Abstract
Chemically modified waxy corn starch is a promising material for biodegradable bioplastic synthesis. This work is to study the film performance and microbial enzymatic biodegradation of the film made from chemically modified waxy corn starch according to the effect of pre-gelatinization and cardanol oil with various ratios as a mixed plasticizer with sorbitol. The preparation of biodegradable bioplastic films from waxy maize acetylated di-starch adipate (WADA) and waxy maize pregelatinized acetylated di-starch adipate (PWADA) blended with polyvinyl alcohol polymer using sorbitol and cardanol oil mixture as plasticizers were performed. Characterization of biodegradability by enzymes mixture of (alpha-amylase and glucoamylase) and soil burial test. The weight loss reached 96% and the degradation percentage reached 95.5% of PWADA and these results were significantly (p < 0.05) lower than WADA; as proved by high-performance liquid chromatography, increasing osmolality is evidence of the degradation as measured by the osmometer and the physical appearance of the film indicated complete degradation after 21 days. The film morphology, chemical structure, crystallinity, transparency, and thermal stability were studied using a scanning electron microscope, Fourier-transformed infrared, X-ray diffraction, UV–Vis spectrophotometer, and thermal gravimetric analysis. As a result, under the electron microscope, PWADA films had a more homogenous surface. The films’ infra-red spectra showed similar patterns, indicating identical chemical structures. Waxy maize starch has an A-type crystalline structure and after the thermoplasticization, the X-ray diffractogram showed new peaks appeared at 2θ of 13.2°, 19.5°, and 20.8°, which attributed to a V-type crystal pattern. The addition of cardanol oil restricts the transmission of light in the UV region by 250 nm by 69 and 63.4%, respectively, indicating UV absorber films. Furthermore, adding oil to (PWADA) and (WADA) films increased onset deterioration from 272 to 318 °C and from 317 to 320 °C, indicating that their thermal stability is improved. The water uptake rate and water vapor permeability increase after the pre-gelatinization of WADA films without cardanol but slightly decrease after the addition of cardanol oil. Also, pre-gelatinization decreases the elongation by 33.3% while increasing the strength by 10.5% of the films. Hence, waxy-modified maize starch film has the potential to be a biodegradable, thermal stable, and UV absorber film in packaging material.
Highlights
-
Completely biodegradable biopolymers
-
Ultraviolet absorber films from modified waxy maize starch
-
Superior thermal stable oil in film composites
-
Competitive mechanical properties from physio-chemical modification of waxy maize starch
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The environmental burden of plastics is boundless; from 1950 to 2018, nearly 6.3 billion tons of plastics have been generated worldwide, with 9% and 12% reused and combusted, respectively. The growing human population and consistent demand for plastics and plastic products are to blame for the continuous increase in plastic production, generation of waste, and accompanying environmental pollution [1]. Plastics can be categorized as degradable and non-degradable polymers based on their chemical properties [2]. Bioplastics are a source of hope because they are biodegradable polymers made from natural components, and researchers have spent a lot of time and effort trying to discover a way to replace petroleum-based plastics [3].
Plastics and bioplastics can degrade through abiotic processes such as photodegradation, oxidation, and hydrolysis [4]. Also, microbial degradation has arisen as one of the potential alternative methods of plastic breakdown, rather than the potential degrading procedures of plastic synthetic polymers due to their effectiveness, affordability, environmental friendliness, and sustainability [5]. Understanding the relationship between microorganisms and polymers is crucial for tackling environmental issues related to plastics since many living entities, particularly microorganisms, have evolved techniques for surviving and decomposing plastics. These microorganisms including bacteria [2] and fungi [6] are useful for green chemistry because of their capacity for bioremediation and plastic breakdown, which will help remove toxic polymers from the environment [7].
Polysaccharides are biopolymers that come in a variety of types, and at reasonable prices, and are spread throughout the world. Nowadays, starch is the most widely used polysaccharide in industries (food, cosmetics, paper, textiles, and pharmaceuticals), but its native form has functional properties and processability limitations; thus, modification of starch by physical, chemical, and physio-chemical modifications has been the suitable solution [8]. Starch is made up of granules that are arranged in a specific pattern and have a particular crystalline structure depending on its source. Starch granules are made up of linear amylose and branched amylopectin macromolecules with different ratios relied on their origin and type. Normal corn starch (25 percent amylose, 75% amylopectin), waxy corn starch (0–2% amylose, 98% amylopectin), and amylose-starch (up to 70% amylose) are examples of different types of starch from corn with different amylose and amylopectin ratios [9]. When starch dispersion is cooked at gelatinization temperature with water and a plasticizer such as glycerol, the resulting films are brittle and have poor mechanical and barrier characteristics [10]. To overcome these limitations various approaches have been researched. Blending thermoplastic starch with another synthetic polymer such as (polyvinyl alcohol, polypropylene, poly acrylic acid, poly lactic acid, and polyethylene) [10,11,12,13,14] is being examined to enhance the mechanical and barrier properties of thermoplastic starch.
PVA (polyvinyl alcohol) is a synthetic polymer with a wide range of applications and a strong affinity for starch due to its abundance of hydroxyl groups. The mechanical qualities of polyvinyl alcohol film are good, such as tensile strength and elongation [10, 15, 16]. Thermoplastic starch mixed with polyvinyl alcohol and various plasticizers such as polyol (glycerol, sorbitol, mannitol, polyethylene glycols, and so on…) [17, 18], inorganic salts (CaCl2, AlCl3, MgCl2) [19], and clays (Na-montmorillonite, hallow) [20, 21], as well as metal oxides (such as TiO2, ZnO2) [22, 23], nanoparticles ( such as graphene nanotubes, cellulose nanofibril, and starch nanoparticles) [24, 25], and oils (olive oil, corn oil, and eugenol oil) [26, 27] all have considerable considerations.
Cardanol oil is one of the most promising natural materials in the oleo chemistry and coating industry due to its chemical structure and properties. It is extracted from cashew nutshell after conversion to Anacardic acid. It is composed of a phenol ring linked aliphatic chain of 15 carbon atoms and has more than the unsaturated position at C1 and C3 as shown in Scheme 1 [28].
