Abstract
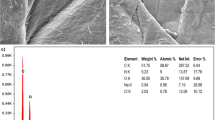
This study presented the pyrolyzed oyster shell (POS) for efficiently removing Cu(II) from aqueous solution. Design of the experiment based on the Taguchi method was implemented to identify the optimum process conditions and explore the influence of process factors (solution temperature, pyrolysis temperature, initial Cu(II) concentration, POS dosage, and reaction time) on copper removal. According to the statistical analysis, pyrolysis temperature was the most effective factor in the Cu(II) removal, and reaction time was the second dominating factor. Optimum Cu(II) removal of 99.82% was acquired at solution temperature 25 ℃, pyrolysis temperature 900 ℃, Cu(II) concentration 100 mg/L, POS dosage 0.2 g, and reaction time 30 min. The Cu(II) adsorption behavior of POS was examined by adsorption kinetics and adsorption isotherm, which displayed that the pseudo-second order model and the Langmuir isotherm model fitted to experimental data with great accuracy. Moreover, POS at 900 ℃ showed the highest adsorption capacity of 1093 mg/g. SEM–EDS, XRD, TG–DTA, FTIR, and BET techniques were applied to investigate the physicochemical features of virgin POS and used POS. The Cu(II) removal mechanism involved electrostatic interaction, cation exchange, posnjakite forming, and precipitation.
Graphical Abstract








Similar content being viewed by others
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Zamora-Ledezma C, Negrete-Bolagay D, Figueroa F, Zamora-Ledezma E, Ni M, Alexis F, Guerrero VH (2021) Heavy metal water pollution: a fresh look about hazards, novel and conventional remediation methods. Environ Technol Inno 22:101504. https://doi.org/10.1016/j.eti.2021.101504
Dong D, Tukker A, Van der Voet E (2019) Modeling copper demand in China up to 2050: a business-as-usual scenario based on dynamic stock and flow analysis. J Ind Ecol 23:1363–1380. https://doi.org/10.1111/jiec.12926
Khan J, Lin S, Nizeyimana JC, Wu Y, Wang Q, Liu X (2021) Removal of copper ions from wastewater via adsorption on modified hematite (α-Fe2O3) iron oxide coated sand. J Clean Prod 319:128687. https://doi.org/10.1016/j.jclepro.2021.128687
Pu X, Yao L, Yang L, Jiang W, Jiang X (2020) Utilization of industrial waste lithium-silicon-powder for the fabrication of novel nap zeolite for aqueous Cu(II) removal. J Clean Prod 265:121822. https://doi.org/10.1016/j.jclepro.2020.121822
Bost M, Houdart S, Oberli M, Kalonji E, Huneau J-F, Margaritis I (2016) Dietary copper and human health: current evidence and unresolved issues. J Trace Elem Med Bio 35:107–115. https://doi.org/10.1016/j.jtemb.2016.02.006
Rais S, Islam A, Ahmad I, Kumar S, Chauhan A, Javed H (2021) Preparation of a new magnetic ion-imprinted polymer and optimization using Box-Behnken design for selective removal and determination of Cu(II) in food and wastewater samples. Food Chem 334:127563. https://doi.org/10.1016/j.foodchem.2020.127563
Ciftci TD (2017) Adsorption of Cu(II) on three adsorbents, Fe3O4/Ni/NixB nanocomposite, carob (ceratonia siliqua), and grape seeds: a comparative study. Turk J Chem 41:760–772. https://doi.org/10.3906/kim-1701-9
Song X, Cao Y, Bu X, Luo X (2021) Porous vaterite and cubic calcite aggregated calcium carbonate obtained from steamed ammonia liquid waste for Cu2+ heavy metal ions removal by adsorption process. Appl Surf Sci 536:147958. https://doi.org/10.1016/j.apsusc.2020.147958
U.S. Environmental Protection Agency (2022) National primary drinking water regulations, https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations [accessed 9 August 2022]
Komkiene J, Baltrenaite E (2016) Biochar as adsorbent for removal of heavy metal ions [cadmium(II), copper(II), lead(II), zinc(II)] from aqueous phase. Int J Environ Sci Te 13:471–482. https://doi.org/10.1007/s13762-015-0873-3
