Abstract
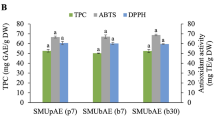
The seeds and peel of pomegranates, which are a byproduct of the fruit processing industry, could be used as a source of antioxidants. This study, therefore, aimed at optimizing experimental conditions to extract antioxidants from the freeze-dried powder of pomegranate seeds and peel. Extraction temperature (°C), time (min), and solvent types (ethanol, methanol, and acetone) were studied as independent variables to optimize the extraction conditions. The interaction between variables was examined using response surface methodology (RSM) with Box-Behnken design (BBD). The total phenolic compounds (TPC), 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity, and ferric reducing antioxidant power (FRAP) were determined to evaluate the antioxidant activities of pomegranate seeds and peel. The TPC of the pomegranate seeds and peel ranged from 1.43 ± 0.19 to 3.98 ± 0.06 mg GAE/100 g DM and 1.37 ± 0.47 to 2.68 ± 0.61 mg GAE/100 g DM, respectively. DPPH values of peel (76.51 ± 1.58 to 85.82 ± 1.97%) were higher than those of the seeds (38.56 ± 4.02 to 70.41 ± 3.64%). The FRAP values for pomegranate seeds and peel were within the range of 144.58 ± 2.57 to 232.61 ± 3.17 mg AAE/100 g DM and 521.42 ± 4.89 to 798.45 ± 4.10 mg AAE/100 g DM, respectively. The results of the RSM optimization demonstrated that under the optimized temperature (50 °C), time (30 min), and solvent (methanol), freeze-dried pomegranate seeds showed the maximum value of TPC (2.91 ± 0.05 mg GAE/100 g DM), DPPH (61.26 ± 2.65%), and FRAP (148.34 ± 4.42 mg AAE/100 g DM). Moreover, 50 °C for 10 min is the optimum temperature and time for maximum antioxidant extraction from pomegranate peel when methanol was used as the solvent. The maximum TPC, DPPH, and FRAP values were 2.79 ± 0.08 mg GAE/100 g DM, 86.19 ± 4.17%, and 785.67 ± 4.92 mg AAE/100 g DM, sequentially. It is clear from the results that pomegranate peel contains more antioxidants than seeds. On an industrial scale, extraction procedures that use more solvent and need time to extract antioxidants may be replaced with the optimized extraction conditions used in the present study.




Similar content being viewed by others
References
Henning SM, Zhang Y, Rontoyanni VG et al (2014) Variability in the antioxidant activity of dietary supplements from pomegranate, milk thistle, green tea, grape seed, goji, and acai: effects of in vitro digestion. J Agric Food Chem 62:4313–4321. https://doi.org/10.1021/jf500106r
Hossain MA, Evan MSS, Moazzem MS et al (2020) Response surface optimization for antioxidant extraction from jackfruit (Artocarpus heterophyllus Lam.) seed and pulp. J Sci Res 12:397–409. https://doi.org/10.3329/jsr.v12i3.44459
Koushesh Saba M, Zarei L (2019) Preharvest methyl jasmonate’s impact on postharvest chilling sensitivity, antioxidant activity, and pomegranate fruit quality. J Food Biochem 43:e12763. https://doi.org/10.1111/jfbc.12763
DiStefano V, Pitonzo R, Novara ME et al (2019) Antioxidant activity and phenolic composition in pomegranate (Punica granatum L.) genotypes from south Italy by UHPLC–Orbitrap-MS approach. J Sci Food Agric 99:1038–1045. https://doi.org/10.1002/jsfa.9270
Turgut SS, Işıkçı F, Soyer A (2017) Antioxidant activity of pomegranate peel extract on lipid and protein oxidation in beef meatballs during frozen storage. Meat Sci 129:111–119. https://doi.org/10.1016/j.meatsci.2017.02.019
