Abstract
This work encompasses the effect of chemical and metabolic stress on lipid content and fatty acid methyl ester (FAME) profile by Chromochloris zofingiensis. To this aim, a control medium amended with specific concentrations of sodium chloride (15 g L−1, 30 g L−1, and 60 g L−1) and of ethanolamine (ETA) and triethylamine (TEA) (0.050 g L−1, 0.100 g L−1, and 0.150 g L−1) has been used for the cultivation of this strain. A better lipid content than the control was obtained under salt stress compared to chemical stress induced by ETA and TEA, while the effect on lipid productivity was negligible. Thirty grams per liter of NaCl allowed to obtain the highest value of the total lipid content (30.33% DW) compared to the control (20% DW). FAME profile revealed a 97.50–98.99% composition in C16–C18 with no statistically meaningful differences among the three concentrations of salt and chemicals tested. The most represented fatty acids were C18:1 oleic (> 45%wt), C16:0 palmitic (> 34%wt), and C18:2 linoleic (> 21%wt) obtained under 60 and 30 mg L−1 of NaCl and 0.150 g L−1 of ETA, respectively. When using 0.150 g L−1 of ETA, unsaturated fatty acids reached the highest portion (67.53%wt) than the control (62.26%wt). A quantitative and qualitative analysis of all FAMEs has been carried out to improve biodiesel properties. Chemical and physical properties of algal lipid–derived biodiesel showed a compliance with ASTM standards for unmixed biodiesel and the main European regulations (EN 14214 and EN 590) for the quality of biodiesel resulted fulfilled. Therefore, a profitable biodiesel can be obtained when cultivating C. zofingiensis under osmotic stress.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the last two centuries, the intensive use of fossil fuels, mostly driven by the development of industrialization, has produced a dramatic increase in carbon dioxide (CO2) emissions. Consequently, there has been a marked increase in the global temperature [1]. The growing concern on the above premises emphasizes at global level the seeking of alternative sustainable and renewable sources of fuel able to substitute those of fossil origin [2].
Microalgae are considered today as cell factories able to produce a wide range of commercially important molecules and other biologically active compounds such as proteins, carbohydrates, lipids, vitamins, and pigments to be exploited in food and health, biofuel, pharmaceutical, and cosmetic markets [3]. Beside their importance in terms of nutritional value, microalgae cultivation shows environmental positive implications due to CO2 uptake from the atmosphere, production of biofuel, agro-fertilizers, bioplastics, and wastewater (WW) treatment [4]. To this aim, the ability of microalgae to combine the biomass growth with the biological WW treatment and biofuel production has been addressed by an extensive literature [5].
The eligibility of microalgae for biodiesel production is based on the fact that this photosynthetic microorganism can sustain a high biomass production characterized by a high lipid content [6]. In this scenario, microalgae can be potentially exploited as renewable and environmentally friendly resources for the production of liquid biofuels. However, economic and technical issues constrain the full development of microalgae mass-production cultivation systems, which limits the full widespread of this existing microalgae-based technology for biofuel production. The economic feasibility of microalgae mass cultivation should be guaranteed by high productivity in terms of biomass and efficient production of lipids [7].
Under photoautotrophic conditions, a series of culture parameters can be modified to tailor cell metabolic pathways toward the production of lipids and the accumulation of triacylglycerol (TAG) in particular. This can be obtained by controlling biotic and abiotic factors such as nutrients (i.e., stress nutrition in the form of nitrogen depletion), light, pH, temperature, oxidative, and salty stress [8].
When microalgae are exposed to stressful conditions, various strategies are adopted by the cells to adapt their metabolism, such as altering the morphology, changing biochemical pathways, and slowing down the growth [9]. At commercial scale, different stressors are available to enhance the productivity of certain algal components, mainly pigments and lipids. In this light, the latest frontier in biochemical field is the use of small chemical molecules as gene modulators for promoting metabolism and lipid accumulation, acting as activators or inhibitors of cell metabolism [10, 11]. Among these modulators, amines such as ethanolamine (ETA) and triethylamine (TEA) are able to influence lipid accumulation and fatty acid methyl ester (FAME) profile when added to the culture medium [12, 13].
Another stressful way adopted to condition the lipid metabolism of microalgae is the increase of salinity, which is able to alter not only the growth but also the biochemical composition of marine and fresh water microalgae [14]. In particular, high salinity is correlated to the accumulation of lipids because it favors the transition from the phase of active cell division to that one characterized by the storage of energy as an adaptation to the stress environment [15]. From a biochemical point of view, the exposition to a saline environment undergoes at the cellular level a survival mechanism of response that implies an increased production of lipids, as energy-rich substances, that allows the cells to survive under extreme environmental conditions [16].
The growing economic and environmental interest in finding robust strains capable of producing biomass that could be exploited as a feedstock for biofuels should be also highlighted [17]. Feedstocks with these characteristics can compete with traditional biodiesel obtained from terrestrial crops in terms of land and water use, carbon sequestration, and reduction of greenhouse gas emissions.
The single-celled green microalgae Chromochloris zofingiensis, which belong to the class of Chlorophyceae [18], represents a valid candidate for this purpose. Nowadays, there is a growing interest toward C. zofingiensis in the frame of a biorefinery approach by producing multiple products from a single source of microalgal biomass. In this scenario, this strain shows great promise due to its high biomass productivity and the accumulation of a diverse range of products such as pigments, carbohydrates, proteins, and essential amino acids [19]. Besides the production of the carotenoid astaxanthin, C. zofingiensis emerges as a potential accumulator of lipids for the production of biodiesel when exposed to suitable stressful conditions such as high light [20] and nitrogen deprivation [21]. Another appealing aspect noteworthy is the ability of C. zofingiensis to modulate its metabolism shifting from an initial photoautotrophy or heterotrophy into a mixotrophic mode, increasing in this way the production of biomass and lipids by coupling two different trophic modes of cell growth [22, 23]. Therefore, based on its ability to growth under these multitrophic culture conditions as well as on its high‑density growth, scalability, and tolerance to a wide range of environmental stresses (primarily pathogens and pollutants), C. zofinginesis can represent a desirable robust strain for biotechnological applications [18, 24, 25].
