Abstract
A significant environmental concern is posed by wastewater containing dyes. When dealing with a polluted medium, adsorption is a beneficial method for the removal of contaminants. This study used argan nutshell as a precursor to synthesize activated carbon via chemical activation with Na2CO3 at 600 °C. The prepared activated carbon (Na@Ac) was characterized using Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), Brunauer–Emmett–Teller (BET), and zeta potential measurements. The results showed that the prepared activated carbon has a high surface area (1543 m2/g) and microporous structure. Furthermore, many parameters for Rhodamine B (RhB) dye removal efficiency, including pH, initial pollutant concentration, and temperature, were optimized by response surface methodology (RSM) with a central composite design. Based on the RSM, the best efficiency was 96.84% for the removal of RhB at pH 5, temperature 20 °C, and initial concentration 33.2 mg/L. The kinetic of RhB dye adsorption onto Na@Ac was effectively explained by a pseudo-second-order model, with a maximum uptake capacity of 35.9 mg/g. Moreover, Density functional theory (DFT) calculations were used to understand the adsorption mechanism of the RhB dye onto the Na@Ac. Based on the DFT calculations, hydrogen bonding and π-π interactions may be preferred mechanisms of RhB dye adsorption onto Na@Ac. The Na@Ac could be a promising adsorbent for the removal of Rhodamine B from polluted wastewater.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, drinking water resources are significantly reduced due to population growth accompanied by robust industrialization and intensive development of agriculture. The release of a wide variety of organic pollutants into the environment is constantly increasing [1, 2]. These organic contaminants have several adverse health impacts. In addition, the deterioration of the quality of different environmental media directly affects the equilibrium of ecosystems (soil, water, air). Dyes are a sort of recalcitrant organic compound, and they are mainly used in the textile and dyeing industry [3]. These areas are among the most water-consuming industries. They generate significant pollution because their various immense effluents are too loaded with acid or basic dyes. In order to protect water resources from pollution, it is necessary to treat contaminated water from its source, which is a significant challenge. Many treatment methods have been employed to eliminate dyes in contaminated water, such as coagulation, chemical oxidation photocatalysis, and adsorption [4,5,6,7,8,9]. Adsorption is one of the most hopeful and promising processes, has attracted considerable attention in the treatment of dye wastewater, and is widely used in industry because it is simple to apply and very effective, even for heavily loaded effluents [10,11,12]. In recent years, significant efforts have been made to develop new materials to improve water quality. The application of activated carbon for dyes removal has been the subject of much research due to its many economic advantages [13, 14]. The performance efficiency is highly dependent on the physical properties and the functional groups of the activated carbon [15, 16]. Activated carbons can be obtained from various types of natural resources (e.g., non-renewable sources like coal and renewable sources like biomass) by two activation processes (physical and chemical) or by a combination of both. For this purpose, we researched to valorize the Argan nutshells constituting an abundant co-product after extracting the almond (source of argan oil) by its use as a precursor for the production of activated carbons [15]. At present, the local residents use spent argan shells as a heating source. However, biomass as a renewable resource like coconut shell, almond, or olive pomace is increasingly employed as a natural-resources to synthesize activated carbon since these resources are inexpensive and sustainable. At the same time, only a few studies use argan nutshells as a precursor of activated carbons synthesized via chemical treatment utilizing K2CO3, KOH, and H3PO4 as activating agents [15, 17]. To the best of our knowledge, this is the first study to use Na@Ac prepared by chemical activation with Na2CO3 to remove Rhodamine B.
In this manuscript, the synthesis of activated carbon from Argan nutshell via chemical activation and its structural, morphological, and characterization have been reported. The performance of adsorption for the removal of Rhodamine B using the prepared Na@Ac material was examined. Furthermore, density functional theory (DFT) calculations were performed to complete the experimental findings and better understand the RhB dye’s uptake mechanism on Na@Ac.