Trigueros L, Wojdyło A, Sendra E (2014) Antioxidant activity and protein–polyphenol interactions in a pomegranate (Punica granatum L.) yogurt. J Agric Food Chem 62:6417–6425. https://doi.org/10.1021/jf501503h
Derakhshan Z, Ferrante M, Tadi M et al (2018) Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem Toxicol 114:108–111. https://doi.org/10.1016/j.fct.2018.02.023
Aboelsoued D, Abo-Aziza FAM, Mahmoud MH et al (2019) Anticryptosporidial effect of pomegranate peels water extract in experimentally infected mice with special reference to some biochemical parameters and antioxidant activity. J Parasit Dis 43:215–228. https://doi.org/10.1007/s12639-018-01078-z
Kharchoufi S, Gomez J, Lasanta C et al (2018) Benchmarking laboratory-scale pomegranate vinegar against commercial wine vinegars: antioxidant activity and chemical composition. J Sci Food Agric 98:4749–4758. https://doi.org/10.1002/jsfa.9011
Amri Z, Ghorbel A, Turki M et al (2017) Effect of pomegranate extracts on brain antioxidant markers and cholinesterase activity in high fat-high fructose diet induced obesity in rat model. BMC Complement Altern Med 17:1–9. https://doi.org/10.1186/s12906-017-1842-9
Pontonio E, Montemurro M, Pinto D et al (2019) Lactic acid fermentation of pomegranate juice as a tool to Improve antioxidant activity. Front Microbiol 10:1550. https://doi.org/10.3389/fmicb.2019.01550
Martinez L, Castillo J, Ros G, Nieto G (2019) Antioxidant and antimicrobial activity of rosemary, pomegranate and olive extracts in fish patties. Antioxidants 8:86. https://doi.org/10.3390/antiox8040086
Fanali C, Belluomo MG, Cirilli M et al (2016) Antioxidant activity evaluation and HPLC-photodiode array/MS polyphenols analysis of pomegranate juice from selected Italian cultivars: a comparative study. Electrophoresis 37:1947–1955. https://doi.org/10.1002/elps.201500501
Orak HH (2009) Evaluation of antioxidant activity, colour and some nutritional characteristics of pomegranate (Punica granatum L.) juice and its sour concentrate processed by conventional evaporation. Int J Food Sci Nutr 60:1–11. https://doi.org/10.1080/09637480701523306
Viyar AH, Qadri R, Iqbal A et al (2017) Evaluation of unexplored pomegranate cultivars for physicochemical characteristics and antioxidant activity. J Food Sci Technol 54:2973–2979. https://doi.org/10.1007/s13197-017-2736-z
Sumaiya K, Jahurul MHA, Zzaman W (2018) Evaluation of biochemical and bioactive properties of native and imported pomegranate (Punica granatum L.) cultivars found in Bangladesh. Int Food Res J 25:737–746
Cano-Lamadrid M, Marhuenda-Egea FC, Hernández F et al (2016) Biological activity of conventional and organic pomegranate juices: antioxidant and antimutagenic potential. Plant foods Hum Nutr 71:375–380. https://doi.org/10.1007/s11130-016-0569-y
Ara KM, Raofie F (2016) Application of response surface methodology for the optimization of supercritical fluid extraction of essential oil from pomegranate (Punica granatum L.) peel. J Food Sci Technol 53:3113–3121. https://doi.org/10.1007/s13197-016-2284-y
Al-Said FA, Opara LU, Al-Yahyai RA (2009) Physico-chemical and textural quality attributes of pomegranate cultivars (Punica granatum L.) grown in the Sultanate of Oman. J Food Eng 90:129–134. https://doi.org/10.1016/j.jfoodeng.2008.06.012
Naik AS, Lele SS (2012) Solid state fermentation of pomegranate seed for lovastatin production: a bioprocessing approach. Adv Biosci Biotechnol 3:643. https://doi.org/10.4236/abb.2012.35083