Li P, Liu H, Zhou M, Lei H, Jian B, Liu R, Li X, Wang Y, Zhou B (2022) Preparation of green magnetic hydrogel from cellulose nanofibril (CNF) originated from soybean residue for effective and rapid removal of copper ions from waste water. Ind Crop Prod 186:115257. https://doi.org/10.1016/j.indcrop.2022.115257
Mejía Ávila J, Rangel Ayala M, Kumar Y, Pérez-Tijerina E, Robles MAR, Agarwal V (2022) Avocado seeds derived carbon dots for highly sensitive Cu(II)/Cr(VI) detection and copper(II) removal via flocculation. Chem Eng J 446:137171. https://doi.org/10.1016/j.cej.2022.137171
Gomes Carvalho Barros GK, Fonseca Melo RP, de Barros Neto EL (2018) Removal of copper ions using sodium hexadecanoate by ionic flocculation. Sep Purif Technol 200:294–299. https://doi.org/10.1016/j.seppur.2018.01.062
Sun H, Zhang X, He Y, Zhang D, Feng X, Zhao Y, Chen L (2019) Preparation of PVDF-g-PAA-PAMAM membrane for efficient removal of copper ions. Chem Eng Sci 209:115186. https://doi.org/10.1016/j.ces.2019.115186
Mulungulungu GA, Mao T, Han K (2021) Efficient removal of high-concentration copper ions from wastewater via 2D g-C3N4 photocatalytic membrane filtration. Colloid Surface A 623:126714. https://doi.org/10.1016/j.colsurfa.2021.126714
Virolainen S, Wesselborg T, Kaukinen A, Sainio T (2021) Removal of iron, aluminium, manganese and copper from leach solutions of lithium-ion battery waste using ion exchange. Hydrometall 202:105602. https://doi.org/10.1016/j.hydromet.2021.105602
Murray A, Ormeci B (2019) Use of polymeric sub-micron ion-exchange resins for removal of lead, copper, zinc, and nickel from natural waters. J Environ Sci 75:247–254. https://doi.org/10.1016/j.jes.2018.03.035
Zhang X-f, Yuan J, Tian J, Han H-s, Sun W, Yue T, Yang Y, Wang L, Cao X-f, Lu C-l (2022) Ultrasonic-enhanced selective sulfide precipitation of copper ions from copper smelting dust using monoclinic pyrrhotite. T Nonferr Metal Soc 32:682–695. https://doi.org/10.1016/S1003-6326(22)65825
Saleh ME, El-Refaey AA, Mahmoud AH (2016) Effectiveness of sunflower seed husk biochar for removing copper ions from wastewater: a comparative study. Soil Water Res 11(1):53–63. https://doi.org/10.17221/274/2014-SWR
Hasanpour M, Hatami M (2020) Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: a review study. Adv Colloid Interfac 284:102247. https://doi.org/10.1016/j.cis.2020.102247
Ma J, Wang D, Zhang W, Wang X, Ma X, Liu M, Zhao Q, Zhou L, Sun S, Ye Z (2022) Development of β-cyclodextrin-modified poly(chloromethyl styrene) resin for efficient adsorption of Cu(II) and tetracycline. Process Biochem 121:298–311. https://doi.org/10.1016/j.procbio.2022.07.017
Wang Q, Li L, Kong L, Cai G, Wang P, Zhang J, Zuo W, Tian Y (2022) Compressible amino-modified carboxymethyl chitosan aerogel for efficient Cu(II) adsorption from wastewater. Sep Purif Technol 293:121146. https://doi.org/10.1016/j.seppur.2022.121146
Rong N, Chen C, Ouyang K, Zhang K, Wang X, Xu Z (2021) Adsorption characteristics of directional cellulose nanofiber/chitosan/montmorillonite aerogel as adsorbent for wastewater treatment. Sep Purif Technol 274:119120. https://doi.org/10.1016/j.seppur.2021.119120
Sitko R, Turek E, Zawisza B, Malicka E, Talik E, Heimann J, Gagor A, Feist B, Wrzalik R (2013) Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton T 42(16):5682–5689. https://doi.org/10.1039/c3dt33097d
Egbosiuba TC, Abdulkareem AS (2021) Highly efficient as-synthesized and oxidized multi-walled carbon nanotubes for copper(II) and zinc(II) ion adsorption in a batch and fixed-bed process. J Mater Res Technol 15:2848–2872. https://doi.org/10.1016/j.jmrt.2021.09.094
