Abstract
The goal of this study is to provide more in-depth study into the biodegradation of phenol and to determine the amount of oxygen required for each function in the bacterial cell which is fundamental in understanding of cell metabolism and biology. The total amount of oxygen consumed by bacteria is determined using manometric technique. In the biodegradation of phenol (less than 150 mg/L) the oxygen consumed up to the plateau (the stage associated with the termination of carbon) is found to be composed of three portions, one is used to directly oxidize portion of the substrate to produce energy to allow normal cell functions to sustain life which is estimated to be 50% of the plateau BOD (biochemical oxygen demand), the second portion is to oxidize energy storage intermediate (most probably carbon mono oxide, CO, is oxidized to CO2) to release energy which is then used to power reproduction which is estimated to be 41.75% of the plateau BOD, third portion is incorporated into the produced new cells which is estimated to be 8.25% of the plateau BOD. The correlation coefficient between the initial phenol concentration and the ultimate BOD values is found to be r = 0.9999. This value of correlation coefficient, r, may indicate that microbes are, in a way, estimating the amount of food available and they grow and reproduce accordingly. This article provides a better understanding of cell metabolism and biology. This understanding of cell metabolism may offer better understanding of human cells. The results of this research paves the way for a similar research on human cells where abnormal oxygen uptake may assist in early prediction of cells dysfunction and diseases and may help in early taking the necessary precautions to avoid illness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, there is a growing concern about potential adverse effects on human health and ecological systems resulting from the production, use and disposal of huge amount of chemicals that offer improvements in industry, agriculture, household conveniences and medical treatment (Osmanet al. 2022; Ojha et al. 2021; Vilardi 2020).
Bacterial degradation of organic chemicals which reach water streams is a very important process in removing several organic chemicals from natural waters (Domde et al. 2023; Park et al. 2023; Eckel et al. 2023). In bacterial degradation, bacteria utilize biodegradable organic pollutants as a source of food, which is then oxidized to produce the necessary energy required for different cell functions.
Currently, there is a serious environmental problem arising from the disposal of hydrocarbons by different industrial activities (Dandessa and Neme 2020; Vuppala et al. 2019). Phenol is an essential material in some industrial products, and it has been used in the synthesis of some resins such as phenol–formaldehyde and phenol–urea–formaldehyde which are used for the production of plastics and strong adhesives (Fan et al. 2014; Musa et al. 2017; Sarika et al. 2020; Ramzan et al. 2022; Lotfy and Rashed 2002). During the production and applications of these resins, phenol finds its way into the plant’s wastewater stream in concentrations ranging from 100 to 2300 mgL−1 (Habibi and Sharifi 2016). Phenol is reported hazardous substance and toxic to humans and to the environment at very small concentrations (Musa et al. 2017), and it has been included into the hazardous priority list of the Environmental Protection Agency (Castillo-Carvajal et al. 2014). Phenol is also known to react with chlorine forming chloro-derivatives which causes unacceptable odor and taste to water and are readily bio-accumulative (Ratnakar et al. 2016). Therefore, wastewater containing phenol must be treated carefully before discharging into the environment. Removal of phenol via adsorption on agricultural byproducts (Ponnuchamy et al. 2020) or carbon which is activated chemically at 700 °C, is reported to reach 84.38% (Al-Doury and Ali, 2015), however, complete removal of phenol by adsorption is still an expensive process. In spite of the many methods (physical, biological and chemical, including adsorption, oxidation and photocatalysis) present in the market for removing toxic matters from water, biological treatment remains the most favored because of its reliability, industrial applicability, simplicity, cost-effectiveness, biodegradation of toxic contaminants to harmless fragments and is environmental friendly (Ramzan et al. 2022; Silva et al. 2023; Iturbe et al. 2023; Yingbo et al. 2023). Knowledge of the biodegradation pathways of pollutants is an important indicator which enables the possibility of predicting the fate of a substance in the environment. Pollutants fate in an ecological system has drawn the attention of many researchers due to its importance in assessing the hazards associated with the use of such materials (Kwon et al. 2023). Biological decomposition of various substrates is performed by a large number of microorganisms (Mattelin et al. 2023) which utilize oxygen for the biological transformation of substrates. Biological transformation of phenolic compounds in wastewater has been reported by different authors (Musa et al. 2017; Lotfy and Rashed 2022). The total amount of oxygen required for biological transformation of phenol is reported (Lotfy and Rashed 2002) however, the exact utilization of absorbed oxygen by bacterial cells is still mysterious. More in-depth investigation into biodegradation and studies that determine the exact use of oxygen by cells, and the amount of oxygen required for each cell function is important in order to advance the understanding of cell biochemistry in normal and pathological situations (Castillo-Carvajal et al. 2014). The objective of this study is to correlate the oxygen uptake by microorganisms, during biodegradation of phenol, to the different functions of cells in order to have a better understanding of cell metabolism and biology. This understanding of oxygen utilization by cells may lead to a better understanding of human cells, abnormal oxygen uptake may help early prediction of cells dysfunction and diseases.
