was 5037.3 mg·Nm−3 to 3566.3 mg·Nm−3 and in SS 7447.7 mg·Nm−3 to 3390.3 mg·Nm−3. Furthermore, the concentrations of the individual tar compounds were determined and subsequently divided into tar classes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Anaerobic digestion is widely used for the disposal of organic waste in biogas plants. The main product from anaerobic digestion is biogas, which can be used for direct combustion after proper purification from acids, for CHP generation in cogeneration units [1] or in natural gas-powered vehicles as an alternative to fossil fuels [2]. A by-product from anaerobic digestion is a digestate. It is an undecomposed residue, which, unlike wood pellets, is rich in nitrogen (N), phosphorus (P), potassium (K), but also in sulphur (S) and chlorine (Cl). Due to this composition, digestate is suitable as an organic fertilizer [3]. However, its use on agricultural land is limited by regulations (location, quantity, composition etc.). In particular, the limits for nitrogen and heavy metals must be complied with in order to avoid water and soil contamination. For this reason, there are some complications, because the number of biogas plants in the Czech Republic is constantly growing, producing more and more digestate, which must be treated accordingly. Therefore, further possibilities of using digestate in thermochemical processes are being sought. One of these processes for the effective use of digestate is gasification. Gasification technology is the most efficient process to utilize biomass (from biomass to electricity) [4]. Gasification can be defined as a process that converts biomass or fossil fuels (generally carbon-based material) into gases. This is achieved by reactions between the fuel and the gasifying agent at high temperature (> 700°C). The gasifying agent can be air, steam, oxygen, carbon dioxide or a mixture of them. The resulting gas mixture is called syngas, synthesis gas or producer gas. The syngas consists mainly of CO, H2, CO2, N2 and some hydrocarbons [5]. In addition, syngas always contains some amount of tar. Tar is the biggest obstacle to the use of syngas in direct applications. The amount of tar depends primarily on the gasification temperature, gasifying agent, the fuel composition and the type of reactor [6]. Tar removal technologies can be divided into primary and secondary methods. The primary measures consist in the removal of tar directly in the gasification reactor, while the secondary measures take place outside of the gasifier [7].
Gasification reactors can be divided into three groups depending on the design of the reactor: fixed bed, fluidized bed and entrained flow reactor [8]. The fluidized bed reactor is used in this study, so some of its advantages are summarized here. In fluidized bed solid particles are kept in suspension by gasifying agent to create a liquid–like gas–solid mixture. Compared to other types of reactors, fluidized bed reactors have almost isothermal temperature distribution and a large gas–solid interface area resulting in efficient heat transfer [9]. Furthermore, the fluidized bed reactor provides good mixing of solid particles and reactor can be operated with a wide range of feedstocks. Because of these facts, high carbon conversion efficiency (CCE) is achieved.
This study [4] investigated the possibility of digestate gasification in a downdraft gasifier with a cold gas efficiency (CGE) of 72%. Chen et al. [10] studied the gasification of digestate in a downdraft fixed bed gasifier under laboratory conditions. An equivalence ratio (ER) with a medium value improved gas quality and CGE. The LHV ranged from 3.42 MJ·Nm−3 to 4.78 MJ·Nm−3 and the CGE ranged from 35.9% to 67.01%. The tar content of the syngas depended on the ER and ranged from 1.61 g·Nm−3 to 6.48 g·Nm−3. Wiśniewski et al. [11] dealt with the gasification of digestate in a batch reactor at a temperature of 850°C, using CO2 as gasifying agent. Under these conditions, a maximum LHV of 5 MJ·Nm−3was achieved. The effect of gasification temperature on the co-gasification of digestate and lignite in a laboratory scale fixed bed gasifier was investigated by Chang et al. [12]. The results show an increase in LHV with increasing gasification temperature. At a temperature of 950°C, the LHV of syngas was highest (6.52 MJ·Nm−3), almost twice that at 650°C. Comprehensive research on the gasification of digestate from wet and dry fermentation compared to the gasification of wood chips was performed by Balas et al. [13]. This research focuses primarily on the effect of gasification temperature on syngas composition.