However, there is little debate about the effect of the pre-gelatinization of starch on the film made from it. Also, the use of blending plasticizers such as sorbitol with hydrophobic oil such as cardanol oil to examine the implications on the physicochemical properties and microbial biodegradability of the film fabricated from modified waxy corn starch blended with polyvinyl alcohol.
This research aims to study the effect of physicochemical modifications on waxy maize starch when blending with polyvinyl alcohol. Also, study the effects of sorbitol-plasticized TPS/PVA and sorbitol-cardanol mixes with varying ratios of cardanol on the film performance and microbial degradation.
2 Materials and methods
2.1 Materials
Cargill provided acetylated di-starch adipate (12,605) and pregelatinized acetylated di-starch adipate (12,616) from an importer (AWA Food Solution, Cairo, Egypt), sorbitol syrup 70% dry substance was supplied from (Egyptian Starch and Glucose Company, Cairo, Egypt), cardanol oil (K2P chemicals, Mumbai, India), commercial polyvinyl alcohol from (M.wt 125,000, viscosity 4% at 20 °C 35–50 cS) (Egyptian British Company for chemicals, Cairo, Egypt), alpha-amylase from Bacillus licheniformis (E.C.3.2.1.1, 1000 RAU (reference amylase unit), where RAU is the amount of enzyme that will convert 1 mg of soluble starch per min. into soluble dextrin and oligosaccharide), and glucoamylase from Aspergillus Niger (E.C.3.2.1.3, 570 GAU/g, 1.16 g/ml, where GAU is the amount of enzyme that will liberate 1 g of glucose per hour from starch), both acquired from (GENEN CORE DePunt, China) and laboratory grade absolute ethanol were used.
2.2 Methods
To create thermoplastic starch and PVA films, 4 g of waxy corn starch powder was first gelatinized in 100 ml of distilled water for 30 min at 95 °C, then preparing a 10% solution of commercial PVA by dissolving 10 g of PVA granules was in 90 ml distilled water and stirred at 200 rpm during heating at 80 °C for 30 min.
Starch, polyvinyl alcohol (60:40) % content, and sorbitol by 25% of the whole mixture were blended to make the films as seemed in Table 1. For 30 min, the entire mixture was brought to a boil in a water bath to complete the starch gelatinization and homogenize the mixture.
Cardanol oil of 0.5% was prepared by dissolving the oil in absolute ethyl alcohol, which was then mixed with various percentages (0.2%, 0.4%, 0.6%). Finally, as indicated in Scheme 2, the final slurry was poured onto a petri dish and dried for 72 h at 50 °C in the oven.
In the synthesis of thermoplastic waxy maize acetylated distarch adipate film (WADA) and waxy maize pregelatinized acetylated distarch adipate film (PWADA), all these steps were completed, and various formulations were chosen for further characterization and evaluations, as indicated in Table 1.
2.3 Characterization of prepared films
2.3.1 Biodegradation
Enzymatic biodegradation
The plastic films were cut into 2 cm × 2 cm squares and placed in a tiny beaker containing 100 ml of 1:1 (amylase and glucoamylase) mixing enzymes, 25 ml of distilled water, and control samples without TPS foils, and incubated in 37 °C water bath. The hydrolysate was removed from the beaker at regular intervals and the solid content was adjusted to 1% using a refractometer (Atago a70000, Japan) and an osmometer (Model 3250 Osmometer, advanced instrument, USA) as a function of dextrose equivalent, which is used in the starch hydrolysis industry as an indicator of full starch hydrolysis [29]. The contents of the beaker were filtered through 0.45 filter paper (Mixed Cellulose Nitrate with Cellulose Ester, Sartorius, Germany) after some time, and the filtrate was injected into HPLC (Agilent 1260 infinity) using a metacarpi 87H column (300 × 7.8 mm) (Varian Inc., Palo Alto, USA) at a flow rate of 0.6 l/min [30]. The dextrose equivalent can be calculated from HPLC according to Delheye G. and Moreels E., 1988 [31]. The degradation percentage calculated was from Eq. (1).
W0 is the weight of starch present in the film before degradation, and DE is the dextrose equivalent acquired by HPLC spectra.
For weight loss estimates, the residue was dried in an air oven at 105°C12 h until it attained a constant weight Eq. (2).
W0 is the weight of the dried plastic film before biodegradation, while Wd is the weight of the residue after biodegradation.
Soil burial degradation
Soil burial tests were done according to the approach reported by Hasan et al. with some modifications [32]. Waxy maize acetylated di-starch adipate (WADA) and waxy maize pregelatinized acetylated di-starch adipate (PWADA) thermoplastic starch (TPS) films were cut into 2 × 2 cm squares and put in the soil at a depth of 10 cm, irrigated at intervals to keep the soil moist. Examine the physical look of the films every 5 days and photograph them with a mobile camera until they have completely degraded. The experiment was set up as a three-replication randomized design. The research was carried out in botany and microbiology department, Faculty of Science, Al-Azhar University Cairo Governorate Egypt during the spring season (April 2021). Vicia faba (Fava bean) seeds were grown in 10 × 40 cm cylindrical plastic pots filled with 250 g of standard soil produced as recorded in Table 2 and replenished according to ISO1755: 2012-soil quality. To allow seeds to germinate, each container was filled with ordinary soil (2/3 by volume). The soil for this investigation came from the herbaceous garden of the Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Cairo Egypt, Table 2. Plastic films containing cross-linked waxy maize starch (WADA) and pregelatinized, stabilized, and cross-linked waxy maize starch (PWADA) combined with polyvinyl alcohol polymer and plasticizers sorbitol and sorbitol-cardanol oil were compared to control films and placed in the experiment soil (free from bio-plastic films). Fava bean seeds were germinated in an incubator for 5 days at 25 °C on moist Whatman filter paper. In each pot, two radicle-emerged seeds were sowed at a depth of 3 cm. The plants were reduced to one plant per pot after seven days. During the experiment, plants were permitted to grow freely in pots for 3 weeks under natural growth conditions. At the end of the study, when the Fava bean plants were 21 days old, both pots were displaying strong growth, and data gathering on plant growth began.