2 Material and methods
2.1 Preparation of activated carbon
The argan nutshell (ANS) was collected from the southwest of Morocco. The material was sieved, and a material smaller than 100 µm was selected. Activated carbon was produced using the chemical treatment using sodium carbonate (Na2CO3) as the activator. In the first stage, the argan nutshell was rinsed with MilliQ water and dried at 110 °C for 24 h, and then the raw ANS was impregnated with Na2CO3 solutions for 24 h with continuous stirring (800 rpm). In the second stage, the mixture was dried for 24 h at 110 °C. This sample was denoted as Na_ANS. Afterwards, the sample was subjected to a carbonization process at 400–700 °C with a heating rate of 10 °C/min for 60 min, under a continuous flow of 60 mL/min of purified nitrogen gas. Following the carbonization, the product was boiled with an HCl acid solution (10 wt %) under reflux for 4 h to eliminate impurities and minimize the ash contents of the activated carbon [18, 19]. Finally, the product was rinsed many times with MilliQ water until chloride ions were not detected and then dried for 24 h at 110 °C. Activated carbon prepared was labeled as Na@Ac.
All chemicals in this present work were used as received without further purification. All experimental solutions were prepared using high-purity deionized water. The activating agent was sodium carbonate Na2CO3 (purity > 99%) purchased from Sigma-Aldrich. Rhodamine B (RhB) was purchased from Sigma-Aldrich.
The scanning electron microscopy (SEM) technique using an SEM Hitachi SU3500 instrument was used to characterize the surface morphology of raw materials and activated carbons. Samples were coated with a thin gold layer during SEM operation. The FTIR spectra Fourier-Transform InfraRed (FTIR) spectra of the raw materials and activated carbon were obtained with a PerkinElmer FT-IR Spectrometer Frontier equipped with the ATR sampling accessory (diamond crystal). The measurements were performed in transmittance mode throughout the range 4000–400 cm−1 with a spectral resolution of 4 cm−1. The specific surface area pores volume and size distribution of the activated carbon were evaluated according to the Brunauer Emmet–Teller (BET) equation with nitrogen adsorption–desorption isotherms at 77 K using a Micrometrics Gemini V device. The isoelectric point titration of the activated carbon was performed using a Zetasizer Nano ZS (Malvern Instruments).
2.2 Adsorption experiments
Different batch adsorption experiments were performed to study the adsorption of RhB from an aqueous solution using the synthesized Na@Ac activated carbon. Firstly, the Na@Ac obtained at different temperatures (400–700 °C) were tested for the RhB removal in a preliminary experiment to select the best adsorbent (see Fig. SI.1). From the preliminary data, the Na@Ac prepared at 600 °C showed much higher RhB adsorption capacity, and was therefore selected for the optimization experiments using RSM. The RSM was applied to optimize the removal efficiency of RhB on Na@Ac. These experiments were performed in 15 mL Corning© tubes with 10 mL of varying initial concentrations (30–70 mg/L) of the aqueous RhB solution at different solution pH (3–7) and temperatures (10–30 °C). In batch adsorption tests, 10 mg of Na@Ac were mixed with 10 mL of RhB solution in 15 mL Corning© tubes. The pH of solutions was adjusted with NaOH/HCl (0.1 M). The tubes were centrifuged after being continuously stirred at 60 rpm for 24 h on an orbital shaker. Kinetics (0–60 min) assays were carried out using the optimum adsorption conditions. The concentration of RhB after adsorption (see Fig. SI.2) was measured using a UV-spectrophotometer (Shimadzu UV–vis spectrometer) at a maximum wavelength of (λmax = 554 nm). The RhB Adsorption percentage (%) and quantity adsorbed qe (mg/g) were determined using Eqs. (1) and (2), respectively:
where C0 and Ce denote the initial RhB dye concentration and equilibrium concentration (mg/L), respectively. Conversely, V (L) denotes the solution volume, while W (g) stands for the quantity of Na@Ac.
3 Results and discussion
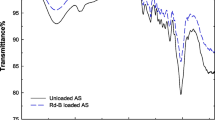
FTIR analyzed the presence of the functional group in ANS, Na_ANS, and Na@Ac, and the spectra are shown in Fig. 1. For all the samples in Fig. 1, bands at 3443 and 2926 cm−1 are attributed to the (O–H) vibrations in the hydroxyl and (C-H) vibrations groups, respectively [20]. The FTIR spectra of ANS and Na_ANS are identical. The bands at 1732 and 1597 cm−1 are related to the C = O stretching vibrations in ketones (RCOR’) and aromatic C = C bonds, respectively. While the band situated around 1505 cm−1 might be attributed to the C = C ring stretch of aromatic rings [21]. The peak at 1462 cm−1 confirms the presence of the C = C of aromatic rings. Furthermore, the peak at 1372 cm−1 could be assigned to the C-O vibrations in carboxylate groups. Besides, the bands in the range of 1000–1300 cm−1 could be attributed to the C-O stretching vibrations. The peak at 591 cm−1 is attributed to the in-plane ring deformation [15, 22]. The FTIR spectrum of Na@Ac showed that the intensity of the bands strongly decreased, and others disappeared compared to the raw argan nutshell. Moreover, these bands disappeared for Na@Ac, as proof of removing most surface functional groups during the activation process.
