Abstract
Sugar beet pulp (SBP) is a by-product formed in large amounts during the production of refined sugar and is currently used as a low-cost feed for livestock. The dry SBP comprises largely three polysaccharides (75–85%), cellulose, hemicellulose and pectin, whose component sugars constitute potential valuable feedstock for producing building blocks for chemicals and materials. In the present study, a simple and integrated process for the fractionation of SBP polysaccharides into streams enriched with corresponding sugars is developed. Initial screening of several pectinase preparations (from Novozymes) for the treatment of dry SBP (at initial pH 4.0, 50 °C) showed Pectinase ME to release the maximum amount of galacturonic acid (GalU) along with high amounts of arabinose (Ara). On the other hand, subjecting the SBP to acid hydrolysis using 0.64 M sulphuric acid at 80 °C was relatively selective in solubilising mainly Ara (71.3% of the initial content), and the subsequent treatment with Pectinase ME solubilised 68.5% of the initial GalU content. Treatment of the residual solid fraction with cellulases released 81.9% of the original glucose content. Size-exclusion chromatography revealed the presence of oligomers ranging from dimers to pentamers in the acid and pectinase hydrolysates. Mass-balance based process analysis of 1000 kgdry/h SBP biorefinery using the three-stage fractionation of sugars and further valorization to arabitol, mucic acid and levulinic acid, respectively, indicated the potential economic feasibility and value addition of SBP, which is currently sold at 200 US$/t as animal feed. Recycling of water and catalysts would be important for reducing waste generation and improving environmental performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The increasing demand for sustainable and bio-based alternatives for petroleum-based chemicals has led to global interest in biorefineries that use residual biomass as feedstock. One such residual stream that has aroused interest for its valorisation potential is sugar beet pulp (SBP), a side-product generated from the industrial production of refined sugar [1, 2]. The European Union is the world-leading producer of beet sugar (19 Mtonnes in 2019/2020), producing about 3.7 Mtonnes of dried SBP [3]. In the same campaign in Sweden, SBP dry matter accounted for 63,000 tonnes obtained from 2.03 Mtonnes sugar beet production [4]. To date, SBP is mainly sold as low-cost animal feed and entails high drying cost [2, 5, 6]. Hence, processing of SBP with resultant added value would be useful for the sugar industry.
On a dry-weight basis, SBP consists primarily of polymeric carbohydrates (75–85% w/w), including 20–25% cellulose (polymer of glucose), 25–36% hemicelluloses (containing mainly arabinose and minor amounts of xylose, mannose and galactose) and 20–25% pectin (having primarily galacturonic acid units), with low lignin content (1–3%) [5, 6], making it an ideal feedstock for the production of biofuels and biochemicals [5, 7, 8]. Additionally, SBP contains about 10–15% protein content [5] and < 1% ferulic acid [9]. Previous studies on SBP utilisation have focused on the solubilisation of the biomass in order to facilitate its fermentation to produce biogas [10], ethanol [11], acetone-butanol-ethanol [12], lactic acid and propylene glycol [13]. Several methods of pre-treatment including steam explosion, hydrothermal treatment, enzymatic hydrolysis, acid hydrolysis and alkaline hydrolysis, and their combinations have been investigated and optimised, depending on the target chemicals to be produced [10, 11, 14].
In order to achieve high resource efficiency, it is important that the sugars present in significant amounts (glucose, arabinose and galacturonic acid) in the hydrolysate are utilised to yield useful products. This may be achieved, e.g. by genetically modifying the microorganisms to enable the utilisation of different sugars, while an alternative is to fractionate the sugars into separate streams that can then be valorised individually into the products of interest to develop a cost-effective biorefinery. Glucose is readily utilised by various microorganisms as a substrate for conversion into a wide range of useful products, such as organic acids and alcohols [15,16,17]. Glucose can also be transformed by chemical catalysis to high-value platform chemicals, such as 5-hydroxymethylfurfural [18] and levulinic acid [19, 20]. L-Arabinose is itself a potentially valuable product for its beneficial effects on lowering blood glucose and insulin responses, when fed together with a sugar and starch rich diets [21]. It can also be converted to other chemicals; an interesting example being arabitol obtained by chemical hydrogenation or yeast fermentation [22,23,24]. L-Arabitol is of interest as a potential sweetener with low glycoemic, antcariogenic and prebiotic effects [24] and is also identified to be among the top 12 value added chemicals from biomass, with potential for transformation to other functional derivatives [20, 24, 25]. More recent studies have shown the biocatalytic conversion of arabinose using transketolases to pharmaceutically active compounds [26, 27]. Galacturonic acid has been used as a substrate for the production of polymer building blocks, adipic acid and 2,5-furan dicarboxylic acid (FDCA) via mucic acid through chemical/biological processes [28, 29]. Even the mucic acid itself can be used as a monomer for bio-based polyesters [30].