Materials and methods
Reagents and chemicals
Phenol was purchased from Sigma-Aldrich and a stock solution of 1000 mg L−1was prepared. All chemicals used were reagent-grade.
Determination of phenol
The brominating mixture method (Yishnoi 1982) for phenol determination was modified (Lotfy and Rashed 2002) in order to make it capable of detecting lower phenol concentrations.
Biodegradation studies
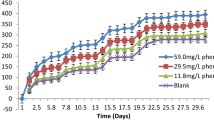
In the biodegradation studies, synthetic wastewater, with composition shown in Table 1 (Trahern et al. 1980), seeded with 10% municipal wastewater to inoculate with microorganisms and dosed with different phenol concentrations (phenol concentrations gradient is based on the concentrations found in the industrial wastewater of phenol-based industries) was used. Blank solution was prepared in the same way but without phenol addition. The experiments were done at 25 °C, magnetic stirring (to ensure homogeneous nutrients and oxygen diffusion) and in dark (required for optimal bacterial growth and to avoid decomposition by direct light) utilizing a respirometric BOD controller. The synthetic wastewater was adjusted to pH 6.8. Blank solution and treatments were kept under the same experimental conditions, BOD values were recorded on regular basis (Fig. 1). After the appropriate time of incubation the supernatant was separated to determine the residual phenol concentration, seed corrected data were obtained by subtracting BOD values of seeded blank from the corresponding results of other seeded and dosed with phenol experiments, which gave values of BOD due to phenol added as a “pure substrate,” all experiments were done in duplicates.
The BOD values of the pure substrate (phenol) obtained as explained above were drawn against time (Fig. 2) and the procedure outlined by Parisod and Schroeder (1978) was followed for the calculation of plateau BOD value. In this method, the plateau value which is associated with the termination of the carbonaceous stage is calculated by line extension of the pre and post plateau curves as exemplified in Fig. 2, the BOD value corresponding to the intersection, of the pre and post extensions, is taken as the plateau value for the corresponding phenol concentration. Plateau BOD is associated with the completion of the carbonaceous stage in which the substrate’s carbon is removed (Parisod and Schroeder, 1978), i.e., the oxygen absorbed for the oxidation of the phenolic carbon, while the BOD of the cells (BODcell) is the amount of oxygen absorbed to oxidize dead cells (which is not part of the plateau BOD). The relative phenol concentration ratios are obtained by dividing the concentration by the smallest concentration and the relative plateau ratios are obtained by dividing each plateau value by the smallest plateau value. A theoretical equation for the complete oxidation of the cell was proposed (Eq. 2) and utilized to calculate the amount of oxygen required for complete oxidation of cells.
Results
The biodegradation of phenol-containing wastewater is studied. Different concentrations of phenol were added to samples of synthetic wastewater. BOD was monitored at 25 °C over long period (30 days), their long period BOD exertion curves are shown in Fig. 1. Measurements of residual phenol showed complete utilization of phenol in all concentrations used. This study is concerned with experiments where complete utilization of phenol is achieved.
The method of Parisod and Schroeder (1978) was followed for the calculation of seed corrected BOD values, as the difference between the oxygen uptake of a blank (prepared the same way as experimental solutions but no phenol was added) and the oxygen uptake of sample dosed with phenol, at the same point in time. The reason for seed corrected data in this method is to subtract oxygen consumption resulting from the presence of any other organic matter exist in the solution. Seed corrected BOD curves obtained as described above where phenol is utilized as the only substrate are shown in Fig. 2.
In the above figure, the line extension of the post- and pre-plateau curves are presented to demonstrate the way used for determining the value of the plateau. The plateau values in Table 2 are taken from the above figure and the correlation between phenol concentration ratios and plateau BOD ratios are shown in Fig. 3.
