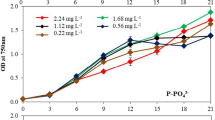
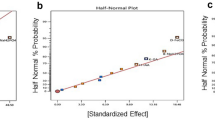
Abstract
Improved productivities of microalgal biomass tend to play a significant role in biorefineries pertaining to multifaceted applications and the inadequate biomass yield in any particular medium is a bottleneck that must be overcome to achieve such sustainability goals. In our present study, we employed new approach to enhance the cell growth of a potential strain Chlorella saccharophila (UTEX 247), i.e., media engineering perspective. For better biomass yields, the fundamental constituents are the macronutrients within the growth medium consisting of nitrogen (as NaNO3, sodium nitrate), phosphorus (as K2HPO4, dipotassium phosphate) with an additional source of carbon supplementation in the form of NaHCO3, sodium bicarbonate. Our preliminary studies by One Factor at a Time demonstrated no effect on growth with additional carbon supplementation but showed that nitrogen and phosphorus ratios play a significant role in the biomass production. Furthermore, we optimized the biomass yields employing the central composite design associated with the response surface methodology tool to illustrate the combinatorial effects of nitrogen (N) and phosphorous (P). Our results have showed an increase up to 131% dcw in biomass production, i.e., 0.84 g L−1 DCW with 26.4 mM and 0.11 mM of NaNO3 and K2HPO4 concentrations, respectively, than the control condition (NaNO3: 17.6 mM; K2HPO4: 0.23 mM) yielding a biomass content of 0.64 g L−1 DCW with a coefficient of variance of 5.12%. In conclusion, the new perspective of media engineering predicts and also evaluates the best condition for the specific strain of interest so that the optimized medium essentially produces higher cell biomass along with other biocommodities of industrial significance.



Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- RSM:
-
Response surface methodology
- CCD:
-
Central composite design
- N:
-
Nitrogen
- P:
-
Phosphorus
- NaNO3:
-
Sodium nitrate
- KH2PO4:
-
Phosphate
- NaHCO3:
-
Bicarbonate
- CV:
-
Coefficient of variance
- EDTA:
-
Fe-Ethylenediaminetetraacetic acid
- O.D. :
-
Optical density
- SPV:
-
Sulpho-phospho-vanillin
- PAM:
-
Pulse amplitude modulation
- ANOVA:
-
Analysis of variance
- VP:
-
Validation point
References
Liu J, Song Y, Qiu W (2017) Oleaginous microalgae Nannochloropsis as a new model for biofuel production: review & analysis. Renew Sust Energ Rev 72:154–162. https://doi.org/10.1016/j.rser.2016.12.120
Kabir G, Hameed B (2017) Recent progress on catalytic pyrolysis of lignocellulosic biomass to high-grade bio-oil and bio-chemicals. Renew Sust Energ Rev 70:945–967. https://doi.org/10.1016/j.rser.2016.12.001
Katiyar R, Gurjar B, Biswas S, Pruthi V, Kumar N, Kumar P (2017) Microalgae: an emerging source of energy based bio-products and a solution for environmental issues. Renew Sus Energ Rev 72:1083–1093. https://doi.org/10.1016/j.rser.2016.10.028
Kumar S, Shrestha P, Salam PA (2013) A review of biofuel policies in the major biofuel producing countries of ASEAN: production, targets, policy drivers and impacts. Renew Sust Energ Rev 26:822–836. https://doi.org/10.1016/j.rser.2013.06.007
Su Y, Zhang P, Su Y (2015) An overview of biofuels policies and industrialization in the major biofuel producing countries. Renew Sust Energ Rev 50:991–1003. https://doi.org/10.1016/j.rser.2015.04.032
Manirafasha E, Ndikubwimana T, Zeng X, Lu Y, Jing K (2015) Phycobiliprotein: potential microalgae derived pharmaceutical and biological reagent. Biochem Eng J 109:282–296. https://doi.org/10.1016/j.bej.2016.01.025
Song C, Liu Q, Ji N, Deng S, Zhao J, Li S, Kitamura Y (2015) Evaluation of hydrolysis-esterification biodiesel production from wet microalgae. Bioresour Technol 214:747–754. https://doi.org/10.1016/j.biortech.2016.05.024
Noraini M, Ong HC, Badrul MJ, Chong W (2015) A review on potential enzymatic reaction for biofuel production from algae. Renew Sust Energ Rev 39:24–34. https://doi.org/10.1016/j.rser.2014.07.089