Yusuf M, Elfghi FM, Zaidi SA, Abdullah EC, Khan MA (2015) Applications of graphene and its derivatives as an adsorbent for heavy metal and dye removal: a systematic and comprehensive overview. RSC Adv 5(62):50392–50420. https://doi.org/10.1039/c5ra07223a
Gu M, Hao L, Wang Y, Li X, Chen Y, Li W, Jiang L (2020) The selective heavy metal ions adsorption of zinc oxide nanoparticles from dental wastewater. Chem Phys 534:110750. https://doi.org/10.1016/j.chemphys.2020.110750
Zonato RdO, Estevam BR, Perez ID, dos Santos Aparecida, Ribeiro V, Boina RF (2022) Eggshell as an adsorbent for removing dyes and metallic ions in aqueous solutions. Clean Chem Eng 2:100023. https://doi.org/10.1016/j.clce.2022.100023
Botta R, Asche F, Borsum JS, Camp EV (2020) A review of global oyster aquaculture production and consumption. Mar Policy 117:103952. https://doi.org/10.1016/j.marpol.2020.103952
Xing R, Qin Y, Guan X, Liu S, Yu H, Li P (2013) Comparison of antifungal activities of scallop shell, oyster shell and their pyrolyzed products. Egypt J Aquatic Res 39(2):83–90. https://doi.org/10.1016/j.ejar.2013.07.003
Chen D, Pan T, Yu X, Liao Y, Zhao H (2019) Properties of hardened mortars containing crushed waste oyster shells. Environ Eng Sci 36(9):1079–1088. https://doi.org/10.1089/ees.2018.0465
Chen Y, Xu J, Lv Z, Xie R, Huang L, Jiang J (2018) Impacts of biochar and oyster shells waste on the immobilization of arsenic in highly contaminated soils. J Environ Manage 217:646–653. https://doi.org/10.1016/j.jenvman.2018.04.007
Mo KH, Alengaram UJ, Jumaat MZ, Lee SC, Goh WI, Yuen CW (2018) Recycling of seashell waste in concrete: a review. Constr Build Mater 162:751–764. https://doi.org/10.1016/j.conbuildmat.2017.12.009
Zhong G, Liu Y, Tang Y (2021) Oyster shell powder for Pb(II) immobilization in both aquatic and sediment environments. Environ Geochem Hlth 43(5):1891–1902. https://doi.org/10.1007/s10653-020-00768-z
Woo-Hang K (2020) Removal of cadmium by sorption from aqueous solution using pre-treated oyster shells. J Korean So Environ Technol 20(3):179–187. https://doi.org/10.26511/JKSET.21.3.1
Kwon HB, Lee CW, Jun BS, Yun JD, Weon SY, Koopman B (2004) Recycling waste oyster shells for eutrophication control. Resour Conserv Recy 41(1):75–82. https://doi.org/10.1016/j.resconrec.2003.08.005
Khirul MA, Kim B-G, Cho D, Yoo G, Kwon S-H (2020) Effect of oyster shell powder on nitrogen releases from contaminated marine sediment. Environ Eng Res 25(2):230–237. https://doi.org/10.4491/eer.2018.395
You K, Yang W, Song P, Fan L, Xu S, Li B, Feng L (2022) Lanthanum-modified magnetic oyster shell and its use for enhancing phosphate removal from water. Colloid Surface A 633:127897. https://doi.org/10.1016/j.colsurfa.2021.127897
Goyal M, Rattan VK, Aggarwal D, Bansal RC (2001) Removal of copper from aqueous solutions by adsorption on activated carbons. Colloid Surface A 190(3):229–238. https://doi.org/10.1016/S0927-7757(01)00656-2
Wang X, Wang C (2016) Chitosan-poly(vinyl alcohol)/attapulgite nanocomposites for copper(II) ions removal: pH dependence and adsorption mechanisms. Colloid Surface A 500:186–194. https://doi.org/10.1016/j.colsurfa.2016.04.034
Durán-Jiménez G, Hernández-Montoya V, Montes-Morán MA, Bonilla-Petriciolet A, Rangel-Vázquez NA (2014) Adsorption of dyes with different molecular properties on activated carbons prepared from lignocellulosic wastes by Taguchi method. Micropor Mesopor Mat 199:99–107. https://doi.org/10.1016/j.micromeso.2014.08.013
Zaluski D, Stolarski MJ, Krzyzaniak M (2022) Validation of the Taguchi method on the example of evaluation of willow biomass production factors under environmental stress. Ind Crop Prod 185:115170. https://doi.org/10.1016/j.indcrop.2022.115170