SS, similar to digestate, is an unavoidable by-product of wastewater treatment, characterized by a high moisture content and a high organic and inorganic matter. Because of its mineral and nutrient content (N, P, K, etc.) it is used for composting or applied as fertilizer to agricultural land [14]. SS also contains toxic substances, especially heavy metals such as mercury (Hg), cadmium (Cd) or lead (Pb), as well as organic pathogens and bacteria. The trace element composition of SS is shown in research [15]. Furthermore, the significant amount of phosphorus in SS ash (up to 14% of total P) has sparked interest in SS ash processing [16]. Increasing urbanization and population growth are leading to an increasing number of wastewater treatment plants. As a result, a large amount of SS is produced annually posing a serious disposal problem. SS disposal in landfills is prohibited in the Czech Republic. Therefore, thermochemical processes are becoming increasingly popular. Technologies based on SS to energy allow not only the reduction of waste volume, but also the direct generation of energy. These technologies include incineration, pyrolysis and gasification. In this study, only gasification is considered.
Gasification of SS has been studied mainly under laboratory conditions, which is confirmed by the following studies. SS gasification in a fluidized bed was studied by Migliaccio et al. [17]. They studied the gasification of SS at a temperature of 850°C with a nitrogen/air mixture at different values of ER. At ER 0.1, a LHV of 12.1 MJ·Nm−3 and a CGE of 50% were obtained, whereas at ER 0.2, the LHV was 5.8 MJ·Nm−3 and the CGE was 57%. Further research on the gasification of SS in a fluidized bed on a laboratory scale was carried out by Gil-Lalaguna et al. [18]. They found that the gasification temperature has the greatest influence on the gasification process of SS. A higher temperature leads to a lower amount of tars and a higher yield of gas (GY), CGE and CCE. On the other hand, the LHV is favoured by increasing the steam content and decreasing the oxygen content in the gasifying agent. The average LHV of the syngas was 5.49 MJ·Nm−3, the tar content at 850°C was 16 g·Nm−3 and the CGE was 55.12%. The effect of bed height in a fluidized bed at 850°C was investigated by Manya et al. [19]. The results show that an increase of bed height improves the CGE, which can be explained by the high ash content of SS, which is an obstacle to gas diffusion. Other research papers dealing with the gasification of SS on a laboratory scale are listed here [20,21,22,23].
While there are many studies on laboratory-scale gasification of SS, there are only sporadic studies on the pilot-scale. Experiments on SS gasification in a circulating fluidized bed pilot plant were performed by Petersen et al. [24]. The average value of LHV was 4.7 MJ·Nm−3 at ER 0.3 and CGE 58%. Contrary to the research [18] they claim that the most important factor is ER, which has the greatest influence on the gas composition. A combined gasification and combustion process of SS was tested in a pilot-scale circulating fluidized bed gasifier by Zhu et al. [25]. High combustion efficiency and low NOx emissions were achieved in this two-stage process for SS disposal. Hydrogen production by co-gasification of SS and industrial wastewater sludge (IS) in a pilot-scale fluidized bed gasifier was carried out by Chen et al. [26]. Experimental results show that hydrogen production was increased with an increase of IS. The LHV of syngas ranged from 4.84 MJ·Nm−3 to 5.11 MJ·Nm−3 with a corresponding CGE of 33.91% to 36.15%.
As can be seen from the introductory section, information on gas composition and tars during gasification of digestate and SS in a pilot-scale fluidized bed gasifier is unfortunately still lacking in the literature. Therefore, in this research, the experimental gasification of SS and digestate is performed and discussed. In particular, the effects of steam addition to the gasifying agent on the gas and tar composition are presented.
2 Materials and methods
2.1 Fuel properties
The alternative biomass fuels chosen for the reactor were SS and digestate. SS came from a wastewater treatment plant in Brno, Czech Republic, and digestate came from a biogas plant in Dolní Němčice, Czech Republic. Both fuels were dried in a belt dryer with hot air, ensuring hygienic safety. The result is a dry granulate of both fuels suitable for gasification. The proximate and ultimate analyses of the fuels are shown in Table 1. SS is characterized by a relatively low moisture content of 5.6% and a high ash content of 44.5%, which reduces the LHV to 10.7 MJ·kg−1, the results are comparable to studies [18, 19]. On the other hand, the digestate was characterized by a higher moisture content of 13.2% and a much lower ash content of 11.7% than SS. The LHV of the digestate was slightly higher (11.8 MJ·kg−1), which is in agreement with the study [27].