The morphology of Na_ANS (Fig. 2a) showed a relative roughness surface caused by chemical activation with no porosity, except for occasional cracks. The morphology of activated carbon Na@Ac (Fig. 2b) showed considerable changes in the surface porosity caused by the activation process. This porosity improvement is consistent with the findings shown by the prepared activated carbon’s textural characteristics.
The textural characteristics of the Na_ANS and Na@Ac were determined by nitrogen physisorption at 77 K in Fig. 3a. It is apparent from the shape of the obtained isotherms that the Na_ANS and the prepared Na@Ac were mainly macroporous (isotherm, type II) and microporous (isotherm, type I), respectively, according to the IUPAC classification [23, 24]. These experiments calculated the specific surface area (BET), and the total pore volume of Na_ANS was 155 m2/g and 0.076 cm3/g, respectively. When considering the prepared activated carbon Na@Ac, a remarkable increase was noted in the specific surface area of 1543 m2/g and total pore volume of 0.96 cm3/g, in accordance with SEM observations. It is possible to deduce that the observed increase in specific surface area and total pore volume is caused by the chemical activation at 600 °C. The isoelectric point (IEP) was determined by zeta potential measurement and found at a pH value of 4.36 (Fig. 3b).
3.1 Response surface methodology modeling
The present design focuses on a three-level central composite design (CCD) in response surface methodology to seek the best conditions for removing RhB onto Na@Ac. Three variables influence the present procedure: (pH―X1, temperature―X2, and initial dye concentration―X3). This research used 17 runs to assess the impact of the three input factors on RhB uptake efficiency. Table 1 depicts the arrangement of the factorial design. Table 2 provides a comprehensive overview of the investigations and their respective responses to RhB removals.
After applying analysis of variance (ANOVA) to the experimental runs, the CCD design table findings were derived and fitted using a suitable polynomial equation. The second-order polynomial (Eq. (3)) model was selected and fairly fitted for all three experimental variables and responses (RhB removal efficiency) based on the model assessment in Table 3, which focuses on maximum R2, predicted R2, and adjusted R2. The predictive of the quadratic (second-order) polynomial response model may thus be defined as follows (Eq. (3)) [25, 26]:
where Y represents the predicted response,β0 βi and represent the intercept and linear coefficients, βii and βij represent the quadratic and interaction coefficients, and Xi and Xj represent the coded values of the experimental variables.
For RhB removal, an ANOVA analysis was made, and the outcomes are shown in Table 4. The results of the ANOVA analysis revealed that the F and P-values were less than 1000 and 0.0500, respectively. This proved the significance of the model terms. Simultaneously, the lack of fit F-value in the ANOVA tables present a minor error concerning the pure error. The quadratic polynomial model in Eq. 4 was generated using the experimental data (CCD data in Table 2) by randomization and multiple regression analysis using the Design Expert® version 11.1.2.0 software.
The statistical diagnostics test is a great and valuable method for validating the supplied model. Figure 4 depicts these diagnostic plots. It is possible to see that the data points are essentially straight-line by categorizing the fraction of predicted probability in terms of residuals (Fig. 4a). The actual responses were compared to their residuals based on predicted responses in the other diagnostic plots, indicating that the second-order polynomial model was essential to forecast retrieval effectiveness in the experimental input conditions (Fig. 4b). Furthermore, as shown in the plots (Fig. 4c), the data was homogeneous.