Haghgir A, Hosseini SH, Tanzifi M, Yaraki MT, Bayati B, Saemian T, Koohi M (2022) Synthesis of polythiophene/zeolite/iron nanocomposite for adsorptive remediation of azo dye: optimized by Taguchi method. Chem Eng Res Des 183:525–537. https://doi.org/10.1016/j.cherd.2022.05.0420263-8762
Maazinejad B, Mohammadnia O, Ali GAM, Makhlouf ASH, Nadagouda MN, Sillanpaa M, Asiri AM, Agarwal S, Gupta VK, Sadegh H (2020) Taguchi L9 (34) orthogonal array study based on methylene blue removal by single-walled carbon nanotubes-amine: adsorption optimization using the experimental design method, kinetics, equilibrium and thermodynamics. J Mol Liq 298:112001. https://doi.org/10.1016/j.molliq.2019.112001
Moamen OAA, Hassan HS, Zaher WF (2020) Taguchi L16 optimization approach for simultaneous removal of Cs+ and Sr2+ ions by a novel scavenger. Ecotox Environ Safe 189:110013. https://doi.org/10.1016/j.ecoenv.2019.110013
Boulahbal M, Malouki MA, Canle M, Redouane-Salah Z, Devanesan S, AlSalhi MS, Berkani M (2022) Removal of the industrial azo dye crystal violet using a natural clay: characterization, kinetic modeling, and RSM optimization. Chemosphere 306:135516. https://doi.org/10.1016/j.chemosphere.2022.135516
Ahmadipouya S, Haris MH, Ahmadijokani F, Jarahiyan A, Molavi H, Moghaddam FM, Rezakazemi M, Arjmand M (2021) Magnetic Fe3O4@UiO-66 nanocomposite for rapid adsorption of organic dyes from aqueous solution. J Mol Liq 322:114910. https://doi.org/10.1016/j.molliq.2020.114910
Panda H, Tiadi N, Mohanty M, Mohanty CR (2017) Studies on adsorption behavior of an industrial waste for removal of chromium from aqueous solution. S Afr J Chem Eng 23:132–138. https://doi.org/10.1016/j.sajce.2017.05.002
Tan YH, Abdullah MO, Nolasco-Hipolito C, Zauzi NSA (2017) Application of RSM and Taguchi methods for optimizing the transesterification of waste cooking oil catalyzed by solid ostrich and chicken-eggshell derived CaO. Renew Energ 114:437–447. https://doi.org/10.1016/j.renene.2017.07.024
Jiang L, Huang G, Shao L, Huang J, Peng S, Yang X (2021) In situ electrochemical synthesis of Zn-Al layered double hydroxides for removal of strontium: optimization and kinetics study. Colloid Surface A 608:125589. https://doi.org/10.1016/j.colsurfa.2020.125589
Vinayagam R, Murugesan G, Varadavenkatesan T, Bhole R, Goveas LC, Samanth A, Ahmed MB, Selvaraj R (2022) Algal biomass-derived nano-activated carbon for the rapid removal of tetracycline by adsorption: experimentation and adaptive neuro-fuzzy inference system modeling. Bioresource Technol Rep 20:101291. https://doi.org/10.1016/j.biteb.2022.101291
Wang W, Zhang X, Zhang Y, Mi X, Wang R, Shi H, Li C, Du Z, Qiao Y (2021) Adsorption of emerging sodium p-perfluorous nonenoxybenzene sulfonate (OBS) onto soils: kinetics, isotherms and mechanisms. Pedosphere 31(4):596–605. https://doi.org/10.1016/S1002-0160(21)60005-X
Yi X, He J, Guo Y, Han Z, Yang M, Jin J, Gu J, Ou M, Xu X (2018) Encapsulating Fe3O4 into calcium alginate coated chitosan hydrochloride hydrogel beads for removal of Cu(II) and U(VI) from aqueous solutions. Ecotox Environ Safe 147:699–707. https://doi.org/10.1016/j.ecoenv.2017.09.036
Veloso CH, Filippov LO, Filippova IV, Ouvrard S, Araujo AC (2020) Adsorption of polymers onto iron oxides: equilibrium isotherms. J Mater Res Technol 9(1):779–788. https://doi.org/10.1016/j.jmrt.2019.11.018
Rae IB, Gibb SW, Lu S (2009) Biosorption of Hg from aqueous solutions by crab carapace. J Hazard Mater 164(2–3):1601–1604. https://doi.org/10.1016/j.jhazmat.2008.09.052
Boddu S, Chandra A, Khan AA (2022) Biosorption of Cu(II), Pb(II) from electroplating industry effluents by treated shrimp shell. Mater Today Proc 57:1520–1527. https://doi.org/10.1016/j.matpr.2021.12.052