In Table 2 above, the phenol concentration ratios are obtained by dividing each phenol concentration by the smallest concentration (which is 11.8 mg/L) and plateau BOD ratios are obtained by dividing each plateau value by the smallest plateau value (which is 20 mg/L).
Results in Table 2, presented in Fig. 3 are supporting the validity of the plateau calculation method although it is not showing a direct relation between the initial phenol concentrations and the ultimate oxygen required till the termination of the phenolic carbon. It was of interest to us to investigate the correlation between the initial phenol concentration and the ultimate oxygen required for complete removal of phenolic carbon and most important, the exact use of the absorbed oxygen inside the cell.
Although in the above experiments, measurements are showing complete removal of phenol at the plateau BOD, it is concluded by Kim et al. (1981) that 64.4% of the phenolic carbon removed is calculated to be used for cell synthesis, then we assume that the remaining 35.6% of phenolic carbon is directly oxidized. The ultimate BOD was calculated by adding the measured plateau BOD values and the theoretical BOD of the cells generated up to the plateau, a cell formula C5H7O2N was used by Parisod and Schroeder (1978), in calculating the cell’s BOD. The complete chemical oxidation of phenol requires 2.383 g of oxygen per gram of phenol and 3.11 g of oxygen per gram of phenolic carbon according to the following oxidation reaction (1):
The oxidation of the cell carbon requires 1.42 mg O2/mg cells, according to the following oxidation reaction (2):
A theoretical carbonaceous BOD of 1.42 g O2/g cells (from Eq. 2 above, by dividing the amount of oxygen used to oxidize carbon, one mole O2 for each one mole C, by the molar mass of the cell) was used in the estimation of the BOD of cells, as shown in Table 3.
Comparison between the oxygen required for complete biological degradation of phenol and the oxygen required for complete chemical oxidation of the same phenol concentrations is shown in Table 3 and the correlation between them is shown in Fig. 4. Looking at the cell formula in Eq. (2) above, there is some oxygen incorporated in the composition. We thought if oxygen incorporated in the composition of cells is calculated and added to the corresponding oxygen used for oxidizing the 35.6% of the phenolic carbon remaining of each phenol concentration (64.4% of the phenolic carbon removed is calculated to be used for cell synthesis), the sum will equal the oxygen consumed until reaching the plateau for that phenol concentration, but it was found that there is still some oxygen missing (Table 4). It was found that the value of missing oxygen is close to the value of oxygen required for cell oxidation, BODcell. Figure 5 represents the correlation between the oxygen missing to reach the measured plateau and the BODcell mg/L.
Discussion
This study provides a rather demanded (Castillo-Carvajal et al. 2014; Wagner et al. 2011) more in-depth investigation into the biodegradation process and the oxygen utilization by microorganisms. The ratios of the measured plateau values in this study are in good agreement with the corresponding initial phenol concentration ratios (Table 2 and Fig. 3), which is supporting the validity of the measured plateau values, and indicating that the amount of oxygen absorbed by micro-organisms is directly related to the amount of phenol (food) available.
If each value of the oxygen used up to the plateau (Table 2) is divided by the corresponding initial phenol concentration that will give 1.69 mg O2/mg phenol, the amount oxygen used for the oxidation of carbon of the cells produced up to the plateau is 1.42 mg O2/mg cells (according to Eq. 2), the sum of the oxygen consumed in the previous two processes (1.42 + 1.69) is 3.11 mg O2/mg phenol, that is the oxygen consumed per unit substrate (phenol) in the complete biodegradation process, which is found to be equal to the oxygen required for the direct chemical oxidation of 1 mg of phenolic carbon, 3.11 mg of oxygen per mg of phenolic carbon, calculated according to Eq. (1) by dividing the amount of oxygen used by the amount of carbon which gives a 3.11 g of oxygen per gram of phenolic carbon. That is to say that the amount of oxygen required for chemical oxidation of the substrate carbon and the amount of oxygen required for metabolism for the same substrate are equal, similar findings were reported by Wagner et al. 2011. Calculating the total oxygen required for complete biodegradation (ultimate BOD mg/L) for each phenol concentration and comparing the results with the values of the oxygen required for chemical oxidation of phenol (Table 3) shows very close values for the same initial phenol concentration, which is supporting the previous conclusion and it shows that at the end of the microbial biodegradation cycle for completely biodegradable substrates, total oxygen consumed will equal the oxygen required for chemical oxidation, Fig. 4 shows the correlation between the total oxygen required for complete biodegradation and the oxygen required for chemical oxidation of the same phenol concentration.