2.3.2 Scanning electron microscope (SEM)
A scanning electron microscope (Philips, model Quanta FEg250, England) was used for observing the fracture surface of the films. The samples for SEM analysis were firstly coated with a thin layer of gold and subsequently were imaged at an operating voltage of 20 kV under a vacuum.
2.3.3 XRD analysis
The X-ray diffractometer used was (LABX XRD-6000, Shimadzu, Japan). X-rays of 1.5410 Å wavelength were generated by the Cu Kα source. The angle of diffraction, 2θ was varied from 4 to 80° to identify any changes in the crystal structure and intermolecular distances between the inter-segmental chains.
2.3.4 Fourier transform infrared spectroscopy (FTIR)
A spectrum 100 spectrophotometer (PerkinElmer Inc., USA) was used to measure the starch/PVA films in total reflection mode. For FTIR measurement, the samples were mixed with anhydrous KBr and then compressed into thin disk-shaped pellets. The spectra were obtained with a resolution of 4 cm−1, and the tests were run from 4000 to 700 cm−1.
2.3.5 Water uptake pattern
The performance of water uptake was evaluated as mentioned by Tian H et al. with some adjustments [33]. The prepared films were divided into 2 cm × 2 cm. Then, they were dried in an oven at 50 °C for 24 h and weighed immediately and recorded as W0. The water uptake of the films was reported after storage in desiccator chambers over the salt solutions at a constant 75% RH for 10 days at 25 °C. Constant 75% RH was achieved with saturated salt solutions of NaCl (∼75% relative humidity) [33].
The water uptake (WU) of TPS of WADA and PWADA starch/PVA composite films was evaluated as (equal 3):
where W0 is the weight of the dried sample and Wt is the weight of the sample after time t.
The second Fick’s law of diffusion can theoretically be used to show the adsorption of moisture into TPS of WADA and PWADA starch/PVA films (Eq. 4):
where C, t, D, and X are the concentration at time t, adsorption time, diffusion coefficient, and film thickness, respectively. The initial adsorption kinetic in a short time can be calculated as in (Eq. 5):
where Mt, M∞, and L are the mass of absorbed water at time t, the mass of absorbed water at thermodynamic equilibrium, and film thickness respectively [34].
The diffusion coefficient D can be obtained from a slope of fitting Mt/M∞ versus √t of R2 over 99% or the straight-line portion.
2.3.6 Water vapor permeability (WVP)
Water vapor permeability (WVP) was assessed according to the method carried out by Zheng et al. with some changes [27]. The films were divided into segments measuring 60 mm in diameter, mounted in permeation tubes with inner diameters of 15 mm and height of 30 mm, filled with granular anhydrous calcium chloride (0% RH), and lined with melt paraffin to ensure closure. After that, a desiccator containing a saturated potassium sulfate solution (95% RH) was used to hold the permeation tubes at this condition for a while.
The weight change of the permeation tubes was measured at different intervals of 24 h and 216 h. The WVP and WVR of the film were then calculated as Eqs. (6, 7) respectively:
where △m was the weight of the permeation tube acquired by desiccant (g), L was the average film thickness (m), A was the film area exposed to moisture transfer (m2), △t was the time of permeation (h), and △P was the water vapor pressure difference between the two sides of the film (Pa).
2.3.7 Thermogravimetric analyses (TGA)
Using 4 mg of material in aluminum pans, the thermal gravimetric analyses (TGA) were performed using a Shimadzu TGA-50 instrument (Japan) at a heating rate of 30 °C/min in a nitrogen environment at 30 ml/min. The onset degradation temperature, mass loss at the onset degradation temperature, second degradation temperatures, and the remaining mass at 650 °C were used to properly express thermal stability.
2.3.8 Mechanical properties
A universal testing machine (LR10K; Lloyd Instruments, Fareham, UK) was used to determine the mechanical properties of the films. Tests were conducted by ASTM D882-09. Strips of samples measuring 10 mm × 60 mm and having a thickness of nearly 0.2 mm were cut off and introduced into the device. Initial grip separation was set to 30 mm and crosshead speed was set to 20 mm/min. Calculations were performed for both the % elongation at break (EB) and the tensile strength (TS).
2.3.9 Transparency
The transparency of the films was measured according to previous research by Dai et al. [35]. Each film sample was sliced into a rectangle portion of 10 mm × 30 mm, which was then inserted into the cuvette’s side. A UV–Vis spectrophotometer (Jenway 6705, UK) was used to measure the absorbance of each film, with a blank cuvette serving as the reference. The number produced by dividing the absorbance at 600 nm by the film thickness served as a measure of the transparency (mm−1) of the film (mm). The results of each measurement were performed three times.
3 Result and discussion
3.1 Film biodegradation
3.1.1 Enzymatic biodegradation
Enzymes secreted by microorganisms degrade the starch plastic polymer into its molecular constituents, which are used as a carbon source for microbial growth [36]. Starch biodegradation has mostly been associated with the activity of alpha-amylase and glucoamylase, a particular enzyme for starch hydrolysis that is regarded as an important contributor to degradation [37, 38]. Hence, alpha amylase is an endo-amylase that hydrolyzes the α-l,4-linkages in starch (amylose and amylopectin) almost at random, while glucoamylase is an exo-amylase that hydrolyzes the α-1,4-linkages as well as the α-1,6-linkages in liquefied starch (amylose and amylopectin) almost at random [39]. The enzyme mixture degraded TPS/PVA films in a short amount of time, and the degradable materials were detected by raising the osmolality, as shown in Fig. 1, which correlates with the degree of starch hydrolysis by a linear relationship, as reported by Kearsley 1976 [29], who establish a linear relationship between the degree of starch hydrolysis and osmolality as feasible, and affordable methods to produce specialty sugars seem to be reverse osmosis and ultrafiltration of glucose syrups. Also, our results agree with contemporary research by Karimi and Biria (2016) [37]; they indicated that amylase may be effectively able to break down saturated linear hydrocarbons. Furthermore, pre-gelatinization slightly increasing the hydrolysis rate than unpregelatinized waxy starch, this might be due to the pre-gelatinization destroying the starch granules leading to ease of hydrolysis. Therefore, as seen in Fig. 2, the percent of weight loss during the experiment reached 96% indicating superior biodegradation of TPS prepared from modified waxy maize starch blended with PVA [40, 41]. Moreover, after complete hydrolysis happened to TPS/PVA films, the filtrate was analyzed by high-performance liquid chromatography, and the peaks appeared specified to saccharides produced from starch hydrolysis as seemed in Fig. 3. However, the addition of cardanol oil very slightly affect the rate of waxy starch hydrolysis; the degradation percentage of films reached 95.5% and decreased to 91.2% and from 90.5 to 89.6% in PWADA and WADA films respectively when cardanol oil increased to 0.6%, and this might be because of oil decrease the rate of starch hydrolysis as shown in Fig. 4 [30, 31, 42].