For many freshwater Chlorella strains, such as Chlorella vulgaris [26,27,28] and Chlorella protothecoides [29], there are examples of modification on lipid content and FAME composition based on the application of an osmotic stress. The possibility to use seawater instead of freshwater for algae cultivation could reduce the footprint of freshwater, helping in this way to preserve a scarse resource on the Earth. On the other hand, there are few examples dealing with the response of C. zofingiensis to salinity levels, showing how biomass production could be severely compromised [30]. Hence, by considering the potential use of chemicals such as NaCl producing a metabolic stress on microalgae, the effect on C. zofingiensis lipid production under a growing salinity environment is investigated in this work. The use of gene modulators as stressors and lipid metabolism inductors is reported as well for the first time for this strain. FAME profile is also analyzed from a quantitative and qualitative point of view to assess a comparison with the standard directives for biodiesel properties.
2 Materials and methods
2.1 Inocula and culture medium preparation
The culture collection of algae at the University of Texas, Austin, USA [31], provided the strain Chromochloris zofingiensis UTEX32 object of this study. The standard Bold basal culture medium (BBM) was used for maintenance and cultivation of the cell cultures. The BBM was prepared using the following six stock solutions: NaNO3 (10 g 400 mL−1 H2O), KH2PO4 (7 g 400 mL−1 H2O), K2HPO4 × 3H2O (3 g 400 mL−1 H2O), MgSO4 × 7H2O (3 g 400 mL−1 H2O), CaCl2 × 2H2O (1 g 400 mL−1 H2O), and NaCl (1 g 400 mL−1 H2O). 10 mL of each stock solution was added to 1 L of distilled water. Three different vitamin stock solutions (thiamine 0.1 g 100 mL−1 H2O, biotin 25 × 10−6 g 100 mL−1 H2O, and vitamin B12 15 × 10−6 g 100 mL−1 H2O) and a PIV metal solution (EDTA-Na2 750 mg L−1, FeCl3 × 6H2O 97 mg L−1, MnCl4 × 4H2O 41 mg L−1, ZnCl2 5 mg L−1, CoCl2 × 6H2O 2 mg L−1, and Na2MoO4 × 2H2O 4 mg L−1) were also prepared. 1 mL of each vitamin stock solution and 6 mL of PIV metal solution were added to 1 L of distilled water after autoclaving it (model 760, ASAL, Cernusco s/N, MI, Italy).
50 mL of BBM and 10 mL of microalgal inocula were poured in 150-mL Erlenmeyer flasks and maintained at room temperature 1 week in cultivation until reaching the end of exponential growth phase. A cotton cup was provided to isolate the flasks to the surrounding environment and to allow air diffusion. Fluorescent lamps (model T8 36 W IP20, CMI, Germany) were adopted to continuously illuminate the flasks providing a light intensity of 50 μmol m−2 s−1 measured with a luxmeter (model HD 2302.0, Delta OHM, Padua, Italy).
2.2 Cultivation conditions and experimental setup
The cultivation of C. zofingiensis took place both in 1-L flasks and 20-L bubble column reactors (thereafter named PBR). The 1-L flasks were filled with 500 mL of BBM, aerated (0.03% CO2 v v−1) by diffusion trough a cotton cup, and daily shaken manually. The flasks were maintained at room temperature and illuminated by white fluorescent lamps with a 50 μmol m−2 s−1 light intensity. The lamps were turned on for 12 h and turned off for the remaining part of the day. The 20-L PBRs (outer diameter 18 cm; inner diameter 17 cm; height 100 cm), were characterized by a working volume of 12 L and were supplemented by 10 L of medium and 2 L of inocula. An air pump (GIS Air Compressor, Carpi, MO, Italy) provided the compressed air while a CO2 cylinder provided the gas, respectively, to obtain a filtered mix of CO2 and compressed air (2–98% v v−1) which was supplied through a perforated rubber stopper to the column. The PBRs were illuminated with a photoperiod of 12 h/12 h at room temperature by one fluorescent panel providing a light intensity of 65 μmol m−2s−1, as reported in Fig. 1.
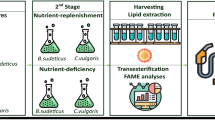
Three chemicals such as NaCl, ETA (Merck KGaA, Germany), and TEA (Merck KGaA, Germany) were added to the BBM as reported in the experimental setup shown in Table 1. Three different concentrations for each source were tested as follows: 0.050 g L−1 (ETA1), 0.100 g L−1 (ETA2), and 0.150 g L−1 (ETA3) for ethanolamine; 0.050 g L−1 (TEA1), 0.100 g L−1 (TEA2), and 0.150 g L−1 (TEA3) for trimethylamine; and 15 g L−1 (SAL1), 30 g L−1 (SAL2), and 60 g L−1 (SAL3) for NaCl. The test under stress was carried out in two stages as follows: (a) growth phase (GP) which lasted 20 days and (b) stress phase (SP) which started on the 20th day and lasted 5 days.
A series of experiments (with an initial concentration of the inocula equal to 0.1 g L−1) were performed to evaluate C. zofingiensis growth, biomass production, and total lipid content as well as FAME profile according to the experimental setup. Optical density (OD) and biomass concentration (g L−1) were collected to monitor microalgae growth. The final dry weight, the total lipid content, and the FAME profile were obtained at the end of the cultivation both at growth and stress phases.
2.3 Cell growth and dry weight determination
The absorbance (ABS) of the culture detected at 720 nm by a spectrophotometer (model ONDA V30 SCAN–UV VIS, ZetaLab, Padua, Italy) was used to monitor C. zofingiensis growth for 25 consecutive days. Distilled water was used as the blank for the absorbance readings. A relationship between dried biomass concentration and ABS was established by building a regression equation, which allowed calculating the biomass of the sample once obtained the value of ABS reading. The detailed procedure adopted for the gravimetrical determination of the dry biomass has been reported in a previous work carried out by our team [22].
