Abstract
Microbial degradation is an important process for removing many organic chemicals from natural waters. The estimation of biodegradability of chemicals which reach the aquatic environment is necessary in assessing the hazard associated with their use. Phenol has many industrial applications and it finds its way into wastewater streams. It is toxic to several biochemical reactions. However, biological transformation of phenol to non-toxic entities is possible. In this article, the data of biodegradation of phenol at different concentrations were studied. For phenol concentrations equal to or less than 147 mg/L, the plateau (the stage associated with the termination of carbon) BOD (biochemical oxygen demand) was calculated to be 169.47% of the corresponding initial phenol concentration, the BOD of the biomass produced up to the plateau was calculated to be 70% of the corresponding initial phenol concentration. This study shows that the biomass produced up to the plateau, the plateau BOD, and the ultimate BOD can all be estimated for phenol concentrations less than 147 mg/L just if the initial phenol concentration is known and without having to repeat the time-consuming BOD experiments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, there are growing concerns about potential adverse human and ecological health effects resulting from the production, use, and disposal of many chemicals necessary for agriculture, industry, household, and medical treatment [2, 23]. A major environmental problems today is the hydrocarbon contamination resulting from different industrial activities [4]. Progress in science and technology should not ignore its likely impact on the environment. Phenol is a key precursor in some industrial manufactures and it has been especially used in the synthesis of some plastics and resins such as phenol–formaldehyde and phenol-urea–formaldehyde [12, 16].
During the production of these adhesive and during their applications, phenol finds its way into the wastewater stream with concentrations ranging from 100 to 2300 mg L−1 phenol [5]. Phenol is reported hazardous substance and is toxic to humans and the environment [12]. Its impact implies the inhibition of biological activity of microorganisms that are responsible for the stabilization of the organic load in industrial and domestic effluents. It reacts with chlorine and produce mono-, di-, or trichlorophenols, which impart tastes and odors of waters [17]. Chlorinated phenols have often been detected in treated wastewaters [18] and have been identified in fish [9] and are readily bio-accumulative [17]. Therefore, this wastewater must be efficiently treated before discharging into the environment. Among the physical, chemical, and biological methods available for the removal of toxic contaminants from wastewater, biological processes are preferred due to their flexibility, industrial applicability, reliability, removal of a wide range of contaminants, simplicity of operation and maintenance, environmentally friendly, degradation of contaminants to less toxic or harmful products rather than transferring them into another phase, and cost-effectiveness [3, 11, 16]. Biodegradation is the use of microorganisms to breakdown or degrade or transform environmental pollutants. Biodegradability is an important ecological indicator of a substance since the value of biodegradability gives possibility of predicting and describing the fate of the substance as a pollutant of an ecologic system (air, water, or soil) [26]. The environmental fate of organic pollutants has become an important research area of increasing concern [25].
Biological decomposition of various substrates is performed by a large number of microorganisms [1, 6, 7, 13, 19, 22, 27]. Microbial degradation of phenolic wastewater has been reported by some authors [3, 20, 21]. Biodegradation studies of organic compounds are tedious, costing, and time-consuming. In this study, the correlations between the initial phenol concentration and different biological parameters are provided, which enables estimating the biodegradation parameters without running the costing and time-consuming BOD test. Results of this study are anticipated to guide subsequent biodegradation experiments of other biodegradable organic compounds.
2 Materials and methods
2.1 Reagents and chemicals
Phenol from Sigma-Aldrich, purity 99%, and a stock solution of 1000 mg L−1 were prepared. Solvents and other chemicals used were all reagent-grade.