The assessed model (Eq. 4) uses the RSM approach to create the 3-D plots and determine the optimum conditions for RhB removal efficiency. Figure 5 shows that the removal of RhB rises as the pH of the solution increases. In the 3D and contour plot presented in Fig. 5a and d, respectively, the interactions between temperature and pH are shown. For example, at the lower value of pH and temperature, the adsorption efficiency of prepared Na@Ac was decreasing. An increase in the pH solution and temperature led to the better removal efficiency. At pH 5 and temperature 20 °C, the adsorption percentage of the Na@Ac was 96.84%. The efficiency decreases significantly in acidic environments or below the optimum pH. It was clear from Fig. 5, that the adsorption percentage of RhB dye is strongly dependent on the solution pH and mainly related to the activated carbon’s zeta potential [27, 28]. Moreover, It was also observed that RhB concentration*temperature interaction affects the RhB adsorption efficiency onto Na@Ac significantly. In summary, based on these outcomes with three variables, the best efficiency was 96.84% for the removal of RhB and was obtained at pH 5, temperature: 20 °C, and initial concentration: 33.2 mg/L (Table 2).
3.2 Kinetics and regeneration studies
This work used the pseudo-first-order and the pseudo-second-order kinetic models to study the kinetic behavior of RhB adsorption onto the Na@Ac. The pseudo-first-order kinetic model [29] is often appropriate for the adsorption process’s first 20 to 30 min [30]. This model assumes the reversibility of the adsorption process [31]. The following equation expresses this model:
In contrast to the pseudo-first-order model, the pseudo-second-order kinetic model [32], predicts the behavior throughout the whole range of adsorption time scales. The pseudo-second-order model assumes that the rate-limiting step may be chemisorption, which implies valence forces by electron sharing or exchange between the sorbate and the sorbent [33]. The following expression gives the pseudo-second-order equation:
where k1 is the rate constant of pseudo-first-order in min−1 and k2 is the rate constant of pseudo-second-order in (g mg−1 min−1), and qt and qe represent the quantity of solute adsorbed at time t and equilibrium, respectively.
Figure 6 depicts the effect of contact time on the adsorption of RhB onto Na@Ac. The result demonstrates that the sorption uptake of RhB increased gradually with time and reached about 96% removal efficiency at 60 min. This relatively fast kinetic may be due to the high number of available binding sites on the surface of the Na@Ac, which reduced drastically as time passed. Other researchers have observed similar findings [12, 34]; and it is explained that as time progresses, the number of unoccupied sites reduces, and hence, the adsorption process approaches saturation. To predict the linear correlations between the experimental data points and equilibrium, the pseudo-first-order (PS1) and pseudo-second-order (PS2) order models were applied (Fig. 6b). The correlation coefficients, R2 for the PS1 and PS2 models were 0.922 and 0.971, respectively. Coupled with its higher R2 value, the PS2 estimated the equilibrium uptake (qe = 35.9 mg/g) slightly above the experimental value of 32.27 mg/g, suggesting that equilibrium was almost reached. These findings revealed that the RhB sorption follows the PS2 kinetic model, and hence portrays the chemical nature of the interaction between the RhB molecules and active sites of Na@Ac.
The reusability of the Na@Ac green-activated carbon is essential to its practical implementation. After each cycle, 10 mg of Na@Ac laden with RhB was agitated with 10 mL ethanol solution for 30 min, and then the mixture was washed three times with milliQ water and finally dried at 110 °C for next use. In this work, the reused performance of Na@Ac is illustrated in Fig. 7. The adsorption performance of Na@Ac was relatively efficient at removing RhB after the five cycles. The adsorption efficiency of RhB on Na@Ac was around 91.8%, up to the fifth cycle. The current study concludes that Na@Ac is an effective green material owing to its outstanding activity and good reuse performance, as well as its good performance for the elimination of Rhodamine B. Therefore, its usage in an industrial process is plausible.
Table 5 reports the maximum RhB dye adsorption capacity of the Na@Ac produced in this paper and those of some usual agricultural wastes-based activated carbons from the literature, chosen for their composition close to the studied activated carbon. Even though the direct comparison of Na@Ac with other adsorbents is fairly challenging owing to the diverse experimental conditions utilized, it has been determined that the Na@Ac adsorption capacity for RhB is among the highest. Therefore, considering the adsorption capacities of other considered agricultural wastes-based activated carbon, low cost, accessibility, and environment-friendly green-material, it can be concluded that the activated carbon produced from argan nutshell via chemical activation at 600 °C (Na@Ac) proved to be a promising low cost-effective adsorbent to remove rhodamine B dye from aqueous solutions.