The proximate analysis was conducted in accordance with the following European standards: EN ISO 18134–3 [28], EN ISO 18123 [29] and EN ISO 18122 [30]. The calorific values were determined in accordance with EN ISO 18125 [31]. The ultimate analysis of the tested fuels was performed using the CHNOS Elemental Analyzer.
2.2 Tar and gas measurement
To determine the tar content, a uniform sampling procedure was established, which is described in the so-called tar protocol [32]. This establishes uniform rules for the sampling and analysis of tar and the simultaneous collection of dust samples so that results can be compared between research sites around the world. This method is based on the solubility of tar in six impingers filled with isopropanol. The first four isopropanol impingers are placed in a warm bath and the remaining two impingers are placed in a cold bath so that the gas sample is cooled in two steps, first to 20°C and finally to − 20°C. The sampling method used in this measurement uses modified setup with 4 impingers. This modification has been used for many years by the staff of University of chemistry and technology Prague and University of technology Brno, and according to our measurements the results are comparable to the results according to the tar protocol. Samples were sent for analysis to determine the gravimetric weight of tar and to determine the individual concentrations of tar compounds using a GC–MS.
The composition of the gas produced was determined by both on-line and off-line methods. The continuous record of the gas composition was measured with the online infrared syngas analyzer Gasboard-3100. It is a stationary syngas analyzer based on NDIR, TCD and electrochemical technology. It can simultaneously measure CO, CO2, CH4, H2, O2 and calculate the calorific values of measured syngas. Online recording was used only to control and monitor the syngas during gasification. The gas composition results reported are from off-line sampling performed in glass sample containers and subsequently analyzed using a gas chromatograph HP 6890 with TCD and FID.
2.3 Fluidized bed operation
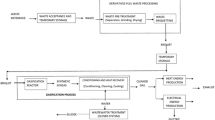
The research was carried out on the Biofluid 2 (see Fig. 1). It is a pilot plant for gasification of different types of biomass fuels with a nominal output of 100 kWt (in produced gas). The maximum parameters for gasification of wood chips are: airflow rate 50 Nm3·h−1 and fuel consumption 40 kg·h−1. Syngas from gasification of wood chips consists mainly of CO (12–17%), CO2 (14–20%), H2 (8–14%), CH4 (3–5%), O2 (up to 0.3%), N2 (50–60%), and other hydrocarbons (up to 1%). The LHV of syngas ranges from 3–7 MJ·Nm−3 depending on the operating parameters.
The fuel is conveyed from a fuel storage tank (1) equipped with a fuel stirrer (2) to the reactor (3) via a screw conveyor (4). The screw conveyor is equipped with a frequency converter (5) to control the amount of fuel. The blower (6) supplies compressed air to the reactor, which is used as the gasifying agent. The primary air is introduced under the grate (7), while the secondary and tertiary nozzles are located above the bed. The air can be heated by electric heating elements downstream of the blower, allowing the effects of air preheating on the gasification process to be observed. The syngas produced passes through a cyclone separator (8) where particles are separated and collected in a particle holder (9). The syngas is then combusted in the flare, which is equipped with a natural gas burner (10) with its own air inlet. The ash produced during gasification can be removed from a container at the bottom of the reactor.
The fluidized bed reactor must first be heated to the required operating temperature. This is achieved by an additional natural gas burner (11) located in the lower part of the gasifier under the grate. The flue gas from the burner flows through the reactor and heats it up to approx. 500°C. After the reactor is heated (approximately 2 h), the burner is removed, and the fuel is fed into the reactor via a screw conveyor. Simultaneously, the gasifying agent is introduced. After heating the reactor to an operating temperature of 750°C, the experiments with the SS and the digestate are initiated. The gasification temperature of ~ 750°C was chosen based on previous experience and on the characteristic temperatures of the ash to avoid slagging and fouling [33]. After stabilization of all operating parameters, especially the gasification temperature, samples (12) of syngas and tar were collected. For each experimental setup, 3 tar and 3 gas samples were taken.