2.2 Analytical determinations
2.2.1 Determination of phenol
The brominating mixture method [28] for phenol determination was modified by authors [10] in order to make it capable of detecting lower phenol concentrations. The modified method is as follows:
In a 250-ml conical flask, pipette out 25 ml brominating mixture and add 25 ml water, 5 ml conc. hydrochloric acid and 5 ml potassium iodide solution. The solution will become dark brown due to liberation of iodine. Titrate this with sodium thiosulphate solution (0.1 N) until the solution is light yellow in color and then add few drops of starch solution, the solution becomes blue. Add sodium thiosulphate very carefully and note the volume of sodium thiosulphate at the end point marked by the disappearance of the blue color, this blank titration is used to determine the volume of brominating mixture equivalent to 1 ml of sodium thiosulphate solution. Then take 25 ml of standard phenol for titration, add 50 ml water and 5 ml conc. hydrochloric acid. To this, add 0.2 N brominating mixture (prepared by dissolving 5.567 g of potassium bromate and 75 g of potassium bromide in 1 L distilled water) from a burette until the solution is light yellow and no more precipitate of tribromophenol separates out; the brominating mixture reacts with existing phenol to form a precipitate of tribromophenol. Add 2 ml more of the brominating mixture, the excess brominating mixture remains in solution, and note the volume added, and then add 2 ml potassium iodide and titrate to the liberated iodide against sodium thiosulphate using starch indicator as described under blank experiment above. Since for lower phenol concentrations, the resulting tribromophenol will be a small amount and the residual bromine would be too high to be consumed by 2 ml KI. It has been found that it is more convenient to add 3 ml of KI instead of 2 ml; reproducible results have been obtained. Repeat the titration with 25 ml of the unknown phenol solution in the same manner as with the standard phenol solution described above. Use the same volume of brominating mixture for the unknown phenol solution.
Calculations:
-
(i) weight of phenol in the standard solution (W g).
-
(ii) volume of sodium thiosulphate used in blank experiment against 25 ml brominating mixture (V ml).
-
(iii) volume of brominating mixture added to 25 ml standard phenol solution (V\ml).
-
(iv) volume of sodium thiosulphate used for known phenol solution (V1ml).
-
(v) volume of sodium thiosulphate used for unknown phenol solution (V2ml).
V ml sodium thiosulphate solution ≡ 25 ml brominating mixture, hence:
1 ml of sodium thiosulphate solution ≡ 25/V ml brominating mixture.
∴ V1ml of sodium thiosulphate solution ≡ (25/V) × V1ml of brominating mixture.
Hence, the volume of brominating mixture used for 25 ml of standard phenol solution = V\—[25 V1/ V] ml. Similarly, the volume of brominating mixture used for 25 ml of unknown phenol solution = V\—[25 V2/V].
Hence, the weight of phenol in the unknown solution = 4 × W × {V\—[25 V2/ V]} / {V\—[ 25 V1/V]} g/L.
2.3 Biodegradation studies
The biodegradation studies were based on using synthetic wastewater (which contains Bacto-peptone which is a common component in microbiological media as an organic nitrogen source for bacteria; in addition to essential growth elements other than carbon which will be driven from phenol, the synthetic wastewater was clear and pale yellow in color) seeded with 10% municipal wastewater (collected from the municipality of Mansoura city, to the north of Egypt, latitude and longitude coordinates are 31.037933, 31.381523) and dosed with phenol. Controls were prepared in the same way but without the addition of phenol. The biodegradability at 25 °C was followed using a respirometric BOD controller. Experiments were done in dark (required for the optimal bacterial growth and to avoid decomposition by direct light). All bottles were dosed with 10% seed (municipal wastewater) to inoculate them with microorganisms; experiments were done in duplicates. Microbial growth medium (Table 1) was adjusted to pH 6.8 (pH 6.8 is suitable for bacterial cells and is the mean pH for natural water streams in the region). In all experiments, treatments and controls were done under the same experimental conditions. Seed-corrected data were obtained by subtracting blank values from corresponding values in other experiments, which gave BOD values corresponding to the phenol added as “pure substrate” only.
After the appropriate time of incubation, the supernatant was separated to determine the final phenol concentration.