Bhuiya M, Rasul M, Khan M, Ashwath N, Azad A (2015) Prospects of 2nd generation biodiesel as a sustainable fuel-Part: 1 selection of feedstocks, oil extraction techniques and conversion technologies. Renew Sust Energ Rev 55:1109–1128. https://doi.org/10.1016/j.rser.2015.04.163
Moncada J, Tamayo JA, Cardona CA (2015) Integrating first, second, and third generation biorefineries: incorporating microalgae into the sugarcane biorefinery. Chem Eng Sci 118:126–140. https://doi.org/10.1016/j.ces.2014.07.035
Caporgno MP, Mathys A (2018) Trends in microalgae incorporation into innovative food products with potential health benefits. Front Nutr 5:1–15. https://doi.org/10.3389/fnut.2018.00058
Matos ÂP (2017) The impact of microalgae in food science and technology. J Am Oil Chem Soc 94:1333–1350. https://doi.org/10.1007/s11746-017-3050-7
Enzing C, Ploeg M, Barbosa M, Sijtsma L, authors Vigani M, Parisi C, Rodriguez Cerezo E, editors. Microalgae-based products for the food and feed sector: an outlook for Europe. EUR 26255. Luxembourg (Luxembourg): Publications Office of the European Union; 2014. JRC85709. https://doi.org/10.2791/3339.
Wijffels RH, Barbosa MJ, Eppink MH (2015) Microalgae for the production of bulk chemicals and biofuels. Biofuel Bioprod Biorefin 4:287–295. https://doi.org/10.1002/bbb.215
Mondal M, Ghosh A, Tiwari O, Gayen K, Das P, Mandal M, Halder G (2017) Influence of carbon sources and light intensity on biomass and lipid production of Chlorella sorokiniana BTA 9031 isolated from coalfield under various nutritional modes. Energy Convers Manag 145:247–254. https://doi.org/10.1016/j.enconman.2017.05.001
Barlow J, Sims RC, Quinn JC (2015) Techno-economic and life-cycle assessment of an attached growth algal biorefinery. Bioresour Technol 220:360–368. https://doi.org/10.1016/j.biortech.2016.08.091
Mondal M, Ghosh A, Sharma AS, Tiwari O, Gayen K, Mandal M, Halder G (2015) Mixotrophic cultivation of Chlorella sp. BTA 9031 and Chlamydomonas sp. BTA 9032 isolated from coal field using various carbon sources for biodiesel production. Energy Convers Manag 124:297–304. https://doi.org/10.1016/j.enconman.2016.07.033
Safi C, Zebib B, Merah O, Pontalier P-Y, Vaca-Garcia C (2015) Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew Sust Energ Rev 35:265–278. https://doi.org/10.1016/j.rser.2014.04.007
Pratt R, Daniels T, Eiler JJ, Gunnison J, Kumler W, Oneto JF, Strait LA, Spoehr H, Hardin G, Milner H (1944) Chlorellin, an antibacterial substance from Chlorella. Science 99:351–352. https://doi.org/10.1126/science.99.2574.351
El-Sheekh M, Abu-Faddan M, Abo-Shady A, Nassar MZA, Labib W (2020) Molecular identification, biomass, and biochemical composition of the marine chlorophyte Chlorella sp. MF1 isolated from Suez Bay. J Genet Eng Biotechnol 8:27. https://doi.org/10.1186/s43141-020-00044-8
Gouveia L, Choubert G, Pereira N, Santinha J, Empis J, Gomes E (2002) Pigmentation of gilthead seabream, Sparus aurata (L. 1875), using Chlorella vulgaris (Chlorophyta, Volvocales) microalga. Aquac Res 33:987–993. https://doi.org/10.1046/j.1365-2109.2002.00751.x
Choix FJ, De-Bashan LE, Bashan Y (2012) Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: II Heterotrophic conditions. Enzyme Microb Technol 51:300–309. https://doi.org/10.1016/j.enzmictec.2012.07.013
Yeh KL, Chang JS (2011) Nitrogen starvation strategies and photobioreactor design for enhancing lipid content and lipid production of a newly isolated microalga Chlorella vulgaris ESP-31: implications for biofuels. Biotechnol J 6:1358–1366. https://doi.org/10.1002/biot.201000433
Ahmad S, Pathak VV, Kothari R, Kumar A, Krishna SBN (2018) Optimization of nutrient stress using C. pyrenoidosa for lipid and biodiesel production in integration with remediation in dairy industry wastewater using response surface methodology. 3 Biotech 8:326. https://doi.org/10.1007/s13205-018-1342-8
Yang J, Astatkie T, He QS (2015) A comparative study on the effect of unsaturation degree of camelina and canola oils on the optimization of bio-diesel production. Energy Rep 2:211–217. https://doi.org/10.1016/j.egyr.2016.08.003