2.4 Operating conditions
Gasification of SS and digestate was carried out under the following operating conditions, see Table 2. The desired gasification temperature was kept at 750°C for both fuels in order to compare the results. The temperature of the gasifying agent air was 150°C (temperature measured 300 mm below the grate). The fluidized bed material was consisted only of the produced ash. The digestate gasification process was stable throughout the measurement period, with slight temperature variations of ± 10°C. The pressure drop of the fluidized bed during gasification with air was 0.5–0.8 kPa, while when steam was injected into the reactor, the pressure drop decreased to 0.2–0.4 kPa.
The gasification process of SS was not as stable as digestate gasification, but the effort was to maintain the required operating temperature. These variations, especially in gasification temperature and pressure drop, may be caused by the inhomogeneity of the fuel (very fine particles vs. large lumps) and the high ash content of 44.5%. The pressure drop of the fluidized bed was 1.5–2.5 kPa, which is much higher compared to digestate.
3 Results and discussion
The effectiveness of the gasification process was evaluated using HHV/LHV, CGE, GY and CCE (see Table 3). As observed in SS gasification, the highest LHV was obtained in the gasification with steam + air, 4.21 MJ·Nm−3, while in the gasification of digestate, the highest LHV was obtained only with air as the gasifying agent, 4.06 MJ·Nm−3, which is a comparable LHV as in the study of Gnanendra et al. [4]. As described in literature [6], the addition of steam should have a positive effect on the calorific value due to reforming reactions, in which mainly H2 is formed. This trend can be seen in Fig. 2, where the addition of steam increases the H2 content in the gas produced by 10% compared to air gasification. In digestate gasification, on the other hand, this trend was not confirmed, and the addition of steam to the reactor did not have a positive effect on LHV or other indicators, see Table 3. For H2, there is a decrease of more than 30%, see Fig. 3. In both cases, the assumption that the addition of steam leads to an increase in CO2 and a decrease in CO was confirmed, mainly due to the predominant water–gas shift reaction, mentioned in [34].
The premise of steam + air gasification was to support reforming reactions, which means to significantly increase the hydrogen content. One of the reasons why this is not the case in digestate gasification may be an inappropriate amount of steam injected into the reactor, which leads to deterioration of the gasification process or cooling of the gasification zone. Another possibility is a lower gasification temperature, so that the complete decomposition of water vapor into hydrogen did not occur. However, here we were limited by a temperature of 750°C, because at higher temperatures the ash could sinter on the walls of the reactor and the grate. Digestate as a fuel was also characterized by a much lower ash content than SS, see Table 1. In the case of SS gasification, a higher content of ash could act as a bed material that promotes gasification reactions, increases heat transfer and overall increases the efficiency of the process.
Variations in ER are due to the fact that ER was based on the desired gasification temperature of 750°C. ER can be defined as the actual volume of air (used in gasification) against the theoretical volume of air in stoichiometric combustion.
GY represents the amount of gas produced per 1 kg of fuel (ar) and can be calculated as follows:
where \({V}_{gas}\) is the airflow in (Nm3·h−1) and \({m}_{fuel}\) is the fuel mass flow in (kg·h−1). As can be seen from Table 3, the highest GY was obtained when SS was gasified with air + steam (1.79 m3·kg−1), in contrast, the lowest GY was obtained when digestate was gasified with air + steam (1.37 m3·kg−1). This corresponds to another indicator, which is CGE and can be calculated as follows:
where \({V}_{gas}\) is the airflow in (Nm3·h−1), \({LHV}_{gas}\) is the lower heating value of gas in (MJ·Nm−3), \({m}_{fuel}\) is the fuel mass flow in (kg·h−1) and \({LHV}_{fuel}\) is the lower heating value of fuel (MJ·kg−1). CGE is an indication of the chemical energy of the gas in comparison to that of the fuel. The last parameter describing the gasification process is CCE, which can be defined as follows [35]:
where \({Y}_{G}\) is the gas yield in (%), CO, CO2 and CH4 are the volumetric percentage in the gas (%) and C (%) is the mass percentage of carbon in the tested fuel.