The cell concentration (dry weight), Xdw (g L−1) was calculated using the following equation:
where W is weight (g) of the dried algal biomass, and V is volume (L) of the algae culture used for the test.
The average biomass productivity (∆X) was expressed as
where max Xmax is maximum biomass (g L−1) obtained at (tmax).
The specific growth rate (μ) was calculated according to the following equation:
where X2 and X1 are dry biomass concentration (g L−1) at time t2 and t1, respectively.
A pH meter (model HI 2210, Hanna Instruments, Woonsocket, RI, USA) was used to measure the pH of culture suspensions.
2.4 Total lipid content determination
A modified version of the classical gravimetric method of Bligh and Dyer [32], tuned by Chen et al. [33], was adopted to execute the measurements of the total cell lipid content. Once the algal biomass was collected, it was centrifuged (model 2560 Nakita, Auxilab S.L., Beriain, Spain) at 6500 g for about 5 min in order to separate supernatant and sediments. The latter were collected and freeze-dried at − 80 °C for about 24 h. The extraction of lipids was carried out on the freeze-dried biomass which had been previously lyophilized (Lio1000P, 5 Pascal, Trezzano s/N, MI, Italy). The detailed steps of the lyophilization process are reported in Vitali et al. [22]. Broadly, two extractive solutions are required, one chloroform/methanol mix and a chloroform + sodium chloride solution, which contribute to get a final volume ratio of about 2:2:1.8 (chloroform/methanol/water). The final mixture was subsequently extracted and centrifuged at 6500 g for about 5 min. At this point, the chloroform phase was transferred into a pre-weighted glass tube (m0, mg), blow-dried under N2 flow gas at 60 °C (HGC 244 HA, China) for 90 min and finally weighted (m1, mg). The percentage (%) of the lipid content (TL) was calculated by multiplying the obtained value of total lipid content (g g−1 biomass) by 100 as follows:
The lipid productivity (LP) was calculated as follows:
2.5 FAME determination
A modified protocol reported by Lage and Gentili [34] was adopted for FAME determination starting from lyophilized biomass. The detailed procedure is reported in Vitali et al. [22]. Briefly, toluene and 1% H2SO4 in anhydrous methanol were used to improve the methylation of non-polar lipids and their trans-methylation, respectively. Tricosanoic acid methyl ester CH3(CH2)21COOCH3 ≥ 99.0% (GC), (Sigma-Aldrich, St. Louis, MO, USA) in hexane was used as an internal standard. The extraction of FAMEs was then performed with a 5% NaCl + hexane extractive solution, and after phase separation, the organic phase was quantitatively analyzed by a 7820A Gas Chromatograph (Agilent Technologies, Palo Alto, CA, USA) coupled to a 5977B Mass Spectrometer (Agilent Technologies, Palo Alto, CA, USA). Data on GC-MS system, capillary column, gas carrier, injector, and detector temperature as well as mode of operation for the chromatogram were reported elsewhere [22]. The individual FAMEs were identified and quantified by using a standard reference solution based on a Supelco 37 Component FAME Mix® (Sigma-Aldrich, Saint Louis, MO, USA), tricosanoic acid methyl ester (TAME) internal standard solution, and hexane.
The content of FAMEs was calculated by manually integrating their peak areas with respect to the internal TAME standard solution, after calculation of the response (RF) using the standard reference solution. Finally, fatty acid (FA) levels were reported in terms of g 100 g−1 total FAs.
The relative content of each FA, presented in a percentage of total FA, was expressed by RF, which was derived by the following equation:
where ms is the mass of internal standard (mg), Ai is peak area of section i, mi is the weight of sample (mg), and As is the area of standard.
2.6 Estimation of fuel biodiesel properties
The quality of biodiesel which can be potentially produced from the lipids extracted, was assessed on the basis of the FAME composition. Density (ρ), kinematic viscosity (υ), saponification value (SV), high heating value (HHV), cetane number (CN), iodine value (IV), long-chain saturation factor (LCSF), degree of unsaturation (DU), cold filter plugging point (CFPP), and oxidative stability (OS) were evaluated through the equations proposed by Senthamilselvi and Kalaiselvi [35]. Allylic position equivalent (APE), cloud point (CP), bis-allylic position equivalent (BAPE), and pour point (PP) were evaluated by means of the software Biodiesel Analyzer© Ver. 2.2. [36].
2.7 Statistical analysis
Each experimental condition was investigated in triplicate. Statistical analysis on biomass and lipid content, specific growth rate, lipid productivity, and FAME profile was performed using MetaboAnalyst 5.0 platform tuned by the McGill University, Montreal, Canada. The one-way analysis of variance (ANOVA) followed by Tukey’s honestly significance different (HSD) test was used to statistically analyze the difference among the groups. Variables were reported as significant at 95% confidence (probability limit of 0.05).