Ahmad S, Kothari R, Pathania D, Tyagi V (2020) Optimization of nutrients from wastewater using RSM for augmentation of Chlorella pyrenoidosa with enhanced lipid productivity, FAME content, and its quality assessment using fuel quality index. Biomass Convers Bioref 10:495–512. https://doi.org/10.1007/s13399-019-00443-z
Saleem A, Hussain A, Chaudhary A, Q-u-A A, Iqtedar M, Javid A, Akram AM (2022) Acid hydrolysis optimization of pomegranate peels waste using response surface methodology for ethanol production. Biomass Convers Bioref 12:1513–1524. https://doi.org/10.1007/s13399-020-01117-x
Dahmen-Ben Moussa I, Masmoudi MA, Choura S, Chamkha M, Sayadi S (2021) Extraction optimization using response surface methodology and evaluation of the antioxidant and antimicrobial potential of polyphenols in Scenedesmus sp. and Chlorella sp. Biomass Convers Bioref. https://doi.org/10.1007/s13399-021-01850-x
Campos L, Moura HO, Cruz AJ, Assumpcao S, de Carvalho LS, Pontes LA (2020) Response surface methodology (RSM) for assessing the effects of pretreatment, feedstock, and enzyme complex association on cellulose hydrolysis. Biomass Convers Bioref. https://doi.org/10.1007/s13399-020-00756-4
Kirrolia A, Bishnoi NR, Singh R (2015) Response surface methodology as a decision-making tool for optimization of culture conditions of green microalgae Chlorella spp. for biodiesel production. Ann Microbiol 64:1133–1147. https://doi.org/10.1007/s13213-013-0752-4
Ghosh S, Roy S, Das D (2015) Improvement of biomass production by Chlorella sp. MJ 11/11 for use as a feedstock for biodiesel. Appl Biochem Biotechnol 175:3322–3335. https://doi.org/10.1007/s12010-015-1503-8
Ho S-H, Chen C-Y, Chang J-S (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour Technol 113:244–252. https://doi.org/10.1016/j.biortech.2011.11.133
Zheng Y, Chen Z, Lu H, Zhang W (2011) Optimization of carbon dioxide fixation and starch accumulation by Tetraselmis subcordiformis in a rectangular airlift photobioreactor. Afr J Biotechnol 10:1888–1901. https://doi.org/10.5897/AJB10.1620
Deamici KM, Santos LO, Costa JAV (2021) Magnetic field as promoter of growth in outdoor and indoor assays of Chlorella fusca. Bioprocess Biosyst Eng 44:1453–1460. https://doi.org/10.1007/s00449-021-02526-6
Cheng K-C, Ren M, Ogden KL (2013) Statistical optimization of culture media for growth and lipid production of Chlorella protothecoides UTEX 250. Bioresour Technol 128:44–48. https://doi.org/10.1016/j.biortech.2012.09.085
Mubarak M, Shaija A, Suchithra T (2019) Cost effective approach for production of Chlorella pyrenoidosa: a RSM based study. Waste Biomass Valori 10:3307–3319. https://doi.org/10.1007/s12649-018-0330-x
Singh R, Paliwal C, Nesamma AA, Narula A, Jutur PP (2020) Nutrient deprivation mobilizes the production of unique tocopherols as a stress-promoting response in a new indigenous isolate Monoraphidium sp. Front Mar Sci 7:575817. https://doi.org/10.3389/fmars.2020.575817
Paliwal C, Jutur PP (2021) Dynamic allocation of carbon flux triggered by task-specific chemicals is an effective non-gene disruptive strategy for sustainable and cost-effective algal biorefineries. Chem Eng J 418:129413. https://doi.org/10.1016/j.cej.2021.129413
Guillard RR, Sieracki MS (2005) Counting cells in cultures with the light microscope. In: Andersen RA (ed) Algal culturing techniques. Elsevier Academic Press, London, pp 239–252
Santos-Ballardo DU, Rossi S, Hernández V, Gómez RV, del Carmen R-U, Caro-Corrales J, Valdez-Ortiz A (2015) A simple spectrophotometric method for biomass measurement of important microalgae species in aquaculture. Aquac Res 448:87–92. https://doi.org/10.1016/j.aquaculture.2015.05.044
Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76:965–977. https://doi.org/10.1016/j.talanta.2008.05.019
Yaakob MA, Mohamed RMSR, Al-Gheethi A, Ravishankar GA, Ambati RR (2021) Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: an overview. Cells 10:393. https://doi.org/10.3390/cells10020393
Box GE, Wilson KB. (1992) On the experimental attainment of optimum conditions. Breakthroughs in statistics, Springer Series in Statistics. Springer, New York, NY, pp. 270–310. https://doi.org/10.1007/978-1-4612-4380-9_23.