The syngas was analysed by gas chromatography. Specifically, the content of the following components was determined: H2, O2, CO, CO2, CH4, C2H2, C2H4 and C2H6. The last three are summed in the results and referred to as CxHy. The following equation determines the LHV of syngas:
where H2, CO, CH4, C2H2, C2H4 and C2H6 are volume fractions in syngas and LHV in (MJ·Nm−3). As can be seen from Table 3, all these parameters are interrelated and influence each other. Even though the gasification of SS was not as stable as the gasification of digestate, where the fluctuations of the gasification temperature were at most ± 10°C, SS shows better values. In the gasification of steam + air mixtures, a positive effect can be observed here, in contrast to gasification with pure air. Compared to the research by Petersen et al. [24], where a CGE of 58% was achieved, a relatively high CGE of 70.43% was obtained in the SS gasification. The GY of SS was 1.79 m3, the CCE reached 86.13% and LHV was 4.21 MJ·Nm−3, which corresponds to a comparable LHV obtained by Chen et al. [26].
On the other hand, digestate gasification was very stable, but the parameters of the gasification process did not reach such values as in SS gasification. During gasification with air, compared to the steam + air mixture as gasifying agent, better results for CGE, GY, CCE and LHV were achieved, which is comparable to the study by Gnanendra et al. [4]. The addition of steam had a negative effect on the gasification process here, and the lowest LHV of the gas produced was 2.84 MJ·Nm−3. Another reason could be a too low ER (0.27). Xiao et al. [35] investigated the effect of ER on the calorific value of the gas and found that the calorific value of the gas produced is lowest in the range ER of 0.25–0.3, while the highest calorific value is obtained at ER of about 0.37.
For both fuels, a high N2 content is observed in the sampled gases, which reduces the overall calorific value of the gas. It is given with air as the gasifying agent. When using air enriched with O2, a lower N2 concentration and, on the contrary, a higher proportion of heating components and thus a higher calorific value can be assumed. The effect of O2 concentration (21%, 30%, 50%, 70% and 99.5%) in the gasifying agent on the syngas composition was investigated by Wang et al. [36]. The content of H2 + CO in the syngas (from pine sawdust pellets gasification) increase from 29 to 71% when O2 level changed from 21% to 99.5%.
The total amount of tars contained in the sampling gas from SS and digestate gasification is shown in Table 4. When air was used as the gasifying agent, the amount of tar was 5 g·Nm−3 for digestate gasification and 7.4 g·Nm−3 for SS gasification. The tar values for digestate are in accordance with research [10], where the amount of tar depended on ER and ranged from 1.61 to 6.48 g·Nm−3. Gasification of SS yielded, significantly lower tar values than in the study [18], where the average value was 14.8 g·Nm−3. The addition of steam had a positive effect on both fuels and led to a significant reduction. In the case of SS, there was a 55% decrease to a value of 3.4 g·Nm−3. Simultaneously, the amount of particles in the gas produced was measured. As can be seen from Table 4, there is no dependence on the use of air or the addition of steam. The PM content in the gas produced ranged from 6.3 g·Nm−3 for digestate to 15.7 g·Nm−3 for SS.
In addition, the tar was analyzed using a GC–MS to determine the individual concentrations, and then divided into individual tar classes, the results are presented in Table 5. This tar classification system has been developed in cooperation with Energy research Center of The Netherlands (ECN), Toegepast Natuurwetenschappelijk Onderzoek and University of Twente. In this research, tar is considered as all organic compounds with a molecular weight larger than benzene.
Class 1 is not listed in Table 5 because this class includes very heavy tars that cannot be detected by GC. Class 2 is composed of heterocyclic compounds, that are highly water soluble due to their polarity. Class 3 is comprised of aromatic components, mainly light hydrocarbons with single rings. Class 4 includes light polyaromatic hydrocarbons (PAH), 2–3 rings PAH’s. These compounds condense at relatively high concentrations and intermediate temperatures. Last class 5 contains heavy PAH’s, 4–5 rings that condense at low concentrations at relatively high temperatures. Tar dew point for different tar classes as a function of tar class concentration is shown in Fig. 4 [37].