Su X, Chen Y, Li Y, Li J, Song W, Li X, Yan L (2022) Enhanced adsorption of aqueous Pb(II) and Cu(II) by biochar loaded with layered double hydroxide: crucial role of mineral precipitation. J Mol Liq 357:119083. https://doi.org/10.1016/j.molliq.2022.119083
Mohammadifard H, Amiri MC (2017) Evaluating Cu(II) removal from aqueous solutions with response surface methodology by using novel synthesized CaCO3 nanoparticles prepared in a colloidal gas aphron system. Chem Eng Commun 204(4):476–484. https://doi.org/10.1080/00986445.2016.1277522
Wang S, Bian S, Liu J, Li J, Xu S, Liang Z (2021) Highly adsorptive pristine and magnetic biochars prepared from crayfish shell for removal of Cu(II) and Pb(II). J Taiwan Inst Chem E 127:175–185. https://doi.org/10.1016/j.jtice.2021.08.004
Yin Z, Liu Y, Liu S, Jiang L, Tan X, Zeng G, Li M, Liu S, Tian S, Fang Y (2018) Activated magnetic biochar by one-step synthesis: enhanced adsorption and coadsorption for 17 beta-estradiol and copper. Sci Total Environ 639:1530–1542. https://doi.org/10.1016/j.scitotenv.2018.05.130
Maaloul N, Oulego P, Rendueles M, Ghorbal A, Diaz M (2017) Novel biosorbents from almond shells: characterization and adsorption properties modeling for Cu(II) ions from aqueous solutions. J Environ Chem Eng 5(3):2944–2954. https://doi.org/10.1016/j.jece.2017.05.037
Mahdi Z, El Hanandeh A (2022) Insight into copper and nickel adsorption from aqueous solutions onto carbon-coated-sand: isotherms, kinetics, mechanisms, and cost analysis. Clean Chem Eng 3:100045. https://doi.org/10.1016/j.clce.2022.100045
Yu J, Chi C, Zhu B, Qiao K, Cai X, Cheng Y, Yan S (2020) High adsorptivity and recycling performance activated carbon fibers for Cu(II) adsorption. Sci Total Environ 700:134412. https://doi.org/10.1016/j.scitotenv.2019.134412
Sun Y, Yang G, Zhang L (2018) Hybrid adsorbent prepared from renewable lignin and waste egg shell for SO2 removal: characterization and process optimization. Ecol Eng 115:139–148. https://doi.org/10.1016/j.ecoleng.2018.02.013
Syazwani ON, Rashid U, Yap YHT (2015) Low-cost solid catalyst derived from waste Cyrtopleura costata (Angel Wing Shell) for biodiesel production using microalgae oil. Energ Convers and Manage 101:749–756. https://doi.org/10.1016/j.enconman.2015.05.075
Deng W, Zhang D, Zheng X, Ye X, Niu X, Lin Z, Fu M, Zhou S (2021) Adsorption recovery of phosphate from waste streams by Ca/Mg-biochar synthesis from marble waste, calcium-rich sepiolite and bagasse. J Clean Prod 288:125638. https://doi.org/10.1016/j.jclepro.2020.125638
Wen T, Zhao Y, Jiao X, Yang G, Zhang Z, Wang W, Zhang T, Zhang Q, Song S (2021) Use of posnjakite containing sludge as catalyst for decoloring dye via photo-fenton-like process. J Clean Prod 293:126184. https://doi.org/10.1016/j.jclepro.2021.126184
Wen T, Zhao Y, Zhang T, Xiong B, Hu H, Zhang Q, Song S (2020) Selective recovery of heavy metals from wastewater by mechanically activated calcium carbonate: inspiration from nature. Chemosphere 246:125842. https://doi.org/10.1016/j.chemosphere.2020.125842
Zhang T, Wen T, Zhao Y, Hu H, Xiong B, Zhang Q (2018) Antibacterial activity of the sediment of copper removal from wastewater by using mechanically activated calcium carbonate. J Clean Prod 203:1019–1027. https://doi.org/10.1016/j.jclepro.2018.08.278
Prabhu P, Rao M, Murugesan G, Narasimhan MK, Varadavenkatesan T, Vinayagam R, Lan Chi NT, Pugazhendhi A, Selvaraj R (2022) Synthesis, characterization and anticancer activity of the green-synthesized hematite nanoparticles. Environ Res 214:113864. https://doi.org/10.1016/j.envres.2022.113864
Al-Hosney HA, Grassian VH (2005) Water, sulfur dioxide and nitric acid adsorption on calcium carbonate: a transmission and ATR-FTIR study. Phys Chem Chem Phys 7(6):1266–1276. https://doi.org/10.1039/b417872f