3.1.2 Soil burial test
Amylose and amylopectin make up the complex polymeric substance known as starch [43]. Numerous organisms including bacteria and fungi found in the soil environment can break down the main components of starch. Because microorganisms have extremely particular enzymes that can be used to hydrolyze the carbohydrate polymers into digestible units, weakening the structure and decreasing the strength of the starch polymer [44, 45]. The biodegradability of our waxy starch films combined with PVA was carried out on a laboratory scale following the soil burial test. The physical appearance results of the test indicate a visible total breakdown in all synthesized waxy starch films after 21-day completion. This occurs due to microbial feeding and biodegradation that cause the mixes’ structural properties to be lost, causing starch sites in the blends to be occupied by water or bacteria, which causes the combinations to break down. Also, the mixes swelled during the experiment due to the water absorption, which accelerated the biodegradation process, as shown in Fig. 5. Our results are in good agreement with Obasi et al. [46]; they synthesize polypropylene (PP)/plasticized from cassava starch (PCS) blended with and without compatibilizer (polypropylene-graft-maleic anhydride (PP-g-MA)) and found matched with the enzymatic degradation and variation occurs in tensile strength, elongation at break, and Young’s modulus with different levels of starch content in the blends.
Furthermore, using synthesized waxy starch films combined with PVA as soil carbon and energy sources has a good impact on the growth parameter of Fava plants as shown in Figs. 5 and 6. Hence, plant height is a primary indicator of plant growth rate among plant health parameters; plant height was compared to the control to see if bio-plastic consumption had any negative effects on fava bean plant development. Data collected at the end of the experiment from the early vegetative stage, when fava bean plants were at the three-leaf growth stage, showed that plant leaves and roots exposed to bio-plastic films (WADA) and (PWADA) grew faster than the (no film) control, while plant stem exposed to bio-plastic film (PWADA) grew slower than the (no film) control and (WADA) as shown in Fig. 6. Overall, the findings demonstrate that using biodegradable materials on fava bean plants is not only safe but also encourages plant growth due to their 100% biodegradability. Our findings are consistent with those of Sforzini et al. [47], who found no major ecotoxic effects for Mater-Bi® (PBAT-corn starch) on Vibrio fischeri bacteria, slime mold, protozoa, green algae, Sorghum and cress plants, and Daphnia and earthworm invertebrates, indicating that it meets an acceptable level of environmental protection. Because of their biodegradability, they are also a feasible option for environmental preservation [48, 49].
3.2 Film morphology
In polymer blends, it is critical to examine the morphology of the finished goods to keep track of the homogeneity of the product components, which influences other product qualities and the preparation process’s performance. Because of the action of physical modification (pre-gelatinization) in destroying starch granules, it leads to the enhancement of the thermoplasticization process of pregelatinized waxy modified starch than the unpregelatinized waxy modified starch. So, SEM images of TPS of PWADA/PVA plasticized with sorbitol are only more homogeneous than the image of WADA/PVA as shown in Fig. 7a, d [50]. Also, the addition of cardanol oil to PWADA film more is homogenous than WADA film due to PWADA films having linear chains after the a pre-gelatinization process which tends to form a complex with cardanol oil leading to compatibility between the matrix. In addition, after the plasticization process, the TPS of PWADA/PVA film has uniform morphology than WADA/PVA, as shown in images b, c and e, f, respectively, Moreover, the phenolic chemical structure of cardanol oil promotes the hydrogen bond formation in the matrix and increases the compatibility of the components of the film generally [18].
3.3 XRD analysis
Starch granules are made up of linear amylose and branched amylopectin macromolecules. They are grouped in a laminar pattern and create growth rings, resulting in a semi-crystalline pattern in starch granules. XRD was carried out to show up variations in the crystalline structures of starch after thermoplasticization and to demonstrate that complete gelatinization was attained at the end of the blending process. Waxy maize starch has a type-A crystalline structure with peaks at 2θ 15°, 17°, and 23° that are characterized by more packed granules, and the disappearance of the later peaks indicated the effect of the thermoplasticization and blending process that altered the crystallinity of waxy corn starch [51]. New peaks appeared at 2θ of 13.2°, 19.5°, and 20.8°, which indicated a V-type crystal pattern. A new structure was formed due to new interactions between the plasticizers, amylose, and amylopectin macromolecules. On the other hand, the crystallinity of TPS of PWADA/PVA is lower than WADA/PVA one as the pre-gelatinization process partially damaged the granule crystalline structure, so as shown in Fig. 8, the peaks of PWADA are more unresolved with low intensity than WADA one and the peaks at 2θ 10° and 20° may ascribe to PVA and sorbitol [50]. The addition of cardanol oil enhances the plasticization of two types of TPS compared with a diffractogram of sorbitol only, as seen in Fig. 8.