Gasification with the addition of steam has a positive effect on all tar classes, see Table 5. The most significant impact can be seen in the second tar class, where there is a 98% reduction in phenol in digestate gasification and a 91% reduction in gasification of SS. The third class of tars is the most numerous and accounts for about 70% ± 5% of all tars. It is referred to as BTEX (benzene, toluene, ethylbenzene and three xylene isomers). The xylene isomers are distinguished by the designations ortho (o), meta (m) and para (p). In classes 4 and 5, a decreasing tendency is observed for almost all compounds when steam is added. As far as condensation is concerned, these are classes which condense at higher temperatures. Therefore, it is important to keep the sampling routes and the routes in front of the burner at a sufficiently high temperature (above 250°C) to avoid condensation.
Furthermore, tar dew points according to the ECN model were calculated, see Table 4. During gasification with air as gasifying agent for both fuels, the dew point temperature of the tar was above 150°C. Gasification with steam addition lowered the dew point temperature to 149.4°C for digestate and 141.2°C for SS. As already mentioned, the addition of steam has a positive effect on the total amount of tars, but also on the dew point temperature. Knowledge of the tar dew point temperature is very important to avoid condensation in the pipes and sampling points.
4 Conclusion
In this study digestate and SS were gasified at 750°C in a fluidized bed gasifier to determine the effects of different gasifying agent on gas composition, PM emissions and tar composition. The research has led to several important findings that are summarized here:
-
1)
Gasification of digestate and SS for efficient utilization of this biodegradable waste is feasible. However, the produced gas must be treated to a level acceptable for subsequent use.
-
2)
The LHV of the gas produced during gasification with air is comparable for both fuels at ~ 4 MJ·Nm−3.
-
3)
The addition of steam during SS gasification has a positive effect on CGE, GY, CCE and LHV. Conversely, the addition of steam to the digestate resulted in a deterioration of the overall process, which was reflected in the LHV of the gas produced. On the other hand, gasification with the addition of steam resulted in a significant reduction in tar content for both fuels, 54.5% in the case of SS and 29% for digestate.
-
4)
The tar dew point temperatures are around 150°C, the addition of steam had a positive effect on both fuels and the tar dew point was reduced.
This research has primarily investigated the applicability of digestate and SS as fuels for fluidized bed gasification. CGE, CCE, LHV and GY were the crucial factors in evaluating the gasification process. Further research will be focused on investigating the effects of gasification temperature, the effects of ER and the ratio of steam to biomass on gas and tar composition.
Availability of data and materials
This declaration is not applicable.
References
Lisý M, Baláš M, Špiláček M, Skála Z (2014) Technical and economic optimization of cogeneration technology using combustion and gasification. Acta Polytech 54(1):42–51. https://doi.org/10.14311/AP.2014.54.0042
Abanades S, Abbaspour H, Ahmadi A (2022) A critical review of biogas production and usage with legislations framework across the globe. Int J Environ Sci Technol 19(4):3377–3400. https://doi.org/10.1007/s13762-021-03301-6
Song S, Lim J, Lee J (2021) Food-waste anaerobic digestate as a fertilizer: The agronomic properties of untreated digestate and biochar-filtered digestate residue. Waste Manag 136:143–152. https://doi.org/10.1016/j.wasman.2021.10.011
Gnanendra P, Ramesha D, Dasappa S (2012) Preliminary investigation on the use of biogas sludge for gasification. Int J Sustain Energy 31(4):251–267. https://doi.org/10.1080/1478646X.2011.559550