3.3 DFT calculations
3.3.1 Geometry optimization
Geometry optimization and frequency of RhB dye were performed by applying the density functional theory (DFT) using Becke-three-Lee–Yang–Parr (B3LYP) with the 6-31G (d, p) basis set [47]. The computations were done utilizing Gaussian 09 [48] software package, and the optimized structure of RhB dye is illustrated in Fig. 8.
3.3.2 Frontier molecular orbital analysis (FMOs)
The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), which are known as molecular orbitals (MOs) as well as their gap (∆E), are very significant factors in quantum chemistry. The HOMO represents the area of a molecule that can donate an electron, while LUMO represents the area of a molecule that can accept an electron [49]. Figure 9 illustrates the plot of MOs (HOMO and LUMO) for the RhB dye. As described in this figure, the HOMO orbital is essentially located on the two benzene rings substituted by +N(Et2) and N(Et2), while the LUMO orbital is situated over the benzene ring substituted by RCOOH. These results indicate that both benzene rings substituted by +N(Et2) and N(Et2) are electron donors and the other benzene ring substituted by RCOOH is an electron acceptor. Therefore, hydrogen bonding and π-π interactions may be preferred onto Na@Ac adsorbent.
3.3.3 Molecular electrostatic potential (MEP) mapping
To better understand the chemical reactivity and electron density change in the RhB dye, the molecular electrostatic potential (MEP) and electrostatic potential (ESP) surfaces were generated. The results are presented in Fig. 10. The MEP characteristic is beneficial for studying reactivity in molecular modeling research [50]. Different colors denote the varied electrostatic potential values of the surface; for example, the dark red areas represent the highest electron density (more negative potential), whereas the blue areas characterize the lowest electron density (more positive potential) [51]. The MEP plot of the RhB dye reveals that the C = O group favors the electrophilic attack, and the O–H group favors the nucleophilic attack. The large yellow area around the two benzene rings substituted by +N(Et2) and N(Et2) indicates that these regions may favor the electrophilic attacks. A red-to-yellow coloration of the carbonyl groups in the ESP plot suggests that the electron density is more concentrated on the oxygen atoms.
Based on the results of MOs and MEP plots and the Mulliken atomic charges of RhB dye (Table 6), we can conclude that the C = O group is an attracting electron or charge part while the O–H group is donating an electron or charge part from the adsorbent. The two remaining benzene groups, substituted by +N(Et2) and N(Et2), may interact as attractive groups. The possible adsorption mechanism of RhB onto Na@Ac is given in Fig. 11.
4 Conclusion
Conventional synthesis of argan nutshell, an abundant co-product, was used as a precursor for activated carbon production and studied to remove RhB dye in an aqueous solution. This manuscript aimed to investigate the performance of the adsorption process towards Rhodamine B dye onto Na@Ac. Kinetic investigation revealed that the adsorption of RhB on Na@Ac followed a pseudo-second-order model, with a maximum uptake capacity of 35.9 mg/g for RhB. Based on RSM, the results demonstrated that the maximum efficiency was 96.84% for removing RhB and was obtained at pH 5. DFT calculations suggest that hydrogen bonding and π-π interactions are likely the main keys controlling the RhB dye adsorption onto Na@Ac. The present research shows that Na@Ac is an effective green material due to its excellent activity and reuse performance and its strong performance in removing Rhodamine B. Therefore, its industrial application is conceivable.
Data availability
The corresponding author will provide the datasets generated for this study on request.