Samsur iS, Li TH, Ruslan MSH, Amran NA (2020) Antioxidant recovery from pomegranate peel waste by integrating maceration and freeze concentration technology. Int J Food Eng 16: https://doi.org/10.1515/ijfe-2019-0232
Alkathiri B, El-Khadragy MF, Metwally DM et al (2017) Pomegranate (Punica granatum) juice shows antioxidant activity against cutaneous leishmaniasis-induced oxidative stress in female BALB/c mice. Int J Environ Res Public Health 14:1592. https://doi.org/10.3390/ijerph14121592
Kaur G, Jabbar Z, Athar M, Alam MS (2006) Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem Toxicol 44:984–993. https://doi.org/10.1016/j.fct.2005.12.001
Singh RP, Chidambara Murthy KN, Jayaprakasha GK (2002) Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem 50:81–86. https://doi.org/10.1021/jf010865b
Abascal K, Ganora L, Yarnell E (2005) The effect of freeze-drying and its implications for botanical medicine: a review. Phyther Res An Int J Devoted to Pharmacol Toxicol Eval Nat Prod Deriv 19:655–660. https://doi.org/10.1002/ptr.1651
Tabaraki R, Heidarizadi E, Benvidi A (2012) Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) peel antioxidants by response surface methodology. Sep Purif Technol 98:16–23. https://doi.org/10.1016/j.seppur.2012.06.038
Rahman MM, Khan FE, Das R, Hossain MA (2016) Antioxidant activity and total phenolic content of some indigenous fruits of Bangladesh. Int Food Res J 23(6):2399–2404
Venkataramanamma D, Aruna P, Singh RP (2016) Standardization of the conditions for extraction of polyphenols from pomegranate peel. J Food Sci Technol 53:2497–2503. https://doi.org/10.1007/s13197-016-2222-z
Eberhardt MV, Lee CY, Liu RH (2000) Antioxidant activity of fresh apples. Nature 405:903–904. https://doi.org/10.1038/35016151
Belwal T, Dhyani P, Bhatt ID et al (2016) Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem 207:115–124. https://doi.org/10.1016/j.foodchem.2016.03.081
Box GEP, Hunter WH, Hunter S (1978) Statistics for experimenters. John Wiley and sons New York
Dean A, Voss D, Draguljić D (1999) Design and analysis of experiments. Springer
Hossain MA, Ahmed T, Hossain MS, Dey P, Ahmed S, Hossain MM (2022) Optimization of the factors affecting BT-2 black tea fermentation by observing their combined effects on the quality parameters of made tea using response surface methodology (RSM), Heliyon, 8(2). https://doi.org/10.1016/j.heliyon.2022.e08948
Marchi LB, Monteiro AR, Mikcha J et al (2015) Evaluation of antioxidant and antimicrobial capacity of pomegranate peel extract (Punica granatum L.) under different drying temperatures. Chem Eng Trans 44:121–126. https://doi.org/10.3303/CET1544021
Anwar F, Jamil A, Iqbal S, Sheikh MA (2006) Antioxidant activity of various plant extracts under ambient and accelerated storage of sunflower oil. Grasasy Aceites 57:189–197. https://doi.org/10.3989/gya.2006.v57.i2.36
Sultana B, Anwar F, Ashraf M (2009) Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 14:2167–2180. https://doi.org/10.3390/molecules14062167
Zzaman W, Biswas R, Hossain MA (2021) Application of immersion pre-treatments and drying temperatures to improve the comprehensive quality of pineapple (Ananas comosus) slices. Heliyon 7:e05882. https://doi.org/10.1016/j.heliyon.2020.e05882
Hossain MA, Hossain MS (2021) Optimization of antioxidant extraction from freeze-dried pulp, peel, and seed of Burmese grape (Baccaurea ramiflora Lour.) by response surface methodology. Biomass Convers Biorefinery. https://doi.org/10.21203/rs.3.rs-347432/v1