3.4 Fourier transform infrared spectroscopy (FTIR)
Infrared spectroscopy is a potent tool for determining changes in chemical structure caused by mixing different molecules [52]. As demonstrated in Fig. 9, the TPS of PWADA and WADA/PVA films with sorbitol alone and sorbitol-cardanol mixture plasticizers can be distinguished. The specific band at 3560 and 3520 cm−1 depicts (O–H stretching) which is a numerous functional group in these formulations PWADA/PVA, WADA/PVA, and sorbitol-cardanol plasticizers which can boost the formation of more hydrogen linkage between the polymeric matrix leading to enhanced compatibility between them [52]. The asymmetric CH2 methyl group is also a distinct band at 2920 cm−1 in all films containing cardanol oil, and the skeletal vibration of the aromatic (–C = C–) linkages is a characteristic band at 1590 cm−1 in all films containing cardanol oil [28]. The presence of a C–O stretching aromatic ring is linked to the band at 1267 cm−1. The side chain (–C = C–) double bonds were found between 991 and 869 cm−1, 695 cm−1, and 781 cm−1, matching the vinyl vibrations [31]. At 1260, 1080, and 1019 cm−1, there are notable band peaks attributable to –CH2 stretching, –CH2OH (side chain) related mode, C = O stretching, and C–O–C stretching, respectively. The presence of the C = O feature band at 1710 cm−1 in all spectrums could be attributed to residual acetate groups in PVA and acetylated and cross-linking bonds in PWADA and WADA [53]. Anhydro glucose ring stretching is defined by the absorbance bands at 992, 929, 861, 765, and 575 cm−1 [54]. The microstructure images of the films under scanning electron microscope endorse the infra-red spectra, strong hydrogen bond formed between modified waxy maize starch with PVA and sorbitol-cardanol mixture which promote the compatibility of the film component.
3.5 Water uptake pattern of films
PWADA/PVA and WADA/PVA films have multifunctional hydroxyl groups and absorb moisture from the environment. As a result, the pregelatinization process during starch modification increases starch swelling power and solubility, causing PWADA/PVA films to uptake more moisture than WADA/PVA films when sorbitol is used as a plasticizer alone, as shown in Fig. 10a [55]. As shown in Fig. 10b, c, d, the addition of cardanol oil slightly decreased the rate of water uptake of TPS of WDADA/PVA and PWADA/PVA films. This could be because of the hydrophobic character of cardanol oil; the presence of an aliphatic group restricts the number of hydroxyl groups exposed to interaction with the hydroxyl group of moisture. Also, the reduction of water uptake by cardanol oil on PWADA films is more than on WADA films; this could be due to the formed curling path for the water molecule to penetrate the PWADA/PVA polymer matrix. The values of WU and diffusion coefficient, which were less than those found in the starch-chitosan matrix made by Hasan et al. and normal corn/PVA matrix made by the author, could be owing to the sorbitol-cardanol mixture forming a wrinkled channel for water molecules to penetrate the starch/PVA matrix [34, 56].
3.6 Water vapor permeability
Because of the pregelatinization procedure, which increases the swelling and solubility of starch, PWADA/PVA films have somewhat higher water vapor permeability than WADA/PVA films, as shown in Table 3. WVP of PWADA/PVA films is unaffected by the addition of cardanol oil; however, WVP of WADA/PVA films is slightly reduced. As a result, when oil is added, the WVP decreases by 0.6%, from 2.95 to 2.7 (10−10 g.Pa−1 S−1 m−1) as shown in Table 3 and this might be due to the hydrophobicity nature of cardanol oil as alkyl chain in its structure. The addition of cardanol oil was believed to create hydrogen bonding between the starch network and cardanol compound, limiting the availability of hydroxyl groups in establishing interactions with water to form a strong structure, lowering free volume within the matrix, and restricting intermolecular hydrogen bonding [26]. However, the WVP of this study is less than that of Hasan et al. who investigated that the composite of starch-chitosan-palm oil has WVP values in the range of 3.51–4.53 × 10−9, and Zheng et al., who added eugenol oil to the starch chitosan composite [27, 34].
3.7 Thermogravimetric analyses (TGA)
Thermal stability is an important property to consider when developing new film formulations, and it is measured by thermogravimetric analysis as a function of weight change as temperature rises. The thermogram can be broken down into three stages: first, the temperature range (0–200 °C), when weight loss is caused by evaporation of water and smaller molecular weight plasticizer; second, the temperature at which the first and second stages of deterioration occur (200–450 °C); finally, the weighted residual (450–600 °C) at the end of the experiment [31]. As shown in Fig. 11, the TPS of PWADA/PVA films has the lowest onset degradation temperature of 272 °C when plasticized with sorbitol alone, but it increased to 318 °C when cardanol oil was added by 0.2%, and the residual from 0 to 26.2%; indicating that cardanol oil increases the thermal stability of PWADA/PVA films. This could be due to the strong hydrogen bond formed in this formulation. Moreover, the cardanol oil slightly increases the onset degradation temperature of WADA/PVA films from 317 to 320 °C [57, 58].
3.8 Mechanical properties analysis
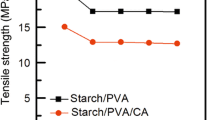
The mechanical properties of thermoplastic PWADA/PVA and WADA/PVA films are shown in Table 4; the pre-gelatinization process slightly enhances the tensile strength by 11.6% of acetylated distarch adipate WADA which consider improvement in thermo-plasticization after physio-chemical modification when compared with chemical modification alone [59]. The addition of cardanol oil by 0.2% has no significant effect on the mechanical properties, but the increase in the oil percent decreases the tensile strength of the films by 17.5 and 55.6 in the case of PWADA/PVA and WADA/PVA films respectively. While the addition of 0.2% cardanol oil mixed with sorbitol plasticizer increases the elongation by 30.6 in the case of PWADA films whereas decreases WADA films by 33.8% indicating the sorbitol-cardanol mixture at cardanol portion 0.2% is a good plasticizer to PWADA films as proved in the water uptake of the films [60].
3.9 Transparency
The safety of products that are susceptible to UV rays is an important need in the packaging sector. As indicated in Table 5, adding cardanol oil to the thermoplastic PWADA and WADA starch films reduces transmission by 69 and 63.4% in the UV area at 250 nm wavelength, showing that cardanol oil is an effective UV absorber. On the other hand, the addition of cardanol oil, on the other hand, reduces the transparency of PWADA/PVA and WADA/PVA films by 5.5 and 28.8%, respectively [61, 62].