References
Tkaczyk A, Mitrowska K, Posyniak A (2020) Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: a review. Sci Total Environ 717:137222. https://doi.org/10.1016/j.scitotenv.2020.137222
Qi C, Liu X, Ma J et al (2016) Activation of peroxymonosulfate by base: implications for the degradation of organic pollutants. Chemosphere 151:280–288. https://doi.org/10.1016/j.chemosphere.2016.02.089
Chennah A, Anfar Z, Amaterz E et al (2020) Ultrasound-assisted electro-oxidation of methylene blue dye using new Zn3(PO4)2 based electrode prepared by electro-deposition. Mater Today Proc 22:32–34. https://doi.org/10.1016/j.matpr.2019.08.067
Carmen Z, Daniela S (2010) Textile organic dyes-characteristics , polluting effects and separation / elimination procedures from industrial effluents – a critical overview
El Ouardi Y, Branger C, Laatikainen K et al (2020) Impact of thermal treatment on bentonite retention ability toward nickel and silver retention. Sep Sci Technol 55:1–11. https://doi.org/10.1080/01496395.2020.1839772
El Ouardi Y, Branger C, Laatikainen K et al (2021) Enhancing clay adsorption properties : a comparison between chemical and combined chemical / thermal treatments. Groundw Sustain Dev 12:100544. https://doi.org/10.1016/j.gsd.2020.100544
El Ouardi Y, Branger C, Toufik H et al (2020) An insight of enhanced natural material (calcined diatomite) efficiency in nickel and silver retention: application to natural effluents. Environ Technol Innov 18:100768. https://doi.org/10.1016/j.eti.2020.100768
Kumbhar P, Patil S, Narale D et al (2022) Biobased carbon for effective removal of rhodamine B and Cr(VI) from aqueous solution: kinetic, isotherm and thermodynamic study. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-02625-8
Bediako JK, Lin S, Sarkar AK et al (2020) Evaluation of orange peel-derived activated carbons for treatment of dye-contaminated wastewater tailings. Environ Sci Pollut Res 27:1053–1068. https://doi.org/10.1007/s11356-019-07031-8
Cheng ZL, Li Y, xiang, Liu Z, (2018) Study on adsorption of rhodamine B onto Beta zeolites by tuning SiO2/Al2O3 ratio. Ecotoxicol Environ Saf 148:585–592. https://doi.org/10.1016/j.ecoenv.2017.11.005
Khamparia S, Jaspal D (2016) Investigation of adsorption of rhodamine B onto a natural adsorbent Argemone mexicana. J Environ Manage 183:786–793. https://doi.org/10.1016/j.jenvman.2016.09.036
Mousavi SA, Kamarehie B, Almasi A, et al (2021) Removal of rhodamine B from aqueous solution by stalk corn activated carbon: adsorption and kinetic study. Biomass Convers Biorefinery 1–10. https://doi.org/10.1007/s13399-021-01628-1
Mezohegyi G, van der Zee FP, Font J et al (2012) Towards advanced aqueous dye removal processes: a short review on the versatile role of activated carbon. J Environ Manage 102:148–164. https://doi.org/10.1016/j.jenvman.2012.02.021
Zare K, Gupta VK, Moradi O et al (2015) A comparative study on the basis of adsorption capacity between CNTs and activated carbon as adsorbents for removal of noxious synthetic dyes: a review. J Nanostructure Chem 5:227–236. https://doi.org/10.1007/s40097-015-0158-x
Zbair M, Ainassaari K, Drif A et al (2018) Toward new benchmark adsorbents: preparation and characterization of activated carbon from argan nut shell for bisphenol A removal. Environ Sci Pollut Res 25:1869–1882. https://doi.org/10.1007/s11356-017-0634-6
Métivier-Pignon H, Faur-Brasquet C, Le Cloirec P (2003) Adsorption of dyes onto activated carbon cloths: approach of adsorption mechanisms and coupling of ACC with ultrafiltration to treat coloured wastewaters. Sep Purif Technol 31:3–11. https://doi.org/10.1016/S1383-5866(02)00147-8
Chafik T (2012) Nanoporous carbonated materials prepared from the shell of the argan fruit. 19
Uçar S, Erdem M, Tay T, Karagöz S (2015) Removal of lead (II) and nickel (II) ions from aqueous solution using activated carbon prepared from rapeseed oil cake by Na2CO3 activation. Clean Technol Environ Policy 17:747–756. https://doi.org/10.1007/s10098-014-0830-8