If 64.4% of the phenolic carbon is used for cell synthesis (Kim et. al 1981), then the rest of the phenolic carbon which is 35.6%, is most probably directly oxidized to provide the cell with the required energy for growth. For each phenol concentration, calculating the oxygen incorporated in the composition of the new cells, according to the cell formula, C5H7O2N, and adding to the oxygen required for oxidizing the 35.6% of the corresponding phenolic carbon, was thought to account for the oxygen consumed until reaching the corresponding plateau. That was not the case, it was found that there still some missing oxygen (Table 4), when calculating the amount of oxygen missing to reach the plateau for each of the given phenol concentrations, it was found to be very close to the value of oxygen required for cell oxidation, BODcell, for the same phenol concentration (Table 4 and Fig. 5). Then, the explanation for the amount of oxygen which cannot be accounted for is that, it must be used to produce energy required for producing new cells, cell division (Place and Adam 2017), since it is almost equal to the amount of oxygen required for cell oxidation, BODcell, for the same phenol concentration. This amount of oxygen, most probably, is used to oxidize an intermediate product (CO is oxidized to CO2, which is considered an excellent source of energy) (Tiquia-Arashiro 2014) to release energy required for new cells construction. That is to say that the amount of oxygen utilized to produce energy for cell construction equal the amount of oxygen used for cell oxidation (destruction) (Table 4 and Fig. 5). The oxygen consumed up to the plateau can now be thought of as composed of three portions, one is used directly to oxidize 35.6% of the substrate to produce energy to allow normal cell functions to sustain life, the second portion is to oxidize an energy storage intermediate product to release energy which is then used to power production of new cells, third is incorporated in the formula of new cells produced, (C5H7O2N). From data in Tables 4 and 3, it can be concluded that the oxygen used to oxidize substrate (to produce energy to sustain life) is estimated to be 50% of the plateau BOD (by dividing the amount of oxygen used to oxidize 35.6% of phenolic carbon, Table 4, by its corresponding measured plateau value, Table 3), the oxygen thought to be used to produce energy to power cell division is estimated to be 41.75% of the plateau BOD (by dividing the amount of oxygen missing to reach the measured plateau value, Table 4, by its corresponding measured plateau value, Table 3), the oxygen incorporated in new cells is estimated to be 8.25% of the plateau BOD (by dividing the amount of oxygen incorporated in new cells, Table 4, by its corresponding measured plateau value, Table 3), i.e., 50% of the absorbed oxygen is used to sustain current life and 50% (41.75% + 8.25%) to generate new life.
Calculating the linear correlation coefficient between the initial phenol concentration and the ultimate BOD values yields a correlation coefficient r = 0.9999. This value of correlation coefficient, r, is showing the linear relationship between the initial phenol concentration and the ultimate BOD values which may indicate that microbes are, in a way, estimating the amount of food available at the beginning and they grow and reproduce accordingly.
Conclusion
The oxygen consumed at the end of the microbial biodegradation cycle for completely biodegradable substrates (metabolism) is equal to the oxygen required for chemical oxidation of its carbon (oxidation). Complete biological degradation of phenol in wastewater is confirmed in this work for phenol concentrations less than 150 mg/L. The oxygen consumed up to the plateau can be thought of as composed of three portions, one is used to directly oxidize substrate to produce energy to allow normal cell functions to sustain life, the second portion is to oxidize energy storage intermediate product to release energy which is then used in the construction of new cells from the 64.4% carbon used for biomass production, third portion of oxygen is incorporated in the composition of the produced new cells. The oxygen used to oxidize substrate is estimated to be 50% of the plateau BOD value, the oxygen used to produce energy to power reproduction is estimated to be 41.75% of the plateau BOD value, the oxygen incorporated in new cells is estimated to be 8.25% of the plateau BOD value, amazingly oxygen is equally divided between sustaining old cells (50%) and generating new cells (41.75% + 8.25%). Amount of oxygen required to oxidize one gram of cells plus the amount of oxygen required to biologically oxidize one gram of phenol up to the plateau equals the amount of oxygen required for chemical oxidation of one gram of phenolic carbon that is (1.42 + 1.69 = 3.11 g of oxygen). Bacterial cells are using oxygen in a very accurate way and that enables calculating biodegradation parameters for completely biodegradable substrates using mathematical models or computer programs without having to run many tedious practical experiments. Calculating the amount of oxygen consumed for each function in the cell may provide better understanding to oxygen consumption in human cells, the understanding of how oxygen is utilized in the cell will guide analyses of data when changes in the oxygen consumption occurs and contribute to the understanding of the redox biochemistry of cells under normal and pathological conditions. Unexpected consumption may help in disease anticipation and the early taking of necessary precaution measures to avoid illness.