Thaipong K, Boonprakob U, Crosby K et al (2006) Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J food Compos Anal 19:669–675. https://doi.org/10.1016/j.jfca.2006.01.003
Koocheki A, Taherian AR, RazaviS MA, Bostan A (2009) Response surface methodology for optimization of extraction yield, viscosity, hue and emulsion stability of mucilage extracted from Lepidium perfoliatum seeds. Food Hydrocoll 23:2369–2379. https://doi.org/10.1016/j.foodhyd.2009.06.014
Wu Y, Cui SW, Tang J, Gu X (2007) Optimization of extraction process of crude polysaccharides from boat-fruited sterculia seeds by response surface methodology. Food Chem 105:1599–1605. https://doi.org/10.1016/j.foodchem.2007.03.066
Jouki M, Mortazavi SA, Yazdi FT, Koocheki A (2014) Optimization of extraction, antioxidant activity and functional properties of quince seed mucilage by RSM. Int J Biol Macromol 66:113–124. https://doi.org/10.1016/j.ijbiomac.2014.02.026
Sood A, Gupta M (2015) Extraction process optimization for bioactive compounds in pomegranate peel. Food Biosci 12:100–106. https://doi.org/10.1016/j.fbio.2015.09.004
Živković J, Šavikin K, Janković T et al (2018) Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep Purif Technol 194:40–47. https://doi.org/10.1016/j.seppur.2017.11.032
Amyrgialaki E, Makris DP, Mauromoustakos A, Kefalas P (2014) Optimisation of the extraction of pomegranate (Punica granatum) husk phenolics using water/ethanol solvent systems and response surface methodology. Ind Crops Prod 59:216–222. https://doi.org/10.1016/j.indcrop.2014.05.011
Jokic S, Velic D, Bilic M, et al (2010) Modelling of the process of solid-liquid extraction of total polyphenols from soybeans. Czech J Food Sci (Czech Republic). https://doi.org/10.17221/200/2009-CJFS
Bouterfas K, Mehdadi Z, Benmansour D, et al (2014) Optimization of extraction conditions of some phenolic compounds from white horehound (Marrubium vulgare L.) leaves. Int J Org Chem 4:292. https://doi.org/10.4236/ijoc.2014.45032
Roy M, Bulbul M, Islam A et al (2022) Study on the drying kinetics and quality parameters of osmotic pre-treated dried Satkara (Citrus macroptera) fruits. J Food Meas Charact 16:471–485. https://doi.org/10.1007/s11694-021-01177-1
Perva-Uzunalić A, Škerget M, Knez Ž et al (2006) Extraction of active ingredients from green tea (Camellia sinensis): extraction efficiency of major catechins and caffeine. Food Chem 96:597–605. https://doi.org/10.1016/j.foodchem.2005.03.015
Akowuah GA, Mariam A, Chin JH (2009) The effect of extraction temperature on total phenols and antioxidant activity of Gynura procumbens leaf. Pharmacogn Mag 5:81
Tsao R (2010) Chemistry and biochemistry of dietary polyphenols. Nutrients 2:1231–1246. https://doi.org/10.3390/nu2121231
Chan SW, Lee CY, Yap CF, et al (2009) Optimisation of extraction conditions for phenolic compounds from limau purut (Citrus hystrix) peels. Int Food Res J 16
Chirinos R, Rogez H, Campos D et al (2007) Optimization of extraction conditions of antioxidant phenolic compounds from mashua (Tropaeolum tuberosum Ruíz & Pavón) tubers. Sep Purif Technol 55:217–225. https://doi.org/10.1016/j.seppur.2006.12.005s
Naczk M, Shahidi F (2004) Extraction and analysis of phenolics in food. J Chromatogr A 1054:95–111. https://doi.org/10.1016/j.chroma.2004.08.059
Arrisujaya D, Susanty D, Hastuti LT (2020) The effect of three variants of extracting solvents on the total phenolic content and antioxidant activity of Diospyros Blancoi seeds. Int J Fruit Sci 20:S1192–S1200. https://doi.org/10.1080/15538362.2020.1782803s