Zhang Y, Cui Y, Chen P (2019) Chapter 14 - gasification technologies and their energy potentials. Elsevier 193–206. https://doi.org/10.1016/B978-0-444-64200-4.00014-1
Basu P (2018) Biomass gasification, pyrolysis and torrefaction: practical design and theory, 3rd edn. Academic Press is an imprint of Elsevier, London
Lisý M, Baláš M, Špiláček M, Skála Z (2015) Operating specifications of catalytic cleaning of gas from biomass gasification. Acta Polytech 55(6):401–406. https://doi.org/10.14311/AP.2015.55.0401
Mahinpey N, Gomez A (2016) Review of gasification fundamentals and new findings: Reactors, feedstock, and kinetic studies. Chem Eng Sci 148:14–31. https://doi.org/10.1016/j.ces.2016.03.037
Rüdisüli M, Schildhauer T, Biollaz S, Ommen J (2012) Scale-up of bubbling fluidized bed reactors—a review. Powder Technol 217:21–38. https://doi.org/10.1016/j.powtec.2011.10.004
Chen G, Guo X, Cheng Z, Yan B, Dan Z, Ma W (2017) Air gasification of biogas-derived digestate in a downdraft fixed bed gasifier. Waste Manag 69:162–169. https://doi.org/10.1016/j.wasman.2017.08.001
Wiśniewski D, Gołaszewski J, Białowiec A (2015) The pyrolysis and gasification of digestate from agricultural biogas plant. Arch Environ Prot 41(3):70–75. https://doi.org/10.1515/aep-2015-0032
Chang S, Zhang Z, Cao L, Ma L, You S, Li W (2020) Co-gasification of digestate and lignite in a downdraft fixed bed gasifier: effect of temperature. Energy Convers Manag 213:112798. https://doi.org/10.1016/j.enconman.2020.112798
Baláš M, Milčák P, Elbl P, Lisý M, Lachman J, Kracík P (2022) Gasification of fermentation residue in a fluidised-bed gasifier. Energy 245:123211. https://doi.org/10.1016/j.energy.2022.123211
Fijalkowski K, Rorat A, Grobelak A, Kacprzak M (2017) The presence of contaminations in sewage sludge – the current situation. J Environ Manag 203:1126–1136. https://doi.org/10.1016/j.jenvman.2017.05.068
Elbl P, Sitek T, Lachman J, Lisý M, Baláš M, Pospíšil J (2022) Sewage sludge and wood sawdust co-firing: Gaseous emissions and particulate matter size distribution. Energy 256:124680. https://doi.org/10.1016/j.energy.2022.124680
Ma P, Rosen C (2021) Land application of sewage sludge incinerator ash for phosphorus recovery: a review. Chemosphere 274:129609. https://doi.org/10.1016/j.chemosphere.2021.129609
Migliaccio R, Brachi P, Montagnaro F, Papa S, Tavano A, Montesarchio P, Ruoppolo G, Urciuolo M (2021) Sewage sludge gasification in a fluidized bed: experimental investigation and modeling. Ind Eng Chem Res 60(13):5034–5047. https://doi.org/10.1021/acs.iecr.1c00084
Gil-Lalaguna N, Sánchez JL, Murillo MB, Rodríguez E, Gea G (2014) Air–steam gasification of sewage sludge in a fluidized bed Influence of some operating conditions. Chem Eng J 248:373–382. https://doi.org/10.1016/j.cej.2014.03.055
Manyà J, Sánchez J, Abrego J, Gonzalo A, Arauzo J (2006) Influence of gas residence time and air ratio on the air gasification of dried sewage sludge in a bubbling fluidised bed. Fuel. 85(14–15):2027–2033. https://doi.org/10.1016/j.fuel.2006.04.008
Nilsson S, Gómez-Barea A, Cano D (2012) Gasification reactivity of char from dried sewage sludge in a fluidized bed. Fuel 92(1):346–353. https://doi.org/10.1016/j.fuel.2011.07.031
Nilsson S, Gómez-Barea A, Ollero P (2013) Gasification of char from dried sewage sludge in fluidized bed: reaction rate in mixtures of CO2 and H2O. Fuel 105:764–768. https://doi.org/10.1016/j.fuel.2012.09.008
De Andrés J, Narros A, Rodríguez M (2011) Behaviour of dolomite, olivine and alumina as primary catalysts in air–steam gasification of sewage sludge. Fuel 90(2):521–527. https://doi.org/10.1016/j.fuel.2010.09.043
Freda C, Cornacchia G, Romanelli A, Valerio V, Grieco M (2018) Sewage sludge gasification in a bench scale rotary kiln. Fuel 212:88–94. https://doi.org/10.1016/j.fuel.2017.10.013