Data availability
All data that are assessed and/or generated during this current study are included in this article.
References
AL-Doury MM, Ali SS (2015) Removal of phenol and para-chlorophenol from synthetic waste water using prepared activated carbon from agricultural wastes. Int J Sustain Green Energy 4(3):92–101. https://doi.org/10.11648/j.ijrse.20150403.14
Castillo-Carvajal LC, Sanz-Martín JL, Barragán-Huerta BE (2014) Bio-degradation of organic pollutants in saline waste water by halophilic micro-organisms: a review. Environ Sci Pollut Res 21:9578–9588. https://doi.org/10.1007/s11356-014-3036-z
Dandessa C, Neme A (2020) Review on bio-degradation of organic compounds and plastics. Int J Curr Res Acad Rev 8(8):38–47. https://doi.org/10.20546/ijcrar.2020.808.006
Domde P, Purohit H, Kashyap R, Wagh S (2023) Bio-degradation of poly-cyclic aromatic hydrocarbon compound by bacterial cultures. Int J Curr Sci Res Rev 06:3246. https://doi.org/10.47191/ijcsrr/V6-i6-18
Eckel F, Sinzinger K, Opdenbosch D, Schieder D, Sieber V, Zollfrank C (2023) Influence of microbial biomass content on bio-degradation and mechanical properties of poly(3-hydroxybutyrate) composites. Biodegradation 35:1–16. https://doi.org/10.1007/s10532-023-10038-1
Fan D-B, Li G-Y, Qin T-F, Chu F-X (2014) Synthesis and structure characterization of phenol–urea–formaldehyde resins in the presence of magnesium oxide as catalyst. Polymers 6(8):2221–2231. https://doi.org/10.3390/polym6082221
Habibi A, Sharifi S (2016) Kinetic modeling for simultaneous biodegradation of phenol and formaldehyde at inhibitory concentrations by ralstonia eutropha. Clean Soil Air Water J 44(9):1113–1122
Iturbe P, Bonte M, Weedon J, Braster M, Brandt B, Spanning R (2023) Correlating the succession of microbial communities from Nigerian soils to petroleum bio-degradation. W J Microbio Biotech 39. https://doi.org/10.1007/s11274-023-03656-7
Kim JW, Humenick MJ, Armstrong NE (1981) A comprehensive study on the biological treatabilites of phenol and methanol analysis of bacterial growth and substrate removal kinetics by a statistical method. Water Res 15:1221–1231
Kwon S, Zambrano M, Pawlak J, Ford E, Venditti R (2023) Aquatic bio-degradation of poly(β-hydroxybutyrate) and polypropylene blends with compatibilizer and the generation of micro- and nano-plastics on bio-degradation. J Polym Environ 31:1–13. https://doi.org/10.1007/s10924-023-02832-y
Lotfy HR, Rashed IG (2002) A method for treating waste-water containing formaldehyde. Water Res 36:633–637
Lotfy HR, Rashed IG (2022) Predicting the fate and the biological treatment parameters of phenol in a wastewater from its initial concentration. Biomass Convers Biorefinary. https://doi.org/10.1007/s13399-022-03153-1
Mattelin V, Verfaille L, Kundu K, Wildeman S, Boon N (2023) A new colorimetric test for accurate determination of plastic bio-degradation. Polymers 15:2311. https://doi.org/10.3390/polym15102311
Musa ZJ, Bandela Bhattacharya A, NN, (2017) Biological removal of phenol from waste water using a bacterial biofilm. Int J Rec Sci Res 8(12):22674–22676. https://doi.org/10.24327/ijrsr.2017.0812.1314
Ojha A, Tiwary D, Oraon R, Singh P (2021) Degradations of endocrine-disrupting chemicals and pharmaceutical compounds in wastewater with carbon-based nanomaterials: a critical review. J Environ Sci Pollut Res 28:30573–30594
Osman AI, Fawzy S, Farghali M et al (2022) Biochar for agronomy; animal farming; anaerobic digestion; composting; water treatment; soil remediation; construction; energy storage, and carbon sequestration: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-022-01424-x