Khan SA, AlKiyumi AR, AlSheidi MS et al (2016) In vitro inhibitory effects on α-glucosidase and α-amylase level and antioxidant potential of seeds of Phoenix dactylifera L. Asian Pac J Trop Biomed 6:322–329. https://doi.org/10.1016/j.apjtb.2015.11.008
Chandrasekara A, Rasek OA, John JA et al (2016) Solvent and extraction conditions control the assayable phenolic content and antioxidant activities of seeds of black beans, canola and millet. J Am Oil Chem Soc 93:275–283. https://doi.org/10.1007/s11746-015-2760-y
Shen L, JiH- F (2012) The pharmacology of curcumin: is it the degradation products? Trends Mol Med 18:138–144. https://doi.org/10.1016/j.molmed.2012.01.004
Al-Rawahi AS, Rahman MS, Guizani N, Essa MM (2013) Chemical composition, water sorption isotherm, and phenolic contents in fresh and dried pomegranate peels. Dry Technol 31:257–263. https://doi.org/10.1080/07373937.2012.710695
Cheung LM, Cheung PCK, Ooi VEC (2003) Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem 81:249–255. https://doi.org/10.1016/S0308-8146(02)00419-3s
Zhao M, Yang B, Wang J et al (2006) Identification of the major flavonoids from pericarp tissues of lychee fruit in relation to their antioxidant activities. Food Chem 98:539–544. https://doi.org/10.1016/j.foodchem.2005.06.028
Atik F, Mohammedi Z (2011) Impact of solvent extraction type on total polyphenols content and biological activity from Tamarix aphylla (L.) Karst. Int J Pharma Bio Sci 2:609–615
La J, Kim M-J, Lee J (2021) Evaluation of solvent effects on the DPPH reactivity for determining the antioxidant activity in oil matrix. Food Sci Biotechnol 30:367–375. https://doi.org/10.1007/s10068-020-00874-9
Victor NE, Justina YT, Sunday AM, Aderonke IO (2012) DPPH radical scavenging capacity of phenolic extracts from African yam bean (Sphenostylis stenocarpa). Food Nutr Sci 2012:. https://doi.org/10.4236/fns.2012.31002
VanTai N, Linh MN, Thuy NM (2021) Optimization of extraction conditions of phytochemical compounds in “Xiem” banana peel powder using response surface methodology. J Appl Biol Biotechnol 9:56–62. https://doi.org/10.7324/JABB.2021.9607
Cracolice MS, Peters EI (2009) Introductory chemistry: an active learning approach: Cengage Learning. Inc, singapore 615–618
Silva EM, Souza JNS, Rogez H et al (2007) Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem 101:1012–1018. https://doi.org/10.1016/j.foodchem.2006.02.055
Pinelo M, DelFabbro P, Manzocco L et al (2005) Optimization of continuous phenol extraction from Vitis vinifera byproducts. Food Chem 92:109–117. https://doi.org/10.1016/j.foodchem.2004.07.015s
Karasu Ç, Cumao\uglu A, Gürpinar AR, et al (2012) Aldose reductase inhibitory activity and antioxidant capacity of pomegranate extracts. Interdiscip Toxicol 5:15. https://doi.org/10.2478/v10102-012-0003-8
Tzulker R, Glazer I, Bar-Ilan I et al (2007) Antioxidant activity, polyphenol content, and related compounds in different fruit juices and homogenates prepared from 29 different pomegranate accessions. J Agric Food Chem 55:9559–9570. https://doi.org/10.1021/jf071413n
Gil MI, Tomás-Barberán FA, Hess-Pierce B et al (2000) Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem 48:4581–4589. https://doi.org/10.1021/jf000404a
Hossain MA, Arafat MY, Alam M, Hossain MM (2021) Effect of solvent types on the antioxidant activity and total flavonoids of some Bangladeshi legumes. Food Res 5:329–335. https://doi.org/10.26656/fr.2017.5(4).035