Petersen I, Werther J (2005) Experimental investigation and modeling of gasification of sewage sludge in the circulating fluidized bed. Chem Eng Process Process Intensif 44(7):717–736. https://doi.org/10.1016/j.cep.2004.09.001
Zhu J, Yao Y, Lu Q, Gao M, Ouyang Z (2015) Experimental investigation of gasification and incineration characteristics of dried sewage sludge in a circulating fluidized bed. Fuel 150:441–447. https://doi.org/10.1016/j.fuel.2015.02.031
Chen Y, Lan T, Chiang K (2021) Enhanced hydrogen production in co-gasification of sewage sludge and industrial wastewater sludge by a pilot-scale fluidized bed gasifier. Int J Hydrogen Energy 46(27):14083–14095. https://doi.org/10.1016/j.ijhydene.2020.10.081
Timofeeva SS, Karaeva JV, Kovalev AA, Kovalev DA, Litti YV (2022) Steam gasification of digestate after anaerobic digestion and dark fermentation of lignocellulosic biomass to produce syngas with high hydrogen content. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2022.11.260
ISO, EN. 18134–3:2015 (2015) Solid biofuels–Determination of moisture content-Oven dry method-Part
ISO, EN. 18123:2015 (2016) Solid biofuels–Determination of the Content of Volatile Matter
ISO, EN. 18122:2015 (2015) Solid biofuels–Determination of Ash Content
ISO, EN. 18125:2017 (2017) Solid biofuels–Determination of Calorific Value
Neeft J, Knoef H, Zielke U, Sjöström K, Hasler P, Simell P, Dorrington M, Greil C (2000) Tar protocol development of a standard method for the measurement of organic contaminants (tar) in biomass producer gases. Proceedings of the 1st World Conference on Biomass for Energy and Industry, Spain
Lachman J, Baláš M, Lisý M, Lisá H, Milčák P, Elbl P (2021) An overview of slagging and fouling indicators and their applicability to biomass fuels. Fuel Process Technol 217:106804. https://doi.org/10.1016/j.fuproc.2021.106804
Singh Siwal S, Zhang Q, Sun CH, Thakur S, Kumar Gupta V, Kumar Thakur V (2020) Energy production from steam gasification processes and parameters that contemplate in biomass gasifier – a review. Bioresour Technol 297:122481. https://doi.org/10.1016/j.biortech.2019.122481
Xiao R, Zhang M, Jin B, Huang Y, Zhou H (2006) High-temperature air/steam-blown gasification of coal in a pressurized spout-fluid bed. Energy Fuels 20(2):715–720. https://doi.org/10.1021/ef050233h
Wang Z, He T, Qin J et al (2015) Gasification of biomass with oxygen-enriched air in a pilot scale two-stage gasifier. Fuel 150:386–393. https://doi.org/10.1016/j.fuel.2015.02.056
Kiel JHA, Paasen SVB, Neeft JPA (2004) Primary measures to reduce tar formation in fluidised-bed biomass gasifiers. Energy Research Centre of the Netherlands (ECN), The Netherlands
Acknowledgements
This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic under OP RDE grant number CZ.02.1.01/0.0/0.0/16_019/0000753 “Research centre for low-carbon energy technologies”.
Funding
Open access publishing supported by the National Technical Library in Prague. This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic under OP RDE grant number CZ.02.1.01/0.0/0.0/16_019/0000753 “Research centre for low-carbon energy technologies”.
Author information
Authors and Affiliations
Contributions
Patrik Elbl: Writing of the original draft, Formal analysis, Editing. Marek Baláš: Supervision, Resources, Investigation. Martin Lisý: Validation, Funding acquisition. Hana Lisá: Investigation, Methodology.
Corresponding author
Ethics declarations
Ethical approval
This declaration is not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elbl, P., Baláš, M., Lisý, M. et al. Sewage sludge and digestate gasification in an atmospheric fluidized bed gasifier. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04276-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04276-9