3.9.1 Comparison of different parameters of prepared films with similar studies
The prepared films from pre-geltinized waxy corn starch/PVA with sorbitol-cardanol oil 0.2 showed superior tensile strength compared with other work as shown in Table 6. Additionally, introducing cardanol oil to modified starch film promotes thermal stability as residual at 600 °C which is a large percentage as seen in Table 6.
4 Conclusion
Biodegradable bioplastic films made from waxy maize starch that had been physio-chemically and chemically modified, blended with polyvinyl alcohol, and plasticized with sorbitol and a sorbitol-cardanol mixture were tested. The pregelatinizing process increases the homogeneity and thermoplasticization of the film matrix. The pre-gelatinization process increased water uptake and WVP of films, whereas cardanol oil slightly decreased the water uptake rate of PWADA, possibly due to a stronger hydrogen bond between the oil and the polymer matrix in PWADA. Furthermore, the addition of cardanol oil improves the thermal stability of the films as well as their ability to absorb ultraviolet light. The pre-gelatinization phase increases tensile strength by 11%, and cardanol oil at 0.2% increases elongation by 30% in the film of PWADA but decreases in the film of WADA by 33.8%. As a result, modified waxy maize starch is a promising biopolymer for making bioplastic films for biodegradable packaging materials.
Data availability
Any data related to this manuscript can be made available on request.
References
Okunola AA, Kehinde IO, Oluwaseun A, Olufiropo EA (2019) Public and environmental health effects of plastic wastes disposal: a review. J Toxicol Risk Assess 5. https://doi.org/10.23937/2572-4061.1510021
Gupta KK, Sharma KK, Chandra H (2022) Micrococcus luteus strain CGK112 isolated from cow dung demonstrated efficient biofilm-forming ability and degradation potential toward high-density polyethylene (HDPE). Arch Microbiol 204:402. https://doi.org/10.1007/s00203-022-03023-4
Queiroz AUB, Collares-Queiroz FP (2009) Innovation and industrial trends in bioplastics. Polym Rev 49:65–78. https://doi.org/10.1080/15583720902834759
Luckachan GE, Pillai CKS (2011) Biodegradable polymers- a review on recent trends and emerging perspectives. J Polym Environ 19:637–676. https://doi.org/10.1007/s10924-011-0317-1
Anani OA, Adetunji CO (2021) Bioremediation of polythene and plastics using beneficial microorganisms. pp 281–302
Sankhla IS, Sharma G, Tak A (2020) Fungal degradation of bioplastics: an overview. In: New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier, pp 35–47
Zeenat, Elahi A, Bukhari DA, Shamim S, Abdul Rehman (2021) Plastics degradation by microbes: a sustainable approach. J King Saud Univ Sci 33:101538. https://doi.org/10.1016/j.jksus.2021.101538
BeMiller JN (2018) Corn starch modification, 3rd ed. Elsevier Inc.
Stepto RFT (2009) Thermoplastic starch. Macromol Symp 279:163–168. https://doi.org/10.1002/masy.200950525
Abral H, Atmajaya A, Mahardika M, Hafizulhaq F, Kadriadi, Handayani D, Sapuan SM, Ilyas RA (2020) Effect of ultrasonication duration of polyvinyl alcohol (PVA) gel on characterizations of PVA film. J Market Res 9:2477–2486. https://doi.org/10.1016/j.jmrt.2019.12.078
Sabetzadeh M, Bagheri R, Masoomi M (2015) Study on ternary low density polyethylene/linear low density polyethylene/thermoplastic starch blend films. Carbohydr Polym 119:126–133. https://doi.org/10.1016/j.carbpol.2014.11.038
Kaseem M, Hamad K, Deri F (2012) Rheological and mechanical properties of polypropylene/thermoplastic starch blend. Polym Bull 68:1079–1091. https://doi.org/10.1007/s00289-011-0611-z
Noivoil N, Yoksan R (2021) Compatibility improvement of poly(lactic acid)/thermoplastic starch blown films using acetylated starch. J Appl Polym Sci 138:49675. https://doi.org/10.1002/app.49675
Temesgen S, Rennert M, Tesfaye T, Nase M (2021) Review on spinning of biopolymer fibers from starch. Polymers (Basel) 13:1121. https://doi.org/10.3390/polym13071121
Abral H, Ariksa J, Mahardika M, Hafizulhaq F, Kadriadi, Handayani D, Sapuan SM Ilyas RA (2020) Highly transparent and antimicrobial PVA based bionanocomposites reinforced by ginger nanofiber. Polym Test 81:106186
Ge C, Lansing B, Lewis CL (2021) Thermoplastic starch and poly(vinyl alcohol) blends centered barrier film for food packaging applications. Food Pack Shelf Life 27:100610. https://doi.org/10.1016/j.fpsl.2020.100610
Aydin AA, Ilberg V (2016) Effect of different polyol-based plasticizers on thermal properties of polyvinyl alcohol: starch blends. Carbohydr Polym 136:441–448. https://doi.org/10.1016/j.carbpol.2015.08.093
Zuo Y, Gu J, Tan H, Zhang Y (2015) Thermoplastic starch prepared with different plasticizers: relation between degree of plasticization and properties. J Wuhan Univ Technol Mater Sci Ed 30:423–428. https://doi.org/10.1007/s11595-015-1164-z
Jiang X, Li H, Luo Y, Zhao Y, Hou L (2016) Studies of the plasticizing effect of different hydrophilic inorganic salts on starch/poly (vinyl alcohol) films. Int J Biol Macromol 82:223–230. https://doi.org/10.1016/j.ijbiomac.2015.11.046
Navarchian AH, Jalalian M, Pirooz M (2015) Characterization of starch/poly(vinyl alcohol)/clay nanocomposite films prepared in twin-screw extruder for food packaging application. J Plast Film Sheet 31:309–336. https://doi.org/10.1177/8756087914568904