Memon RA, Bhatti GR, Khalid S et al (2007) A survey of weeds found in cotton fields of the Khairpur district, Sindh, Pakistan. Pak J Bot 39:2265–2274
Dailey A, Vuong QV (2015) Effect of extraction solvents on recovery of bioactive compounds and antioxidant properties from macadamia (Macadamia tetraphylla) skin waste. Cogent Food \& Agric 1:1115646. https://doi.org/10.1080/23311932.2015.1115646
Zazouli S, Chigr M, Jouaiti A (2016) Effect of polar and nonpolar solvent on total phenolic and antioxidant activity of roots extracts of Caralluma europaea. Der Pharma Chem 8:191–196s
Yim HS, Chye FY, Rao V et al (2013) Optimization of extraction time and temperature on antioxidant activity of Schizophyllum commune aqueous extract using response surface methodology. J Food Sci Technol 50:275–283. https://doi.org/10.1007/s13197-011-0349-5
González-Montelongo R, Lobo MG, González M (2010) The effect of extraction temperature, time and number of steps on the antioxidant capacity of methanolic banana peel extracts. Sep Purif Technol 71:347–355. https://doi.org/10.1016/j.seppur.2009.12.022
Olczyk P, Komosinska-Vassev K, Ramos P et al (2017) Free radical scavenging activity of drops and spray containing propolis—an EPR examination. Molecules 22:128. https://doi.org/10.3390/molecules22010128
Permukaan KGB (2021) Optimization of extraction temperature and time on phenolic compounds and antioxidant activity of Malaysian propolis Trigona spp. aqueous extract using response surface methodology. Malaysian J Anal Sci 25:649–660
Erdogan S, Ates B, Durmaz G et al (2011) Pressurized liquid extraction of phenolic compounds from Anatolia propolis and their radical scavenging capacities. Food Chem Toxicol 49:1592–1597. https://doi.org/10.1016/j.fct.2011.04.006
Yusof N, Munaim MSA, Veloo Kutty R (2021) Optimization of total phenolic compounds extracted from propolis by ultrasound-assisted extraction. Chem Eng Commun 208:564–572. https://doi.org/10.1080/00986445.2020.1761799
Mashkor AL (2014) Phenolic content and antioxidant activity of fenugreek seeds extract. Int J Pharmacogn Phytochem Res 6:841–844
Noureen S, Noreen S, Ghumman SA et al (2018) Seeds of giant dodder (Cuscuta reflexa) as a function of extract procedure and solvent nature. Not Bot Horti Agrobot Cluj-Napoca 46:653–662. https://doi.org/10.15835/nbha46211088
Thouri A, Chahdoura H, ElArem A et al (2017) Effect of solvents extraction on phytochemical components and biological activities of Tunisian date seeds (var. Korkobbi and Arechti). BMC Complement Altern Med 17:1–10. https://doi.org/10.1186/s12906-017-1751-y
Nakilciouglu-Tacs E, Ötlecs S (2021) Influence of extraction solvents on the polyphenol contents, compositions, and antioxidant capacities of fig (Ficus carica L.) seeds. An Acad Bras Cienc 93:. https://doi.org/10.1590/0001-3765202120190526
Kim G-H, Duan Y, Lee S-C, Kim H-S (2016) Assessment of antioxidant activity of garlic (Allium sativum L.) peels by various extraction solvents. J Korean Appl Sci Technol 33:204–212. https://doi.org/10.12925/jkocs.2016.33.1.204
Jarungjitaree P, Naradisorn M (2019) Evaluation of antioxidant and antifungal activities of pumpkin by-product and its application in banana. J Food Sci Agric Technol 4:129–133
Hassan IM, Ibrahim HM, Abdel Fattah A et al (2017) Citrus sinensis and Citrus aurantiifolia peel extracts: antibacterial, antioxidant activity and total phenolic. Int J Curr Microbiol App Sci 6:3983–3998