3 Results and discussion
3.1 Influence of metabolic and chemical stress on C. zofingiensis growth and lipid content
The growth and metabolic functions of microalgae are markedly influenced by stress factors, such as salinity, pH of the growth medium, nutrient depletion, and high light intensity. The effect of metabolic stress on C. zofingiensis biomass and lipid content was evaluated by adding some chemicals to the standard BBM. Figure 2 shows the effect of osmotic stress by the addition to the culture medium of three NaCl concentrations, 15 g L−1, 30 g L−1 g L−1, and 60 g L−1, respectively. Chemicals were added to the control at the 20th day of cultivation, and the algal behavior under salt stress was monitored for 5 days (from the 20th to 25th day). Upon salt addition, no increase in biomass concentration (Fig. 2a) and specific growth rate (Fig. 2b) is observed compared to the control for all the three salt concentration investigated. The biomass concentration exhibited a progressive slight decrease compared to the control as the concentration of the salt increased. This pattern was in agreement with the reduction of biomass concentration under increased salinity of the culture media reported for the marine strains Tisochrysis lutea [37], Tetraselmis suecica [38], Chlorella vulgaris, and Isochrysis galbana [39]. The addition of salt on microalgae growth medium took place at the 20th day of cultivation when the cells were in their exponential phase to submit the whole cell population to the osmotic stress. As it can be seen in Fig. 3, cell growth was progressively affected by changes in the osmolarity and the inhibition was more accentuated under 30 and 60 mg L−1 of NaCl. In this experiment, at the end of the 5 days of salt stress, C. zofingiensis was able to attain a biomass concentration of 1.07, 0.92, and 0.99 g L−1 under 15, 30, and 60 mg L−1 of NaCl, respectively (Fig. 3). The values of biomass obtained at the 20th day of cultivation (when the salt stress was applied) were 1.21, 1, and 1.22 g L−1, respectively. There was therefore an inhibitory effect of cell growth during the 5 days of monitored salt stress that produced a 15%, 12%, and 21% reduction on biomass concentration. It has been suggested that microalgae belonging to the Chlorella genus could have a mechanism to deal with the salinity stress [26]. Therefore, the ability of C. zofingiensis to cope with different salinity stresses could be somehow related to the activation of some of these mechanisms. On the other hand, there are microalgae that experience modification in cell morphology, low photosynthetic rate, and growth inhibition when cultured at low salinity probably due to osmotic and ionic imbalance. For example, T. suecica exhibited a low growth when cultured in 10 mg L−1 and 20 mg L−1 [38]. Conversely, it should be underlined that the growth of some species at high salt concentrations lowers the efficiency of photosynthesis and decreases the biomass accumulation. A high external ionic concentration as well as an excessive ion flux into the cells might play an inhibitory effect of cell growth under high salinity. In fact, osmotic stress under high salinity can decrease the cellular water potential, leading to an excessive uptake of salt ions into the cells that in turn produces a cellular ion imbalance [40]. Although some microalgae and cyanobacteria are reported to be tolerant up to 100 g L−1 of salt concentration, in general, salinity above 35 g L−1 progressively causes negative effects on both cell growth and efficiency of photosynthesis, with photosynthesis process being inhibited by osmotic stress. These findings confirm that microalgae response to variation of salinity changes depends on species, growth phase, and environmental conditions.
Effect of osmotic stress, expressed in terms of NaCl concentration, on final biomass concentration and total lipid content (a) and on specific growth rate and lipid productivity (b). Control medium (CTRL) was the BBM. SAL1 = BBM + 15 g L−1 NaCl, SAL2 = BBM + 30 g L−1 NaCl, and SAL3 = BBM + 60 g L−1 NaCl. Asterisks (*) indicate a statistically meaningful difference (p ≤ 0.05) of the result from the corresponding one of control condition
In a different way compared to biomass accumulation, the total lipid content (TL) resulted statistically (p < 0.05) enhanced in accordance with the increased salinity of the medium. In particular, when the salinity was increased up to 30 mg L−1 (Fig. 2a), the TL increased up to 30.33% DW compared to the control (20% DW), while lipid productivities remained unaffected under the whole range of salinity tested (Fig. 2b).
The highest salt concentration tested (60 mg L−1) did not correspond to the highest TL. A similar trend was also reported by Haris et al. [39] for C. vulgaris under a salinity range 0–40 mg L−1, where the highest TL of 15.06% DW was obtained with 24 mg L−1 while under a maximum salinity of 40 mg L−1, the TL was 7.80% DW and in the control was 6.72% DW. It should be kept in mind that stress conditions may elevate the lipid content in microalgae to guarantee the survival in those culture conditions. When microalgae are grown under salinity stress, the biochemical pathways in the FA synthesis are directly involved because there is a depletion of the major photosynthesis electron acceptors of light and dark reaction [14]. Considering that the extent of this acceptor depletion is species specific, in general, the effect of salinity level toward growth and proximate composition in terms of lipids, proteins, and carbohydrates varies between different microalgae strains.
Recently, metabolomic analysis provided a new insight to elevate lipid yields in the process of biodiesel production using photosynthetic organisms as feedstock [41]. To this aim, the effect on C. zofingiensis biomass and lipid content was investigated by adding to the control two chemicals as triggers of metabolism such as ETA and TEA. ETA is recognized as a metabolite indirectly associated with the FA synthetic pathway. It can have a negative effect on microalgal growth and cell division since it can increase the supply of acetyl-CoA and favor the generation of phosphatidylethanolamine (PE), that is one of the main components of the cytoplasmic membrane [41]. TEA, a tertiary amine with three ethyl groups, is thought as a possible chemical that induces the promotion and accumulation of lipids [42].
In Fig. 4, the effect of three ETA concentrations, 0.050 g L−1, 0.100 g L−1, and 0.150 g L−1, respectively, on C. zofingiensis growth is reported. It can be inferred how biomass concentration and TL remain unaffected by the addition of ETA for all the three ETA concentrations investigated compared to the control (Fig 4a). A different trend was observed for lipid productivity, which exhibited a progressive gradual decrease as the ETA concentration increased compared to the control (Fig. 4b). Identical considerations can be made when the same three TEA concentrations were added to the control BBM. As it can be seen from Fig. 5a, the gradual increase in TEA concentration produced a meager decrease in biomass concentration and increase in TL compared to the control, even if the difference was statistically insignificant between the three TEA concentrations investigated (Fig. 5a). These results were somehow in disagreement with other data available in literature where the lipid content resulted increased once ETA and TEA were added to the culture medium. Cheng et al. [41] reported an increase up to 22% DW of the TL for Scenedesmus obliquus when this strain was cultivated in BG11 control medium with increasing concentration of ETA (0 mM, 0,5 mM, and 2 mM). In this case, the TL was positively correlated to the enhanced availability of ETA, while as reported in Fig. 4a, biomass content and ETA concentration were negatively correlated. The TL was also enhanced by 22.45% DW when ETA was supplemented to Crypthecodinium cohnii [43]. Xue et al. [13] reported the effect of TEA addition on Dunaliella tertiolecta growth and lipid production. In a similar way as reported for C. zofingiensis in Fig. 5b, the addition of TEA in the range 50–100 mg L−1 did not affect lipid productivity while when 150 mg L−1 of TEA was added, the lipid productivity decreased.