Santschi C, Rossi MJ (2006) Uptake of CO2, SO2, HNO3 and HCl on calcite (CaCO3) at 300 K: mechanism and the role of adsorbed water. J Phys Chem A 110(21):6789–6802. https://doi.org/10.1021/jp056312b
Hong S-H, Ndingwan AM, Yoo S-C, Lee C-G, Park S-J (2020) Use of calcined sepiolite in removing phosphate from water and returning phosphate to soil as phosphorus fertilizer. J Environ Manage 270:110817. https://doi.org/10.1016/j.jenvman.2020.110817
Andersen FA, Brecevic L (1992) Cheminform abstract: infrared spectra of amorphous and crystalline calcium carbonate. ChemInform 23(9). https://doi.org/10.1002/chin.199209005
Derkani MH, Fletcher AJ, Fedorov M, Abdallah W, Sauerer B, Anderson J, Zhang ZJ (2019) Mechanisms of surface charge modification of carbonates in aqueous electrolyte solutions. Colloids Interfaces 3(4):62. https://doi.org/10.3390/colloids3040062
Xu Q, Zhou Q, Pan M, Dai L (2020) Interaction between chlortetracycline and calcium-rich biochar: enhanced removal by adsorption coupled with flocculation. Chem Eng J 382:122705. https://doi.org/10.1016/j.cej.2019.122705
Harvey OR, Herbert BE, Rhue RD, Kuo L-J (2011) Metal interactions at the biochar-water interface: energetics and structure-sorption relationships elucidated by flow adsorption microcalorimetry. Environ Sci Technol 45(13):5550–5556. https://doi.org/10.1021/es104401h
Moraes DS, Rodrigues EMS, Lamarao CN, Marques GT, Rente AFS (2019) New sodium activated vermiculite process. Testing on Cu2+ removal from tailing dam waters. J Hazard Mater 366:34–38. https://doi.org/10.1016/j.jhazmat.2018.11.086
Lakhi KS, Cha WS, Choy J-H, Al-Ejji M, Abdullah AM, Al-Enizi AM, Vinu A (2016) Synthesis of mesoporous carbons with controlled morphology and pore diameters from SBA-15 prepared through the microwave-assisted process and their CO2 adsorption capacity. Micropor Mesopor Mat 233:44–52. https://doi.org/10.1016/j.micromeso.2016.06.040
Jeppu GP, Clement TP (2012) A modified Langmuir-Freundlich isotherm model for simulating pH-dependent adsorption effects. J Contam Hydrol 129:46–53. https://doi.org/10.1016/j.jconhyd.2011.12.001
Qian C, Ren X, Rui Y, Wang K (2021) Characteristics of bio-CaCO3 from microbial bio-mineralization with different bacteria species. Biochem Eng J 176:108180. https://doi.org/10.1016/j.bej.2021.108180
Wen T, Zhao Y, Zhang T, Xiong B, Hu H, Zhang Q, Song S (2019) Effect of anions species on copper removal from wastewater by using mechanically activated calcium carbonate. Chemosphere 230:127–135. https://doi.org/10.1016/j.chemosphere.2019.04.213
Funding
This work was supported by the Education Department of Fujian Province (JAT200473) and the Research Climbing Program of Xiamen University of Technology (XPDKT20015).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Shujian Wu, Zheng Liu, and Rongmei Mou. The first draft of the manuscript was written by Zheng Liu, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Statement of Novelty
Oyster shells (OS) are generally discarded and buried. Their rapid growth is becoming a social, environmental, and health problem. However, there needs to be more work on the detailed evaluations of copper removal by using OS in former reports. In this work, we used the pyrolyzed OS (POS) to remove copper ions from water. Taguchi method was used as a design of experiment to optimize process factors and explore their influence on copper removal. Also, the physicochemical properties of virgin POS and used POS were characterized to investigate the removal mechanism.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Z., Wu, S. & Mou, R. Efficient removal of Cu(II) from aqueous solution using pyrolyzed oyster shells by Taguchi method. Biomass Conv. Bioref. 14, 1175–1186 (2024). https://doi.org/10.1007/s13399-023-03884-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-03884-9