Magangana TP, Makunga NP, Amos Fawole O, Opara UL (2021) Effect of solvent extraction and blanching pre-treatment on phytochemical, antioxidant properties, enzyme inactivation and antibacterial activities of ‘Wonderful’ pomegranate peel extracts. Processes 9:1012. https://doi.org/10.3390/pr9061012
Srivastava R, Mishra N, Mishra N (2021) Evaluation of the extraction hours and solvent concentrations on secondary metabolites and antioxidant activity of Feronialimonia fruit. Indian J Nat Prod Resour 12:463–471
SulaimanIS C, BasriM FM et al (2017) Effects of temperature, time, and solvent ratio on the extraction of phenolic compounds and the anti-radical activity of Clinacanthus nutans Lindau leaves by response surface methodology. Chem Cent J 11:1–11. https://doi.org/10.1186/s13065-017-0285-1
Taylor R (1990) Interpretation of the correlation coefficient: a basic review. J diagnostic Med Sonogr 6:35–39. https://doi.org/10.1177/875647939000600106
Mohd-Esa N, Hern FS, Ismail A, Yee CL (2010) Antioxidant activity in different parts of roselle (Hibiscus sabdariffa L.) extracts and potential exploitation of the seeds. Food Chem 122:1055–1060. https://doi.org/10.1016/j.foodchem.2010.03.074
Liu Q, Tang G-Y, Zhao C-N et al (2018) Comparison of antioxidant activities of different grape varieties. Molecules 23:2432. https://doi.org/10.3390/molecules23102432
Ghasemi K, Ghasemi Y, Ebrahimzadeh MA (2009) Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak J Pharm Sci 22:277–281
Zarfeshany A, Asgary S, Javanmard SH (2014) Potent health effects of pomegranate. Adv Biomed Res 3:. 10.4103%2F2277–9175.129371
Zaporozhets OA, Krushynska OA, Lipkovska NA, Barvinchenko VN (2004) A new test method for the evaluation of total antioxidant activity of herbal products. J Agric Food Chem 52:21–25. https://doi.org/10.1021/jf0343480
Pochapski MT, Fosquiera EC, Esmerino LA, et al (2011) Phytochemical screening, antioxidant, and antimicrobial activities of the crude leaves’ extract from Ipomoea batatas (L.) Lam. Pharmacogn Mag 7:165. https://doi.org/10.4103/0973-1296.80682
Kim J, Choi JN, Kang D, et al (2011) Correlation between antioxidative activities and metabolite changes during Cheonggukjang fermentation. Biosci Biotechnol Biochem 1102282394. https://doi.org/10.1271/bbb.100858
Xu BJ, Chang SKC (2007) A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J Food Sci 72:S159–S166. https://doi.org/10.1111/j.1750-3841.2006.00260.xs
Acknowledgements
The authors express their gratitude to the Department of Food Engineering and Tea Technology and Department of Chemistry of Shahjalal University of Science and Technology, Sylhet, Bangladesh, for continuous material support and technical assistance.
Author information
Authors and Affiliations
Contributions
Md. Ar Rafi Himel: methodology, formal data analysis, writing—original draft.
Tanvir Ahmed: methodology, data calculation, writing—original draft.
Mohammad Afzal Hossain: conceptualization, investigation, methodology, validation, data curation, formal data analysis, visualization, project administration, writing—review and editing.
Md. Shakir Moazzem: methodology, formal data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Himel, M.A.R., Ahmed, T., Hossain, M.A. et al. Response surface optimization to extract antioxidants from freeze-dried seeds and peel of pomegranate (Punica granatum L.). Biomass Conv. Bioref. 14, 9707–9722 (2024). https://doi.org/10.1007/s13399-022-03074-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03074-z