Fazal-ur-Rehman M (2018) Methodological trends in preparation of activated carbon from local sources and their impacts on production: a review. Chem Int 4:109–119
Dao TM, Le Luu T (2020) Synthesis of activated carbon from macadamia nutshells activated by H2SO4 and K2CO3 for methylene blue removal in water. Bioresour Technol Reports 12:100583. https://doi.org/10.1016/j.biteb.2020.100583
Bouchelta C, Medjram MS, Zoubida M et al (2012) Effects of pyrolysis conditions on the porous structure development of date pits activated carbon. J Anal Appl Pyrolysis 94:215–222. https://doi.org/10.1016/j.jaap.2011.12.014
Ji Y, Li T, Zhu L et al (2007) Preparation of activated carbons by microwave heating KOH activation. Appl Surf Sci 254:506–512. https://doi.org/10.1016/j.apsusc.2007.06.034
Stafford K, Sing W, Rouquerol J (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 57:603–619
Thommes M, Kaneko K, Neimark AV et al (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution ( IUPAC Technical Report ). Pure Appl Chem. https://doi.org/10.1515/pac-2014-1117
Issam, Jilal, Soufian, El Barkany, Zahra B, Youssef, El Ouardi, Mohamed, Loutou, Hassan A, Mohamed, Abou-Salama, Amin, Salhi, Abderrahmane EI and KL (2021) New ethylenediamine crosslinked 2D-cellulose adsorbent for nanoencapsulation removal of Pb (II) and Cu (II) heavy metal ions: synthesis, characterization application, and RSM-modeling. In: cellulose. IntechOpen
Bediako JK, Wei W, Yun YS (2016) Low-cost renewable adsorbent developed from waste textile fabric and its application to heavy metal adsorption. J Taiwan Inst Chem Eng 63:250–258. https://doi.org/10.1016/j.jtice.2016.03.009
Li Y, Du Q, Liu T et al (2013) Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem Eng Res Des 91:361–368. https://doi.org/10.1016/j.cherd.2012.07.007
Mozhiarasi V, Natarajan TS (2022) Bael fruit shell–derived activated carbon adsorbent: effect of surface charge of activated carbon and type of pollutants for improved adsorption capacity. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-03211-8
Lagergren SK (1898) About the theory of so-called adsorption of soluble substances. Sven Vetenskapsakad Handingarl 24:1–39
Aksu Z (2001) Equilibrium and kinetic modelling of cadmium(II) biosorption by C. Tulgaris in a batch system: effect of temperature. Sep Purif Technol 21:285–294. https://doi.org/10.1016/j.desal.2006.04.052
Guimarães D, Leão VA (2014) Batch and fixed-bed assessment of sulphate removal by the weak base ion exchange resin Amberlyst A21. J Hazard Mater 280:209–215. https://doi.org/10.1016/j.jhazmat.2014.07.071
HO YS, Mckay G (1998) Kinetic models for the sorption of dye From aqueous solution by wood. Process Saf Environ Prot 76:183–191. https://doi.org/10.1205/095758298529326
Ho YS (2006) Review of second-order models for adsorption systems. J Hazard Mater 136:681–689. https://doi.org/10.1016/j.jhazmat.2005.12.043
Gharbani P, Mehrizad A (2022) Preparation and characterization of graphitic carbon nitrides/polyvinylidene fluoride adsorptive membrane modified with chitosan for Rhodamine B dye removal from water: adsorption isotherms, kinetics and thermodynamics. Carbohydr Polym 277:118860. https://doi.org/10.1016/j.carbpol.2021.118860
Karthikeyan S, Babu Rajendran A (2010) Adsorption of basic dye (Rhodamine B) by a low cost activated carbon from agricultural solid waste: Leucaena leucocephala seed shell waste. Nat Environ Pollut Technol 9:461–472
Kannan K, Venugopal T, Kannan K, Kannan K (2014) An innovative and novel method of removal of Rhodamine-B dye by activated carbon prepared from Seeds of Prosopis juliflora. Enviro Geo Chem Acta 1:140–146
Harouna M, Baiboussa G, Abia D et al (2015) Adsorption of rhodamine B in aqueous solution by activated carbon from the seed husks of Moringa oleifera. Int J Eng Res Sci Technol 4:70–79
Nguyen VH, Nguyen DT, Nguyen TT et al (2021) Activated carbon with ultrahigh surface area derived from sawdust biowaste for the removal of rhodamine B in water. Environ Technol Innov 24:101811. https://doi.org/10.1016/j.eti.2021.101811