Effect of ethanolamine (ETA) concentration on final biomass concentration and total lipid content (a) and on specific growth rate and lipid productivity (b). Control medium (CTRL) was the BBM. ETA1 = BBM + 0.050 g L−1 ethanolamine, ETA2 = BBM + 0.100 g L−1 ethanolamine, and ETA3 = BBM + 0.150 g L−1 ethanolamine. Asterisks (*) indicate a statistically meaningful difference (p ≤ 0.05) of the result from the corresponding one of control condition
Effect of triethylamine (TEA) concentration on final biomass concentration and total lipid content (a) and on specific growth rate and lipid productivity (b). Control medium (CTRL) was the BBM. TEA1 = BBM + 0.050 g L−1 triethylamine, TEA2 = BBM + 0.100 g L−1 triethylamine, and TEA3 = BBM + 0.150 g L−1 triethylamine. Asterisks (*) indicate a statistically meaningful difference (p ≤ 0.05) of the result from the corresponding one of control condition
It has been reported that an increased concentration of ETA might promote the formation of FAs by depressing the synthesis of PE [41]. In this light, the involvement of ETA in FA metabolism can explain why when ETA is added to the culture medium, reproductive cycles of cells are slowed down and lipid metabolism is promoted. The direct consequence inside the cytoplasm could be a negative effect on biomass accumulation with a correspondent increase in lipid production, due to an important shift toward the synthesis of lipids.
It should be considered that in our study, three increasing concentrations of ETA did not produce a positive trend on C. zofingiensis biomass (which decreased correspondingly), while only 0.050 g L−1 of ETA produced a slight increase in the TL. Where a similar test was carried out on another algal species (S. obliquus), the TL was significantly up-regulated but only with the highest ETA concentration tested (2 mmol L−1), though the biomass was anyway reduced [41]. These findings may suggest that lipid metabolism can be stimulated depending on the ETA concentration and on the microalgal species involved. This aspect seems to be confirmed by a recent work on the effect of ETA on the production of metabolites in Haematococcus pluvialis [44]. The authors found out that ETA treatment improved the amount of carotenoids and carbohydrates in red cells, but the amount of proteins and biomass decreased in green cells. The ETA pre-treatment did not have significant effects on biomass of red cells, while it increased the amounts of carotenoids, carbohydrates, and proteins.
3.2 Influence of metabolic and chemical stress on FAME profile
The quality of a biodiesel is determined by a comparison between the chemical structure of lipids and the biodiesel standards. In particular, a good match in terms of similarity is obtained if FAs are characterized by a specific length of carbon chain and degree of chain branching as well as of unsaturation. This represents a fundamental prerequisite for considering microalgal biomass as a suitable feedstock for biodiesel production [7, 45]. Therefore, FAs were esterified to obtain the FAME profile of C. zofingiensis as reported in Table 2. FAME profile was obtained after 20 days of cultivation for the control and 25 days under ETA, TEA, and osmotic stress, respectively.
As it can be seen, the main FAs represented in all the media investigated including the control are 16–18 atoms of carbon long-chain compounds with differences not statistically relevant between them (p < 0.05). The most represented are oleic (C18:1), palmitoleic (C16:0), linoleic (C18:2), and hexadecatrienoic (C16:3) acids.
By comparing in detail the effect of osmotic and metabolic stress on FAME, it is interesting to note how the single FAs varied compared to the control based on the stress-inducer chemical considered. In particular, oleic acid increased considerably under osmotic stress with the highest percentage (45.32% DW) obtained with SAL3, while the highest percentage of palmitoleic acid was obtained under SAL2 (34.82% DW) and SAL1 (34.41% DW). Conversely, compared to the control, higher percentages of linoleic acid were obtained under ETA (ETA3 > ETA2 > ETA1) and TEA (TEA1 > TEA2 > TEA3), while lower percentages under osmotic stress. Interestingly, all TEA and ETA conditions produced almost doubled percentages of hexadecatrienoic acid compared to those obtained under osmotic stress.
Unsaturated fatty acids (UFA) represented the main components of FAMEs for all the culture media. Saturated fatty acids (SFA), UFA, and monunsaturated and polyunsaturated fatty acids (MUFA and PUFA, respectively) did not show significant percentage differences (p < 0.05) for the three TEA media compared to the control. UFA and MUFA components resulted statistically increased under all the ETA conditions and osmotic stress, respectively, compared to the control. The high UFA/SFA ratio (greater than 2) was obtained when C. zofingiensis was cultivated in ETA media, being the lowest saturation degree found in all the culture media.
Almutairi et al. [37] reported the FAME composition of the haptophyte microalga Tisochrysis lutea under a range of salt concentrations 0.4–1.0 M. In this context, the authors found that the relationship between SFA and UFA, in terms of increase of one component and corresponding decrease of the other, was not proportional to the increase of salinity in the culture medium. In particular, SFA increased under a salinity of 0.4–0.6 M and decreased under 0.8–1.0 M, with the UFA counterpart that decreased and increased accordingly. A similar trend was also found in this work for C. zofingiensis were SFA increased under 30 g L−1 of salt and decreased when the salinity was increased up to 60 g L−1, while UFA component decreased until 30 g L−1 but increased with 60 g L−1.
The UFA/SFA ratio relies on the cellular internal distribution of SFA and UFA which is linked to the nutritional requirements of microalgae. This last aspect depends in turn on the culture medium. The lipid composition in terms of SFA and UFA can be rearranged by microalgae depending on growth and environmental conditions. For example, an increase in the synthesis of neutral triglycerides leads to elevate the SFA portion inside the cell affecting the synthesis of polar membrane lipids (rich in UFA) which are partially degraded to sustain the triglyceride metabolism [46, 47]. The condition of nutrient starvation is a typical situation that favors this metabolic rearrangement of FAMEs.