Slathia PS, Raina N, Kiran A, Kour R, Bhagat D, Sharma P (2020) Dilute acid pretreatment of pine needles of Pinus roxburghii by response surface methodology for bioethanol production by separate hydrolysis and fermentation. Biomass Convers Bioref 10:95–106. https://doi.org/10.1007/s13399-019-00433-1
Baş D, Boyacı IH (2007) Modeling and optimization I: usability of response surface methodology. J Food Eng 78:836–845. https://doi.org/10.1016/j.jfoodeng.2005.11.024
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Meth Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Mishra SK, Suh WI, Farooq W, Moon M, Shrivastav A, Park MS, Yang J-W (2015) Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour Technol 155:330–333. https://doi.org/10.1016/j.biortech.2013.12.077
Zhou Y, Schideman L, Park D, Stirbet A, Rupassara S, Krehbiel J, Seufferheld M (2015) Characterization of a Chlamydomonas reinhardtii mutant strain with improved biomass production under low light and mixotrophic conditions. Algal Res 11:134–147. https://doi.org/10.1016/j.algal.2015.06.001
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113. https://doi.org/10.1146/annurevplant59.032607.092759
Agarwal A, Patil S, Gharat K, Pandit RA, Lali AM (2019) Modulation in light utilization by a microalga Asteracys sp. under mixotrophic growth regimes. Photosynth res 139:553–567. https://doi.org/10.1007/s11120-018-0526-8
Khuri AI, Mukhopadhyay S (2015) Response surface methodology. Wiley Interdiscip Rev Comput Stat 2:128–149. https://doi.org/10.1002/wics.73
McGaughy K, Abu Hajer A, Drabold E, Bayless D, Reza MT (2019) Algal remediation of wastewater produced from hydrothermally treated septage. Sustainability 11:3454. https://doi.org/10.3390/su11123454
Chinnasamy S, Ramakrishnan B, Bhatnagar A, Das KC (2009) Biomass production potential of a wastewater alga Chlorella vulgaris ARC 1 under elevated levels of CO2 and temperature. Int J Mol Sci 10:518–532. https://doi.org/10.3390/ijms10020518
Zhang X, Yuan H, Guan L, Wang X, Wang Y, Jiang Z, Cao L, Zhang X (2019) Influence of photoperiods on microalgae biofilm: photosynthetic performance, biomass yield, and cellular composition. Energies 12:3724. https://doi.org/10.3390/en12193724
Romanowska-Duda Z, Szufa S, Grzesik M, Piotrowski K, Janas R (2021) The promotive effect of cyanobacteria and Chlorella sp. foliar biofertilization on growth and metabolic activities of willow (Salix viminalis l.) plants as feedstock production, solid biofuel and biochar as C carrier for fertilizers via torrefaction process. Energies 14:5262. https://doi.org/10.3390/en14175262
Doušková I, Kaštánek F, Maléterová Y, Kaštánek P, Doucha J, Zachleder V (2015) Utilization of distillery stillage for energy generation and concurrent production of valuable microalgal biomass in the sequence: biogas-cogeneration-microalgae-products. Energy Convers Manag 51:606–611. https://doi.org/10.1016/j.enconman.2009.11.008
Chia MA, Lombardi AT, Melao MDGG (2013) Growth and biochemical composition of Chlorella vulgaris in different growth media. An Acad Bras Cienc 85:1427–1438. https://doi.org/10.1590/0001-3765201393312
Blair MF, Kokabian B, Gude VG (2015) Light and growth medium effect on Chlorella vulgaris biomass production. J Environ Chem Eng 2:665–674. https://doi.org/10.1016/j.jece.2013.11.005
Couto C, Hernández CP, Alves Sobrinho RCM, Mendes CRB, Roselet F, Abreu PC (2021) Optimization of a low-cost fertilizer-based medium for large-scale cultivation of the coastal diatom Conticribra weissflogii using response surface methodology and its effects on biomass composition. J Appl Phycol 33:2767–2781. https://doi.org/10.1007/s10811-021-02519-8