Gad HMH, El-Sayed AA (2009) Activated carbon from agricultural by-products for the removal of Rhodamine-B from aqueous solution. J Hazard Mater 168:1070–1081. https://doi.org/10.1016/j.jhazmat.2009.02.155
Lacerda DS, Viviane Juan BL-S, Adriana C-G, Mercedes H-N, Sánchez-Báscones Luis MN-G, Pablo M-R, Jesús M-G (2015) Rhodamine B removal with activated carbons obtained from lignocellulosic waste. J Environ Manage 155:67–76. https://doi.org/10.1016/j.jenvman.2015.03.007
Mohammadi M, Hassani AJ, Mohamed AR, Najafpour GD (2010) Removal of rhodamine B from aqueous solution using palm shell-based activated carbon: adsorption and kinetic studies. J Chem Eng data 55:5777–5785
Li Y, Yan X, Hu X et al (2019) Trace pyrolyzed ZIF-67 loaded activated carbon pellets for enhanced adsorption and catalytic degradation of Rhodamine B in water. Chem Eng J 375:122003. https://doi.org/10.1016/j.cej.2019.122003
Kadirvelu K, Karthika C, Vennilamani N, Pattabhi S (2005) Activated carbon from industrial solid waste as an adsorbent for the removal of rhodamine-B from aqueous solution: kinetic and equilibrium studies. Chemosphere 60:1009–1017. https://doi.org/10.1016/j.chemosphere.2005.01.047
Bordoloi N, Dey MD, Mukhopadhyay R, Kataki R (2018) Adsorption of methylene blue and rhodamine B by using biochar derived from Pongamia glabra seed cover. Water Sci Technol 77:638–646. https://doi.org/10.2166/wst.2017.579
Li X, Shi J, Luo X (2022) Enhanced adsorption of rhodamine B from water by Fe-N co-modified biochar: preparation, performance, mechanism and reusability. Bioresour Technol 343:126103. https://doi.org/10.1016/j.biortech.2021.126103
Vigneshwaran S, Sirajudheen P, Karthikeyan P, Meenakshi S (2021) Fabrication of sulfur-doped biochar derived from tapioca peel waste with superior adsorption performance for the removal of malachite green and rhodamine B dyes. Surf Interfaces 23:100920. https://doi.org/10.1016/j.surfin.2020.100920
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Caricato M, Frisch MJ, Hiscocks J (2013) Gaussian 09 IOps reference second edition
Fukui BK (1982) Angew Chem Int Ed Engl Fukui. Angew Chemie Int Ed English 21:801–809
Chanajaree R, Sriuttha M, Lee VS, Wittayanarakul K (2021) Thermodynamics and kinetics of cationic/anionic dyes adsorption on cross-linked chitosan. J Mol Liq 322:114507. https://doi.org/10.1016/j.molliq.2020.114507
Shankar Rao YB, Prasad MVS, Udaya Sri N, Veeraiah V (2016) Vibrational (FT-IR, FT-Raman) and UV-Visible spectroscopic studies, HOMO-LUMO, NBO, NLO and MEP analysis of Benzyl (imino (1H-pyrazol-1-yl) methyl) carbamate using DFT calculaions. J Mol Struct 1108:567–582. https://doi.org/10.1016/j.molstruc.2015.12.008
Acknowledgements
The authors would like to immensely thank the Department of Separation Science, Lappeenranta-Lahti University of Technology LUT, and the Etelä-Karjala Säästöpankki Foundation, Finland, for their financial support.
Funding
Open Access funding provided by LUT University (previously Lappeenranta University of Technology (LUT)). This research work was mainly supported and funded Etelä-Karjala Säästöpankki Foundation, Finland.
Author information
Authors and Affiliations
Contributions
YE: experimental part, investigation, writing-review, and editing. AE: theoretical study, writing. AC: characterization part, investigation, validation. AO: data curation, validation, Writing—review and editing. KL: Project administration, Writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• High surface area activated carbon (Na@Ac) was synthesized via chemical activation with Na2CO3 at 600 °C.
• The adsorption process was analyzed through experiment optimization and DFT calculations.
• Rhodamine B dye removal mechanism is mostly driven by hydrogen bonding and the π–π interaction.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ouardi, Y.E., Aissouq, A.E., Chennah, A. et al. Synthesis, characterization, and DFT investigation of rhodamine B dye removal by activated carbon produced from argan nutshell. Biomass Conv. Bioref. 14, 15107–15118 (2024). https://doi.org/10.1007/s13399-022-03706-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03706-4