The C16:0 FA is particularly suitable for making biodiesel. Therefore, the oil obtained by C. zofingiensis rich in C16:0 would possess high potential as a feedstock for biodiesel synthesis. In particular, C16:0 content was increased from 32.68% in BBM to 34.82% in SAL2, suggesting that the increased salt concentration in the medium can be used as an effective way to induce the accumulation of specific FAs, such as those involved in biodiesel synthesis.
3.3 Analysis of biodiesel properties based on FAME profile
The biodiesel quality is greatly affected by fuel properties. Among these last ones, oxidative stability and ignition are influenced by the lipid component of the biomass as well as by the FAME profile. In particular, the presence of long-chain C16–18 FAs, the high content of C18:1, and the level of UFA positively influence these two parameters. [48]. As it can be seen in Table 2, beside the control, more than 96% of C. zofingiensis total FAs were represented by C16–C18 groups, C18:1 was in the range 30.64% (TEA2)–45.32% (SAL2), and the degree of unsaturation was in the range 57.90% (SAL2)–67.53% (ETA3).
The feasibility of obtaining biodiesel from the extracted lipids was further evaluated on an additional analysis of the FAME profile by taking advantage of the software Biodiesel Analyzer© Ver. 2.2 which implements the equations reported in previous studies [36]. The latter one, by means of suitable mathematical relationships, allows to evaluate the relevant characteristics of biodiesel which can be obtained from the concerned FAME mixture. The obtained results are summarized in Table 3.
What emerges is an almost full compliance of all the physical parameters that characterize the obtainable biodiesel with the range of values prescribed by the ASTM standards (ASTM 6751-12) for unmixed biodiesel. Furthermore, most of the requirements of the European regulation for quality biodiesel (EN 14214 and EN 590) are satisfied by the biodiesel obtained using the culture media studied.
Based on the ASTM directives on biodiesel standards, 40 is the minimum value that should be reported for the cetane number (CN), a value that was actually reported for all the media where C. zofingiensis was cultivated. In addition to CN, the cloud point (CP), the pour point (MP), the lubricity (L), the viscosity (υ), and the density (ρ) represent additional parameters that are carefully considered to guarantee the biodiesel quality. In particular, the values denoting CP and PP should remain low, as found for ETA2 compared to the control. This particular condition is obtained by ensuring a high level of UFA in the FAME, as found for all the three ETA concentrations tested (Table 2). A high content of PUFA C18:2 instead guarantees a reduction of PP, as observed for ETA and for ETA2 in particular. Two parameters which differ somewhat from the prescribed standards are viscosity and density (in bold in Table 3) which were slightly lower. In fact, the values of these parameters should be in the range 3.5–5 mm2 s−1 and 0.86–0.9 t m−3, respectively, according to the European standards. However, what can be noted is that the difference between the reported and prescribed values is very small and could be easily adjusted by adding specific additives to the biodiesel.
4 Conclusion
This work is aimed at evaluating how two chemicals such as ethanolamine (ETA) and trimethylamine (TEA) and osmotic stress obtained by the addition of sodium chloride to the culture medium are able to modify the metabolism of C. zofingiensis toward an enhancement of growth, lipid productivity, and FAME profile. The growth remained unaffected by the chemical concentrations investigated while the salt stress produced a decrease in biomass accumulation. On the other hand, the whole range of salinity tested produced an increase in the lipid content. In particular, the highest lipid content (30.33% DW) was obtained under SAL2 compared to the control (20% DW), while TEA and ETA had a negligible effect.
The addition of ETA on culture medium produced a modification on FAME profile since UFA and in particular PUFA resulted significantly increased compared to the same FAs under osmotic stress. The assessment of FAME composition of C. zofingiensis cultivated under stressful conditions demonstrated that biofuels with characteristics very close to the ones required by the relevant standards for the quality of biodiesel can be produced by using salt and chemicals acting as gene modulators.
Data availability
Original data will be available upon request.
References
Elias SA (2021) History of greenhouse gas warming: CO2. In: Elias SA, Alderton D (eds) Encyclopedia of geology, 2nd edn. Academic Press, Cambridge, MS, USA, pp 444–455
Malins C (2017) What role is there for electrofuel technologies in European transport’s low carbon future? Transport and Environment. Cerulogy, Brussels, Belgium, pp 1–86
Rejmanji M, Suresh S, Nesamma AA, Jutur PP (2021) Microalgal cell factories, a platform for high-value added biorenewables to improve the economics of the biorefinery. In: Das S, Dash HR (eds) Microbial and natural macromolecules – synthesis and applications. Academic Press, Cambridge, MS, USA, pp 689–731
Lutzu GA, Ciurli A, Chiellini C, Di Caprio F, Concas A, Dunford NT (2021) Latest developments in wastewater treatment and biopolymer production by microalgae. J Environ Chem Eng 9(1):104926. https://doi.org/10.1016/j.jece.2020.104926
Hussain F, Shah SZ, Ahmad H, Abubshait SA, Abubshait HA, Laref A et al (2021) Microalgae an ecofriendly and sustainable wastewater treatment option: biomass application in biofuel and bio-fertilizer production. A review Renew Sust Energy Rev 137:110603. https://doi.org/10.1016/j.rser.2020.110603
Ananthi V, Sasikumar P, Pugazhendhi A (2020) Analysis of the limiting factors for large scale microalgae cultivation: a promising future for renewable and sustainable biofuel industry. Renew Sustain Energy Rev 134:110250. https://doi.org/10.1016/j.rser.2020.110250