3 Results and discussion
In this study, the biodegradability data of wastewaters containing phenol, monitored on long period BOD exertions at 25 °C, is studied.
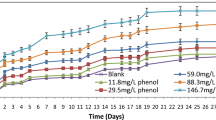
Different doses of phenol ranging from 11.8 to 146.7 mg/L were added to synthetic wastewater samples; their long period BOD exertion curves are shown in Figs. 1 and 2. It was found that phenol is completely utilized in all bottles, whereas partial utilization of phenol was found at concentrations above the reported values.
In this article, we are concerned only with experiments where a complete removal of phenol has been achieved. The plateau BOD values were calculated according to the procedure outlined by Parisod and Schroeder [15], as the oxygen uptake at the plateau minus the oxygen uptake of a seeded blank flask at the same point in time. The purpose of the seed correction in this method is to account for oxygen uptake resulting from extraneous organics present in the used seed solution. The form of the seed-corrected BOD progression curves as found by the previous method where phenol is used as pure substrate is given in Fig. 3.
In this figure, the line extension of the pre- and post- plateau curves is shown to demonstrate the method of determining the plateau value. From this figure, the data in Table 2 are extracted.
The data given above shows that there is a correspondence between substrate ratios and plateau ratios. This correspondence provides excellent evidence of the stoichiometric validity of the plateau concept for phenol. When calculating the correlation coefficient, r, between the initial phenol concentration and the seed-corrected plateau BOD values, using Eq. (1) [14], it was found that the correlation coefficient r = 0.9999.
where:
- n:
-
number of pairs of values
- X:
-
plateau BOD value
- Y:
-
phenol concentration
This value of the correlation coefficient, r, is indicating a complete correlation between plateau BOD values and the initial phenol concentrations, Fig. 4.
These results are supporting the validity of the plateau calculation method, and the plateau concept as associated with the termination of the biodegradation of the substrates’ carbon. It was of interest to investigate the relation between the initial phenol concentration and the ultimate oxygen required for complete removal of the phenol. Although our measurements are showing a complete removal of phenol at the plateau BOD, it is concluded by Kim et al. [8] that 64.4% of the phenolic carbon removed is calculated to be used for cell synthesis. Parisod and Schroeder [15] indicated that the ultimate BOD of a wastewater can be determined by adding the plateau BOD and the BOD of the cells produced up to the plateau. A theoretical BOD of 1.42 g O2/g cells was used in the estimation of the BOD of cells produced up to the plateau based on a cell formula C5H7O2N [15]. The complete chemical oxidation demand of phenol (COD) requires 2.383 g of oxygen per gram of phenol according to the following oxidation reaction, Eq. (2):
Values of the theoretical COD for different phenol concentrations which are calculated based on complete chemical oxidation of phenol are shown in Table 3.
Values of the ultimate BOD for different phenol concentrations are calculated by adding BODcell produced up to the plateau and the measured plateau BOD as indicated in Table 4.
Comparing the results of ultimate BOD mg/L (Table 4) with the values of the oxygen required for chemical oxidation of phenol (Table 3) shows very close values for the same initial phenol concentration. Calculating the linear correlation coefficient, using Eq. (1), between the initial phenol concentration and the ultimate BOD values yields r = 0.9999, Fig. 5.
Calculating the linear correlation coefficient between the initial phenol concentration and the calculated biomass produced up to the plateau, using Eq. (1), is giving r = 1.0002, Fig. 6.
Calculating the linear correlation coefficient, r, between BODultimate and CODtheoretical, using Eq. (1), yields r = 0.9999 indicating a complete correlation between BODultimate and CODtheoretical values, Fig. 7.