Parisod FP, Schoeder ED (1978) Biochemical oxygen demand progression in mixed substrates. Water Pollut Control Fed 50:1827–1834
Park W, Hwangbo M, Chu KH (2023) Plastisphere and microorganisms involved in polyurethane biodegradation. Sci Total Environ 886:163932. https://doi.org/10.1016/j.scitotenv.2023.163932
Place T, Case A (2017) Limitations of oxygen delivery to cells in culture: an underappreciated problem in basic and translational research. Free Radic Biol Med 113:311–322. https://doi.org/10.1016/j.freeradbiomed.2017.10.003
Ponnuchamy M, Kapoor A, Pakkirisamy B et al (2020) Optimization; equilibrium; kinetic and thermodynamic studies on adsorptive remediation of phenol onto natural guava leaf powder. Environ Sci Pollut Res 27:20576–20597. https://doi.org/10.1007/s11356-019-07145-z
Ramzan U, Shakoori FR, Shakoori AR et al (2022) Biodegradation and decolorization of reactive red 2 azo dye by Paramecium jenningsi and Paramecium multimicronucleatum in industrial wastewater. Biomass Conv Bioref. https://doi.org/10.1007/s13399-022-02817-2
Ratnakar A, Shankar S, Shikha S (2016) An overview of bio-degradation of organic pollutants. Int J Sci Innov Res 4(1):73–91
Sarika PR, Nancarrow P, Khansaheb A, Ibrahim T (2020) Bio-based alternatives to phenol and formaldehyde for the production of resins. Polymers 1210:2237. https://doi.org/10.3390/polym12102237
Silva R, Marques C, Arruda T, Teixeira S, Oliveira T (2023) Bio-degradation of polymers: stages; measurement; standards and prospects. Macromol 3:371–399. https://doi.org/10.3390/macromol3020023
Tiquia-Arashiro Sonia (2014) Microbial CO Metabolism. Springer International Publishing, Cham. https://doi.org/10.1007/978-3-319-11873-4_2
Trahern PG, Knocke WR Sherrard JH (1980) The effect of Ni(II) on nitrification in the activated sludge process. Water 77(209):171–176
Vilardi G (2020) P-aminophenol catalysed production on supported nano-magnetite particles in fixed-bed reactor: kinetic modeling and scale-up. Chemosphere 250:126237. https://doi.org/10.1016/j.Chemosphere.2020.126237
Vuppala S, Bavasso I, Stoller M, Di Palma L, Vilardi G (2019) Olive mill waste water integrated purification through pre-treatments using coagulants and biological methods: experimental, modeling and scale-up. J Clean Prod 236:117622. https://doi.org/10.1016/j.jclepro.2019.117622
Wagner B, Venkataraman S, Buettner G (2011) The rate of oxygen utilization by cells. Free Radic Biol Med 51:700–712. https://doi.org/10.1016/j.freeradbiomed.2011.05.024
Yingbo Y, Zhou W, Liang Q, Dian L, Tianyuan S, Qi Q (2023) Advances in biodegradation of polyolefin plastics. Chinese J biotechnol 39:1930. https://doi.org/10.13345/j.cjb.221024
Yishnoi NK (1982) Advanced practical organic chemistry. Vikas publishing house PVT Ltd, India
Acknowledgements
The author wish to thank Prof. Dr. Susana Rodriguez Couto, Lappeenranta University of Technology—Lahti: LUT University—Lahti Campus, Finland and Dr. Ahmed Hosny, Faculty of Engineering, Delta University for Science and Technology, for their assistance during the preparation of this manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Ethical approval
Not Applicable.
Consent to participate
Author agreed to participate.
Consent for publication
Author agreed to publish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lotfy, H.R. Determination of the amount of oxygen required for each function in the bacterial cell during phenol biodegradation in wastewater: a unique concept. Appl Water Sci 14, 165 (2024). https://doi.org/10.1007/s13201-024-02218-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02218-y