Morales M, Aflalo C, Bernard O (2021) Microalgal lipids: a review of lipids potential and quantification for 95 phytoplankton species. Biomass Bioenergy 150:106108. https://doi.org/10.1016/j.biombioe.2021.106108
Bibi F, Jamal A, Huang Z, Urynowicz M, Ali MI (2022) Advancement and role of abiotic stresses in microalgae biorefinery with a focus on lipid production. Fuel 316:123192. https://doi.org/10.1016/j.fuel.2022.123192
Song X, Liu B-F, Kong F, Ren N-Q, Ren H-Y (2022) Overview on stress-induced strategies for enhanced microalgae lipid production: application, mechanisms and challenges. Resourc Conserv Recycl 183:106355. https://doi.org/10.1016/j.resconrec.2022.106355
Rawat J, Gupta PK, Pandit S, Prasad R, Pande V (2021) Current perspectives on integrated approaches to enhance lipid accumulation in microalgae. 3 Biotech 11(6):303. https://doi.org/10.1007/s13205-021-02851-3
Zhao Y, Ngo HH, Yu X (2022) Phytohormone-like small biomolecules for microalgal biotechnology. Trends Biotechnol 40(9):1025–1028. https://doi.org/10.1016/j.tibtech.2022.06.008
Li T-S, Wu J-Y (2015a) Effect of carbon sources on growth and lipid accumulation of newly isolated microalgae cultured under mixotrophic condition. Bioresour Technol 184:100–107. https://doi.org/10.1016/j.biortech.2014.11.005
Xue L-L, Jiang J-G (2017) Cultivation of Dunaliella tertiolecta intervened by trimethylamine enhances the lipid content. Algal Res 25:136–141. https://doi.org/10.1016/j.algal.2017.04.019
Fal S, Aasfar A, Rabie R, Smouni A, Arrousi HEL (2022) Salt induced oxidative stress alters physiological, biochemical and metabolomic responses of green microalga Chlamydomonas reinhardtii. Helyon 8(1):e08811. https://doi.org/10.1016/j.heliyon.2022.e08811
BenMoussa-Dahmen I, Chtourou H, Rezgui F, Sayadi S, Dhouib A (2016) Salinity stress increases lipid, secondary metabolites and enzyme activity in Amphora subtropica and Dunaliella sp. for biodiesel production. Bioresour Technol 218:816–825. https://doi.org/10.1016/j.biortech.2016.07.022
Hounslow E, Evans CA, Pandhal J, Sydney T, Couto N, Pham TK et al (2021) Quantitative proteomic comparison of salt stress in Chlamydomonas reinhardtii and the snow alga Chlamydomonas nivalis reveleas mechanisms for salt/triggered fatty acid accumulation via reallocation of carbon sources. Biotechnol Biofuels 14:121. https://doi.org/10.1186/s13068-021-01970-6
Adegboye MF, Ojuederie OB, Talia PM, Babalola OO (2021) Bioprespecting of microbial strains for biofuels production: metabolic engineering, applications, and challenges. Biotechnol Biofuels 14:5. https://doi.org/10.1186/s13068-020-01853-2
Zhang Y, Ye Y, Bai F, Liu J (2021) The oleaginous astaxanthin-producing alga Chromochloris zofingiensis: potential from production to an emerging model for studying lipid metabolism and carotenogenesis. Biotechnol Biofuels 14:119. https://doi.org/10.1186/s13068-021-01969-z
Wood EE, Ross ME, Jubeau S, Montalescot V, Stanley MS (2022) Progress towards a targeted biorefinery of Chromochloris zofingiensis: a review. Biomass Conv Bioref. https://doi.org/10.1007/s133999-02955-7
Sun Z, Zhang Y, Sun LP, Liu J (2019) Light elicits astaxanthin biosynthesis and accumulation in the fermented ultrahigh-density Chlorella zofinginesis. J Agric Food Chem 67(19):5579–5586. https://doi.org/10.1021/acs.jafc.9b01176
Wang X, Wei H, Mao X, Liu J (2019) Proteomics analysis of lipid droplets from the oleaginous alga Chromochloris zofingiensis reveals novel proteins for lipid metabolism. Genomics Proteomics Bioinformatics 17(3):260–272. https://doi.org/10.1016/j.gpb.2019.01.003
Vitali L, Lolli V, Sansone F, Concas A, Lutzu GA (2022) Effect of mixotrophy on lipid content and fatty acids methyl esters profile by Chromochloris zofingiensis grown in media containing sugarcane molasses. Bioenergy Res. https://doi.org/10.1007/s12155-022-10534-x
Chowdhary AK, Kishi M, Toda T (2022) Enhanced growth of Chromochloris zofingiensis through the transition of nutritional modes. Algal Res 65:102723. https://doi.org/10.1016/j.algal.2022.102723
Liu J (2018) Batch cultivation for astaxanthin analysis using the green microalga Chlorella zofingiensis under multitrophic growth conditions. Methods Mol Biol 1852:97–106. https://doi.org/10.1007/978-1-4939-8742-9_5
Kozlova TA, Kartashov AV, Zadneprovskaya E, Krapivina A, Zaytsev P, Chivkunova OB, Solovchenko AE (2023) Effect of abscisic acid on growth, fatty acid profile, and pigment composition of the Chlorophyte Chlorella (Chromochloris) zofingiensis and its co-culture microbiome. Life 13(2):452. https://doi.org/10.3390/life13020452
Duan X, Ren GY, Liu LL, Zhu WX (2012) Salt-induced osmotic stress for lipid overproduction in batch cultures of Chlorella vulgaris. Afr J Biotechnol 11(27):7072–7078. https://doi.org/10.5897/AJB11.3670
Pandit PR, Fulekar MH, Karuna MSL (2017) Effect of salinity stress on growth, lipid productivity, fatty acid composition, and biodiesel properties in Acutodesmus obliquus and Chlorella vulgaris. Environ Sci Pollut Res 24:13437–13451. https://doi.org/10.1007/s11356-017-8875-y
Mirizadeh S, Nosrati M, Shojaosadati SA (2020) Synergistic effect of nutrient and salt stress on lipid productivity of Chlorella vulgaris through two-stages cultivation. Bioenergy Res 13:507–515. https://doi.org/10.1107/s12155-019-10077-8
Bajwa K, Bishnoi NR (2015) Osmotic stress induced by salinity for lipid overproduction in batch cultures of Chlorella pyrenoidosa and effect on others physiological as well as physicochemical attributes. J Algal Biomass Util 6(4):26–34
Mao X, Zhang Y, Wang X, Liu J (2020) Novel insight into salinity induced lipogenesis and carotenogenosis in the oleaginous astaxanthin-producing alga Chromochloris zofingiensis: a multi-omics study. Biotechnol Biofuels 13:73. https://doi.org/10.1186/s13068-020-01714-y
UTEX, Culture collection of algae at University of Texas, https://utex.org/, 2020.