4 Conclusion
This study is providing a correlation between the initial phenol concentration and the corresponding plateau BOD, the biomass produced up to the plateau, the ultimate BOD, and the oxygen required for chemical oxidation, which enables a good BOD estimation and in turn a good prediction of the fate of phenol in wastewater, by knowing the initial phenol concentration, without having to run the costing, time-consuming BOD experiments. It paves the way for establishing machine learning programs that provide enough data about the biodegradation and fate of pollutants in the environment from their concentrations. The data obtained in this study shows that 9 days are enough for complete phenol degradation and for phenol concentrations less than 150 mg/L; using the data in Table 4, it can be concluded that:
-
The BOD of the biomass produced up to the plateau is calculated to be 70% of the corresponding initial phenol concentration.
-
The plateau BOD value mg/L is calculated to be 169.47% of the corresponding initial phenol concentration.
-
The ultimate BOD mg/L is calculated to be 239.47% of the corresponding initial phenol concentration.
-
The biomass produced up to the plateau is calculated to be 49.32% of the corresponding initial phenol concentration.
-
The ultimate BOD mg/L is calculated to be 141.3% of the corresponding plateau BOD value.
-
The oxygen required for ultimate biological degradation of phenol was found to be very close to the oxygen required for chemical oxidation; it gives a way for calculating biological treatment parameters from the oxygen required for the chemical oxidation of the substrate even if the initial concentration of the substrate is not known.
-
The modification introduced to the brominating mixture method for phenol determination makes it capable of detecting lower phenol concentrations in wastewater samples.
References
Bulai IM, Venturino E (2016) Biodegradation of organic pollutants in a water body. J Math Chem 54:1387–1403
Catania V, Diliberto CC, Cigna V, Quatrin P (2020) Microbes and persistent organic pollutants in the marine environment. Water Air Soil Pollut 231:354
Dabhade MA, Saidutta MB, Murthy DVR (2009) Continuous phenol removal using nocardia hydrocarbonoxydans in spouted bed contactor: shock load study. Afr J Biotechnol 8:644–649
Dandessa C, Neme A (2020) Review on biodegradation of organic compounds and plastics. Int J Curr Res Aca Rev 8(8):38–47. https://doi.org/10.20546/ijcrar.2020.808.006
Habibi A, Sharifi S (2016) Kinetic modeling for simultaneous biodegradation of phenol and formaldehyde at inhibitory concentrations by ralstonia eutropha. Clean – Soil Air Water J 44(9):1113–1122
Hussain S, Hartley CJ, Shettigar M and Pandey G (2016). Bacterial biodegradation of neonicotinoid pesticides in soil and water systems. FEMS Microbiol Lett 363(23)
Karpinska J, Kotowska U (2019) Removal of organic pollution in the water environment. Water 11:2017. https://doi.org/10.3390/w11102017
Kim JW, Humenick MJ, Armstrong NE (1981) A comprehensive study on the biological treatabilites of phenol and methanol - I. Analysis of bacterial growth and substrate removal kinetics by a statistical method. Water Research 15:1221–1231
Kuehl DW, Veith GD and Leonard EN (1978). Brominated compounds found in water - treatment effluents and their capacity to bioaccumulate, In water Chlorination Environmental Impact and Health Effects. ( Edited by Jolley RL, Gorchev H and Hamilton DH Jr. ) 2:175-192 Ann Arbor Science Publishers, ML
Lotfy HR, Rashed IG (2002) A method for treating wastewater containing formaldehyde. Water Res 36:633–637
Mbachu AE, Chukwura EI, Mbachu NA (2020) Role of microorganisms in the degradation of organic pollutants: a review. Energy and Environ Eng 7(1):1–11
Musa ZJ, Aditi Bhattacharya, Bandela NN (2017) Biological removal of phenol from waste water using a bacterial biofilm. Int J Recent Sci Res 8(12):22674–22676. https://doi.org/10.24327/ijrsr.2017.0812.1314