Costa SS, Miranda AL, Andrade BB, de Jesus AD, Souza CO, de Morais MG, Costa JAV, Druzian JI (2018) Influence of nitrogen on growth, biomass composition, production, and properties of polyhydroxyalkanoates (PHAs) by microalgae. Int J Biol Macromol 116:552–562. https://doi.org/10.1016/j.ijbiomac.2018.05.064
Arumugam M, Agarwal A, Arya MC, Ahmed Z (2013) Influence of nitrogen sources on biomass productivity of microalgae Scenedesmus bijugatus. Bioresour Technol 131:246–249. https://doi.org/10.1016/j.biortech.2012.12.159
Solovchenko A, Khozin-Goldberg I, Selyakh I, Semenova L, Ismagulova T, Lukyanov A, Mamedov I, Vinogradova E, Karpova O, Konyukhov I (2019) Phosphorus starvation and luxury uptake in green microalgae revisited. Algal Res 43:101651. https://doi.org/10.1016/j.algal.2019.101651
Lavrinovičs A, Murby F, Zīverte E, Mežule L, Juhna T (2021) Increasing phosphorus uptake efficiency by phosphorus-starved microalgae for municipal wastewater post-treatment. Microorganisms 9:1598. https://doi.org/10.3390/microorganisms9081598
Singh P, Kumar D (2021) Biomass and lipid productivities of cyanobacteria-Leptolyngbya foveolarum HNBGU001. BioEnergy Res 14:278–291. https://doi.org/10.1007/s12155-020-10170-3
Suthar S, Verma R (2018) Production of Chlorella vulgaris under varying nutrient and abiotic conditions: a potential microalga for bioenergy feedstock. Process Saf Environ Prot 113:141–148. https://doi.org/10.1016/j.psep.2017.09.018
Hernandez J-P, de-Bashan LE, Bashan Y (2006) Starvation enhances phosphorus removal from wastewater by the microalga Chlorella spp. co-immobilized with Azospirillum brasilense. Enzyme Microb Technol 38:190–198. https://doi.org/10.1016/j.enzmictec.2005.06.005
Powell N, Shilton AN, Pratt S, Chisti Y (2008) Factors influencing luxury uptake of phosphorus by microalgae in waste stabilization ponds. Environ Sci Technol 42:5958–5962. https://doi.org/10.1021/es703118s
Achbergerová L, Nahálka J (2011) Polyphosphate-an ancient energy source and active metabolic regulator. Microb cell factories 10:63. https://doi.org/10.1186/1475-2859-10-63
Ernst A, Deicher M, Herman PM, Wollenzien UI (2005) Nitrate and phosphate affect cultivability of cyanobacteria from environments with low nutrient levels. Appl Environ Microbiol 71:3379–3383. https://doi.org/10.1128/aem.71.6.3379-3383.2005
Zhang Q, Hu G (2011) Effect of nitrogen to phosphorus ratios on cell proliferation in marine micro algae. Chin J Oceanol Limnol 29:739–745. https://doi.org/10.1007/s00343-011-0503-y
Molina E, Martínez E, Sańchez S, García F, Contreras A (1991) The influence of temperature and the initial N: P ratio on the growth of microalgae Tetraselmis sp. Process Biochem 26:183–187. https://doi.org/10.1016/0032-9592(91)80016-I
Armitage AR, Frankovich TA, Heck KL, Fourqurean JW (2005) Experimental nutrient enrichment causes complex changes in seagrass, microalgae, and macroalgae community structure in Florida Bay. Estuaries 28:422–434. https://doi.org/10.1007/BF02693924
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 111:1–61. https://doi.org/10.1099/00221287-111-1-1
Thomas DL, Mantes JG (1978) Spectrophotometrically assayed inhibitory effects of mercuric compounds on anabaena flos-aquae and anacystis nidulans (cyanophyceae). J Phycol 14:494–499. https://doi.org/10.1111/j.1529-8817.1978.tb02475.x
Cordeiro RS, Vaz IC, Magalhaes S, Barbosa FA (2017) Effects of nutritional conditions on lipid production by cyanobacteria. An Acad Bras Cienc 89:2021–2031. https://doi.org/10.1590/0001-3765201720150707
White D, Pagarette A, Rooks P, Ali S (2013) The effect of sodium bicarbonate supplementation on growth and biochemical composition of marine microalgae cultures. J Appl Phycol 25:153–165. https://doi.org/10.1007/s10811-012-9849-6