Abdullah ZW, Dong Y (2019) Biodegradable and water resistant poly(vinyl) alcohol (PVA)/starch (ST)/glycerol (GL)/halloysite nanotube (HNT) nanocomposite films for sustainable food packaging. Front Mater 6. https://doi.org/10.3389/fmats.2019.00058
Sreekumar PA, Al-Harthi MA, De SK (2012) Reinforcement of starch/polyvinyl alcohol blend using nano¢ titanium dioxide. J Compos Mater 46:3181–3187. https://doi.org/10.1177/0021998312436998
Sani IK, Pirsa S, Tağı Ş (2019) Preparation of chitosan/zinc oxide/Melissa officinalis essential oil nano-composite film and evaluation of physical, mechanical, and antimicrobial properties by response surface method. Polym Test 79:106004. https://doi.org/10.1016/j.polymertesting.2019.106004
Jose J, Al-Harthi MA, AlMa’adeed MAA, Dakua JB, De SK (2015) Effect of graphene loading on thermomechanical properties of poly(vinyl alcohol)/starch blend. J Appl Polym Sci 132:1–8. https://doi.org/10.1002/app.41827
Nazrin A, Sapuan SM, Zuhri MYM et al (2020) Nanocellulose reinforced thermoplastic starch (TPS), polylactic acid (PLA), and polybutylene succinate (PBS) for food packaging applications. Front Chem 8. https://doi.org/10.3389/fchem.2020.00213
Giannakas A, Patsaoura A, Barkoula NM, Ladavos A (2017) A novel solution blending method for using olive oil and corn oil as plasticizers in chitosan-based organoclay nanocomposites. Carbohydr Polym 157:550–557. https://doi.org/10.1016/j.carbpol.2016.10.020
Zheng K, Xiao S, Li W, Wang W, Chen H, Yang F, Qin C (2019) Chitosan-acorn starch-eugenol edible film: physico-chemical, barrier, antimicrobial, antioxidant and structural properties. Int J Biol Macromol 135:344–352. https://doi.org/10.1016/j.ijbiomac.2019.05.151
Mele G, Bloise E, Cosentino F, Lomonaco D, Avelino F, Marcianò T, Massaro C, Mazzetto S, Tammaro L, Scalone A, Schioppa M, Terzi R (2019) Influence of cardanol oil on the properties of poly(lactic acid) films produced by melt extrusion. ACS Omega 4:718–726. https://doi.org/10.1021/acsomega.8b02880
Kearsley MW (1976) Reverse osmosis of glucose syrups. Starch - Stärke 28:138–145. https://doi.org/10.1002/star.19760280408
Salazar-Leyva JA, Osuna-Ruiz I, Rodríguez-Tirado VA, Zazueta-Patrón IE, Brito-Rojas HD (2016) Optimization study of fructans extraction from Agave tequilana weber azul variety. Food Sci Technol 36:631–637. https://doi.org/10.1590/1678-457X.11216
Delheye G, Moreels E (1988) Dextrose equivalent measurements on commercial syrups. Starch - Stärke 40:430–432. https://doi.org/10.1002/star.19880401107
Hasan M, Deepu A, Gopakumar DA, Olaiya NG, Zarlaida F, Alfian A, Aprinasari C, Alfatah T, Rizal S, Abdul Khalil HPS (2020) Evaluation of the thermomechanical properties and biodegradation of brown rice starch-based chitosan biodegradable composite films. Int J Biol Macromol 156:896–905. https://doi.org/10.1016/j.ijbiomac.2020.04.039
Tian H, Yan J, Rajulu AV, Xiang A, Luo X (2017) Fabrication and properties of polyvinyl alcohol/starch blend films: effect of composition and humidity. Int J Biol Macromol 96:518–523. https://doi.org/10.1016/j.ijbiomac.2016.12.067
Hasan M, Rusman R, Khaldun I, Ardana L, Mudatsir M, Fansuri H (2020) Active edible sugar palm starch-chitosan films carrying extra virgin olive oil: barrier, thermo-mechanical, antioxidant, and antimicrobial properties. Int J Biol Macromol 163:766–775. https://doi.org/10.1016/j.ijbiomac.2020.07.076
Dai L, Zhang J, Cheng F (2019) Effects of starches from different botanical sources and modification methods on physicochemical properties of starch-based edible films. Int J Biol Macromol 132:897–905. https://doi.org/10.1016/j.ijbiomac.2019.03.197
Mohanan N, Montazer Z, Sharma PK, Levin DB (2020) Microbial and enzymatic degradation of synthetic plastics. Front Microbiol 11. https://doi.org/10.3389/fmicb.2020.580709
Karimi M, Biria D (2016) The synergetic effect of starch and alpha-amylase on the biodegradation of n-alkanes. Chemosphere 152:166–172. https://doi.org/10.1016/j.chemosphere.2016.02.120
Valachová K, Horváthová V (2007) Starch degradation by glucoamylase Glm from Saccharomycopsis fibuligera IFO 0111 in the presence and absence of a commercial pullulanase. Chem Biodivers 4:874–880. https://doi.org/10.1002/cbdv.200790074
Cuevas-Carballo ZB, Duarte-Aranda S, Canché-Escamilla G (2017) Properties and biodegradability of thermoplastic starch obtained from granular starches grafted with polycaprolactone. Int J Polym Sci 2017. https://doi.org/10.1155/2017/3975692
Rong Y, Sillick M, Gregson CM (2009) Determination of dextrose equivalent value and number average molecular weight of maltodextrin by osmometry. J Food Sci 74:33–40. https://doi.org/10.1111/j.1750-3841.2008.00993.x
Cuevas-Carballo ZB, Duarte-Aranda S, Canché-Escamilla G (2017) Properties and biodegradability of thermoplastic starch obtained from granular starches grafted with polycaprolactone. Int J Polym Sci 2017:1–13. https://doi.org/10.1155/2017/3975692
Alghamdi BA, Alshumrani ES, Saeed MSB, Rawas GM, Alharthi NT, Baeshen MN, Helmi NM, Alam MZ, Suhail M (2020) Analysis of sugar composition and pesticides using HPLC and GC–MS techniques in honey samples collected from Saudi Arabian markets. Saudi J Biol Sci 27:3720–3726. https://doi.org/10.1016/j.sjbs.2020.08.018