Nayak JK, Gautam R and Ghosh UK (2022). Bioremediation potential of bacterial consortium on different wastewaters for electricity and biomass feedstock generation. Biomass Conv Bioref https://doi.org/10.1007/s13399-022-02992-2
Obilor EI, Amadi E (2018) Test for significance of Pearson’s correlation coefficient (r). Int J Innov Math Stat Energy Policies 6(1):11–23
Parisod FP and Schoeder ED (1978). Biochemical oxygen demand progression in mixed substrates”. Water Poll Control Fed 182-1834
Ramzan U, Shakoori FR, Shakoori AR et al. (2022). Biodegradation and decolorization of Reactive Red 2 azo dye by Paramecium jenningsi and Paramecium multimicronucleatum in industrial wastewater. Biomass Conv Bioref https://doi.org/10.1007/s13399-022-02817-2
Ratnakar A, Shankar S, Shikha (2016) An overview of biodegradation of organic pollutants. Int J Sci Innov Res 4(1):73–91
Rockwell AL and Larson RA (1987). Aqueous chlorination of some phenolic acids. In water chlorination environmental impact and health effects “edited by Jolloy RL, Gorchev H and Hamilton DH Jr. 2:67-74 Ann Arbor Science publishers, MI
Rozalska S and Iwanicka-Nowicka R (2016). Organic pollutants degradation by microorganisms. Genomics, metagenomics and metatranstriptomics: backgrounds. Microbial biodegradation, from omics to function and application. Jerzy Dlugonski (edn.) Caister Academic Press, 1–12
Samimi M, Shahriari Moghadam M (2020) Phenol biodegradation by bacterial strain O-CH1 isolated from seashore. Global J Environ Sci Manag 6(1):109–118. https://doi.org/10.22034/GJESM.2020.01.09
Sarkar P, Show S, Tiwari H. et al. (2022). Strategic utilization of groundnut shell (Arachishypogaea) immobilized bacterial consortium for enhanced 4-nitrophenol remediation: statistical optimization, kinetic modeling, and reusability. Biomass Conv Bioref https://doi.org/10.1007/s13399-022-02458-5
Selvan BK, Pandiyan R, Vaishnavi M et al. (2022). Ameliorative biodegradation of hazardous textile industrial wastewater dyes by potential microalgal sp. Biomass Conv Bioref https://doi.org/10.1007/s13399-022-02725-5
Takagi K (2020) Study on the biodegradation of persistent organic pollutants, accepted manuscript, J Pestic Sci. https://doi.org/10.1584/jpestics.J19-06
Trahern PG, Knocke WR and Sherrard JH (1980). The effect of Ni(II) on nitrification in the activated sludge process. Water 77(209)
Trautwein C, Kummerer K (2012) Ready biodegradability of trifluoro methylated phenothiazine drugs, structural elucidation of their aquatic transformation products, and identification of environmental risks studied by LC-MSn and QSAR. Environ Sci Pollut Res 19:3162–3177
Toropov AA and Toropova AP (2018). Improved model for biodegradability of organic compounds: the correlation contributions of rings. Chapter in Methods in Pharmacol and Toxicol https://doi.org/10.1007/978-1-4939-7425-2_8
Verma N, Kumar V and Kesari KK (2022). Microbial and lignocellulosic biomass based dye decolourization. Biomass Conv. Bioref. https://doi.org/10.1007/s13399-022-02537-7
Yishnoi NK (1982) Advanced practical organic chemistry. Text Book, Vikas Publishing House PVT Ltd, India
Acknowledgements
The authors wish to thank Prof. Dr. A. A. Al-Sarawy, Faculty of Engineering, Mansoura University, Egypt, and Dr. Asmaa Ahmed, from the Faculty of Engineering, Delta University for Science and Technology, for their assistance during the preparation of this manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Dr. Hesham R. Lotfy did the practical work, the data analysis, and writing; Prof. Ibrahim G. Rashed supervised the practical work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lotfy, H.R., Rashed, I.G. Predicting the fate and the biological treatment parameters of phenol in a wastewater from its initial concentration. Biomass Conv. Bioref. 14, 12357–12364 (2024). https://doi.org/10.1007/s13399-022-03153-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03153-1