Nayak M, Suh WI, Lee B, Chang YK (2018) Enhanced carbon utilization efficiency and FAME production of Chlorella sp. HS2 through combined supplementation of bicarbonate and carbon dioxide. Energy Convers Manag 156:45–52. https://doi.org/10.1016/j.enconman.2017.11.002
Richmond A, Karg S, Boussiba SJP (1982) Effects of bicarbonate and carbon dioxide on the competition between Chlorella vulgaris and Spirulina platensis. Plant Cell Physiol 23:1411–1417. https://doi.org/10.1093/oxfordjournals.pcp.a076489
Chi Z, Elloy F, Xie Y, Hu Y, Chen S (2015) Selection of microalgae and cyanobacteria strains for bicarbonate-based integrated carbon capture and algae production system. Appl Biochem Biotechnol 172:447–457. https://doi.org/10.1007/s12010-013-0515-5
Zarrinmehr MJ, Farhadian O, Heyrati FP, Keramat J, Koutra E, Kornaros M, Daneshvar E (2020) Effect of nitrogen concentration on the growth rate and biochemical composition of the microalga, Isochrysis galbana. Egypt J Aquat Res 46:153–158. https://doi.org/10.1016/j.ejar.2019.11.003
Taziki M, Ahmadzadeh H, Murry MA (2015) Growth of Chlorella vulgaris in high concentrations of nitrate and nitrite for wastewater treatment. Curr Biotechnol 4:441–447. https://doi.org/10.2174/2211550104666150930204835
Aguirre AM, Bassi A (2013) Investigation of biomass concentration, lipid production, and cellulose content in Chlorella vulgaris cultures using response surface methodology. Biotechnol Bioeng 110:2114–2122. https://doi.org/10.1002/bit.24871
Kim S, Lee Y, Hwang S-J (2013) Removal of nitrogen and phosphorus by Chlorella sorokiniana cultured heterotrophically in ammonia and nitrate. Int Biodeterior Biodegr 85:511–516. https://doi.org/10.1016/j.ibiod.2013.05.025
Rodrigues-Sousa AE, Nunes IV, Muniz-Junior AB, Carvalho JCM, Mejia-da-Silva LC, Matsudo MC (2021) Nitrogen supplementation for the production of Chlorella vulgaris biomass in secondary effluent from dairy industry. Biochem Eng J 165:107818. https://doi.org/10.1016/j.bej.2020.107818
Davis R, Aden A, Pienkos PT (2011) Techno-economic analysis of autotrophic microalgae for fuel production. Appl Energy 88:3524–3531. https://doi.org/10.1016/j.apenergy.2011.04.018
Hildebrand M, Abbriano RM, Polle JE, Traller JC, Trentacoste EM, Smith SR, Davis AK (2013) Metabolic and cellular organization in evolutionarily diverse microalgae as related to biofuels production. Curr Opin Chem Biol 17:506–514. https://doi.org/10.1016/j.cbpa.2013.02.027
Acknowledgements
Senior Research Fellowships for A.M. supported by the Council for Scientific Research (CSIR) (Award No. 09/512(0232)/2017-EMR-I), New Delhi, India, and funding by the Department of Biotechnology (DBT) (Ref ID: DBT/2017/ICGEB/930), New Delhi, India, for S.U.Z. are duly acknowledged.
Funding
The work was supported by the grants provided from the Department of Biotechnology (DBT) and Biotechnology Industry Research Assistance Council (BIRAC), India (Grant No. BT/PB/Center/03/2011-Phase II, BT/SB0078/02/19).
Author information
Authors and Affiliations
Contributions
Conceptualization, analysis, writing—original draft, A.M., S.U.Z; conceptualization, funding acquisition, supervision, project administration, writing—review and editing, P.P.J. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehra, A., Zafar, S.U. & Jutur, P.P. Optimization of biomass production by Chlorella saccharophila UTEX 247 employing response surface methodology. Biomass Conv. Bioref. 14, 8549–8561 (2024). https://doi.org/10.1007/s13399-022-02966-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02966-4