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–917. https://doi.org/10.1139/o59-099
Chen L, Liu T, Zhang W, Chen X, Wang J (2012) Biodiesel production from algae oil high in free fatty acids by two step catalytic conversion. Bioresour Technol 111:208–214. https://doi.org/10.1016/j.biortech.2012.02.033
Lage S, Gentili FG (2018) Quantification and characterization of fatty acid methyl esters in microalgae: comparison of pretreatment and purification methods. Bioresour Technol 257:121–128. https://doi.org/10.1016/j.biortech.2018.01.153
Senthamilselvi D, Kalaiselvi T (2022) Fuel properties of fatty acids methyl esters (FAME) produced with fats of gamma – irradiated mutants of oleaginous microalga - Chlorella sp. KM504965. Madras Agric J. https://doi.org/10.29321/MAJ.10.000574
Talebi AF, Tabatabaei M, Chisti Y (2014) BiodieselAnalyzer: a user-friendly software for predicting the properties of prospective biodiesel. Biofuel Res J 1:55–57. https://doi.org/10.18331/BRJ2015.1.2.4
Almutairi AW, El-Sayed AE-KB, Reda MM (2020) Combined effect of salinity and pH on lipid content and fatty acid composition of Tisochrysis lutea. Saudi J Biol Sci 27:3553–3558. https://doi.org/10.1016/j.sjbs.2020.07.027
Venckus P, Cicchi B, Chini Zitelli G (2021) Effects of medium salinity on growth and biochemical composition of the green microalga Tetraselmis suecica. J Appl Phycol 33:3555–3563. https://doi.org/10.1007/s10811-021-02560-7
Haris N, Manan H, Jusoh M, Khatoon H, Katayama T, Kasan NA (2022) Effect of different salinity on the growth performance and proximative composition of isolated indigenous microalgae species. Aquac Rep 22:100925. https://doi.org/10.1016/j.aqrep.2021.100925
Chen GQ, Jiang Y, Chen F (2008) Salt-induced alterations in lipid composition of diatom Nitzschia laevis (Bacillariophyceae) under heterotrophic culture condition. J Phycol 44:1309–1314. https://doi.org/10.1111/j.1529-8817.2008.00565.x
Cheng J-S, Niu Y-H, Lu S-H, Yuan Y-J (2012) Metabolome analysis reveals ethanolamine as potential marker for improving lipid accumulation of model photosynthetic organisms. J Chem Technol Biotechnol 87(12):1409–1418. https://doi.org/10.1002/jctb.3759
Lin Z, Bao M, Yu Z, Xue L, Ju C, Zhang C (2019) The development of tertiary amine cationic lipids for safe and efficient siRNA delivery. Biomater Sci 7:2777–2792. https://doi.org/10.1039/C9BM00494G
Li J, Niu X, Pei G, Sui X, Zhang X, Chen L, Zhang W (2015b) Identification and metabolomic analysis of chemical modulators for lipid accumulation in Crypthecodinium cohnii. Bioresour Technol 191:362e368. https://doi.org/10.1016/j.biortech.2015.03.068
Rezazadeh H, Mansoori H, Amandadi M (2022) Effects of treatment and pre-treatment of ethanolamine on production of metabolites in Haematococcus pluvialis. Iran J Sci Technol Trans A Sci. https://doi.org/10.1007/s40995-022-01378-3
Miotti T, Pivetti L, Lolli V, Sansone F, Concas A, Lutzu GA (2022) On the use of agro-industrial wastewaters to promote mixotrophic metabolism in Chlorella vulgaris. Effect on FAME profile and biodiesel properties. Chem Eng Trans 92:55–60. https://doi.org/10.3303/CET2292010
Guihéneuf F, Stengel D (2013) LC-PUFA-enriched oil production by microalgae: accumulation of lipid and triacylglycerols containing n-3 LC-PUFA is triggered by nitrogen limitation and inorganic carbon availability in the marine haptophyte Pavlova lutheri. Mar Drugs 11(11):4246–4266. https://doi.org/10.3390/md11114246
Xin Y, Shen C, She Y, Chen H, Wang C, Wei L et al (2019) Biosynthesis of triacylglycerol molecules with a tailored PUFA profile in industrial microalgae. Mol Plant 12(4):474–488. https://doi.org/10.1016/j.molp.2018.12.007
Tamilselvan P, Sassykova L, Prabhahar M, Baskar K, Kannayiram G, Subramanian S, Prakash S (2020) Influence of saturated fatty acid material composition in biodiesel on its performance in internal combustion engines. Mater Today Proc 33:1181–1186. https://doi.org/10.1016/j.matpr.2020.07.626
Acknowledgements
The authors wish to thank Dr. Eya Damergi from École Polytechnique Fédérale de Lausanne, Switzerland, for her precious suggestions and advises in improving the discussion. Prof. Augusta Caligiani from the University of Parma, Italy, is also acknowledged for her helpful discussion on the characterization experiments.
Funding
Open access funding provided by Università degli Studi di Parma within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Vitali L, Sansone F, Lutzu GA, and Concas A contributed to the study conception and design. Material preparation and data collection were performed by Vitali L and Lutzu GA. Analysis was performed by Vitali L, Lolli V, and Sansone F. The first draft of the manuscript was written by Lutzu GA, Kumar A, and Concas A, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable since this work deals with vegetal cells.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Salinity can be used by Chromochloris zofingiensis to enhance lipid accumulation.
• Salt stress triggered lipid content better than control medium.
• Internal cell metabolism was modified by salt to improve lipid production.
• Saturation and unsaturation levels in FAMEs were directly influenced by salinity.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vitali, L., Lolli, V., Sansone, F. et al. Lipid content and fatty acid methyl ester profile by Chromochloris zofingiensis under chemical and metabolic stress. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04153-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04153-5