Nawaz H, Waheed R, Nawaz M, Shahwar D (2020) Physical and chemical modifications in starch structure and reactivity. In: Chemical Properties of Starch. IntechOpen
Wang B-T, Hu S, Yu X-Y, Jin L, Zhu Y-J, Jin F-J (2020) Studies of cellulose and starch utilization and the regulatory mechanisms of related enzymes in fungi. Polymers (Basel) 12:530. https://doi.org/10.3390/polym12030530
Dida G (2018) Isolation and characterization of starch degrading rhizobacteria. Open Access J Microbiol Biotechnol 3. https://doi.org/10.23880/OAJMB-16000129
Obasi HC, Igwe IO, Madufor IC (2013) Effect of soil burial on tensile properties of polypropylene/plasticized cassava starch blends. Adv Mater Sci Eng 2013:1–5. https://doi.org/10.1155/2013/326538
Sforzini S, Oliveri L, Chinaglia S, Viarengo A (2016) Application of biotests for the determination of soil ecotoxicity after exposure to biodegradable plastics. Front Environ Sci 4:1–12. https://doi.org/10.3389/fenvs.2016.00068
Ryan CA, Billington SL, Criddle CS (2017) Assessment of models for anaerobic biodegradation of a model bioplastic: poly(hydroxybutyrate-co-hydroxyvalerate). Bioresour Technol 227:205–213. https://doi.org/10.1016/j.biortech.2016.11.119
Emadian SM, Onay TT, Demirel B (2017) Biodegradation of bioplastics in natural environments. Waste Mana 59:526–536. https://doi.org/10.1016/j.wasman.2016.10.006
Li J, Luo X, Lin X, Zhou Y (2013) Comparative study on the blends of PBS/thermoplastic starch prepared from waxy and normal corn starches. Starch/Staerke 65:831–839. https://doi.org/10.1002/star.201200260
Zhang Y, Rempel C, Liu Q (2014) Thermoplastic starch processing and characteristics-a review. Crit Rev Food Sci Nutr 54:1353–1370. https://doi.org/10.1080/10408398.2011.636156
Mustafa P, Niazi MBK, Jahan Z, Samin G, Hussain A, Ahmed T, Naqvi SR (2020) PVA/starch/propolis/anthocyanins rosemary extract composite films as active and intelligent food packaging materials. J Food Saf 40. https://doi.org/10.1111/jfs.12725
Junlapong K, Boonsuk P, Chaibundit C, Chantarak S (2019) Highly water resistant cassava starch/poly(vinyl alcohol) films. Int J Biol Macromol 137:521–527. https://doi.org/10.1016/j.ijbiomac.2019.06.223
Sánchez-Rivera MM, Flores-Ramírez I, Zamudio-Flores PB, González-Soto RA, Rodríguez-Ambríz SL, Bello-Pérez LA (2010) Acetylation of banana (Musa paradisiaca L.) and maize (Zea mays L.) starches using a microwave heating procedure and iodine as catalyst: Partial characterization. Starch/Staerke 62:155–164. https://doi.org/10.1002/star.200900209
Liu Y, Chen J, Luo S, Li C, Ye J, Liu C, Gilbert RG (2017) Physicochemical and structural properties of pregelatinized starch prepared by improved extrusion cooking technology. Carbohydr Polym 175:265–272. https://doi.org/10.1016/j.carbpol.2017.07.084
Yahia R, Owda ME, Abou-Zeid RE Abdelhai F, Gad ES, Saleh AK, El-Gamil HY (2022) Synthesis and characterization of thermoplastic starch/PVA/cardanol oil composites loaded with in-situ silver nanoparticles. J Appl Polym Sci 139:51511. https://doi.org/10.1002/app.51511
Turco R, Ortega-Toro R, Tesser R Mallardo S, Collazo-Bigliardi S, Boix AC, Malinconico M, Rippa M, Di Serio M, Santagata G (2019) Poly (lactic acid)/thermoplastic starch films: effect of cardoon seed epoxidized oil on their chemicophysical, mechanical, and barrier properties. Coatings 9:1–20. https://doi.org/10.3390/coatings9090574
Salleh MSN, Nor NNM, Mohd N, Draman SFS (2017) Water resistance and thermal properties of polyvinyl alcohol-starch fiber blend film. p 020045
Garg S, Jana AK (2014) Preparation of LDPE-acetylated/butyrylated starch blend blow films and characterization. Chin J Polym Sci (Engl Ed) 32:268–279. https://doi.org/10.1007/s10118-014-1403-3
Guimarães M, Botaro VR, Novack KM, Teixeira FG, Tonoli GHD (2015) Starch/PVA-based nanocomposites reinforced with bamboo nanofibrils. Ind Crops Prod 70:72–83. https://doi.org/10.1016/j.indcrop.2015.03.014
Khalil HPSA, Yap SW, Owolabi FAT et al (2019) Techno-functional properties of edible packaging films at different polysaccharide blends. J Phys Sci 30:23–41. https://doi.org/10.21315/jps2019.30.s1.2
Yan Q, Hou H, Guo P, Dong H (2012) Effects of extrusion and glycerol content on properties of oxidized and acetylated corn starch-based films. Carbohydr Polym 87:707–712. https://doi.org/10.1016/j.carbpol.2011.08.048
Barıs O, Tolga G, Ayse A (2019) Studies on compatibilization of recycled polyethylene/thermoplastic starch blends by using different compatibilizers. Open Chem 17(1):557–563. https://doi.org/10.1515/chem-2019-0064
Cano A, Cháfer M, Chiralt A, González-Martínez C (2015) Physical and antimicrobial properties of starch-pva blend films as affected by the incorporation of natural antimicrobial agents. Foods 5:3. https://doi.org/10.3390/foods5010003
Acknowledgements
The authors express their gratitude to the National Research Centre, and Al-Azhar University, for the technical support of this work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing of interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yahia, R., Owda, M.E., Abou-Zeid, R.E. et al. Biodegradable, UV absorber and thermal stable bioplastic films from waxy corn starch/polyvinyl alcohol blends. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-022-03683-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03683-8