The concept of a biorefinery based on SBP has been explored in a number of studies [14, 27, 31]. Use of centrifugal partition chromatography (CPC) to simultaneously separate the sugars in SBP (after steam explosion followed by acid/hydrothermal treatment) in one step using ethanol-ammonium sulphate solution as liquid stationary phase has been reported [27, 31]. Alexandri et al. [14] proposed a SBP processing strategy to produce succinic acid, pectins and phenolic compounds, which indicated profitability potential according to a preliminary assessment. An early stage techno-economic assessment of emerging biorefinery processes is encouraged to consider whether a developed process strategy has the potential for economic viability or not [32,33,34,35].
In this study, the effect of acid and enzymatic hydrolysis of polysaccharides on the release of sugars from milled SBP was monitored to propose a simple route for its processing to obtain streams enriched in the respective sugars, arabinose, galacturonic acid and glucose. The conditions providing the highest yields of sugars in different fractions were tested in lab-scale. Owing to our interest in biobased polymer building blocks, a conceptual design of a SBP-based biorefinery was developed for further transformation of the sugars to arabitol, mucic acid and levulinic acid, respectively. Preliminary technical feasibility and environmental impact of the proposed biorefinery was assessed by the determination of mass, energy and sustainability indicators. Stoichiometric-economic targeting was also performed in order to assess the potential economic viability of the process.
2 Materials and methods
2.1 Materials
SBP with a dry matter content of 25.4% (w/w) was received from Nordic Sugar A/S (Copenhagen, Denmark) from the campaign 2016/2017 and was stored at − 20 °C. Commercial enzymes were kindly provided by Novozymes (Bagsvaerd, Denmark): Viscozyme Flex (800 fungal xylanase unit (FXU)/g), Cellic CTEC2 (223 filter paper unit (FPU)/mL), Pectinase ME111 GLY PF (20 200 pectinase unit (PECTU)/g), Pectinase ARA GLY PF (32 400 polygalacturonase unit (PGNU)/g), Pectinex® UF (3300 PGNU/g) and Neopectinase — TLG PF (300 pectinase unit (PL)/g, with 5% w/w enzyme content and density 1.17 g/mL. Analytical grade D-glucose, D-xylose, L-arabinose, D-galacturonic acid, D-mannose, D-galactose and D-galacturonic acid were purchased from Sigma-Aldrich Merck (Darmstadt, Germany). All other chemicals were used as received without further purification, unless otherwise stated.
2.2 Compositional analysis of SBP
For compositional analysis of SBP and the screening experiments for acid and enzymatic hydrolysis at 30 mL scale, the pulp was prepared by drying at 45 °C for at least 24 h prior to milling (0.5 mm mesh size). The milled SBP was stored at − 20 °C until further use. The dry matter content of SBP was obtained based on gravimetry, by measuring the mass before and after drying at 105 °C until constant weight was reached.
Extractives or non-structural material of SBP were recovered according to the laboratory analytical procedure (LAP) of National Renewable Energy Laboratory (NREL) [36]. Water extraction followed by ethanol extraction was carried out in a Büchi B-811 Soxhlet apparatus (Flawil, Switzerland), while evaporation of the extracts was carried out in a Büchi Multivapor™ equipment (Flawil, Switzerland). Ash content, acid-soluble and acid-insoluble lignin and structural carbohydrates were determined according to the NREL LAP for determination of structural carbohydrates and lignin in biomass [37].
Protein extraction and determination were performed based on a modified version of the technique used by Hamley-Bennett et al. [11]. Untreated and extractive-free SBP samples, the insoluble fraction from acid-hydrolysed SBP and the insoluble residue from enzyme-treated SBP were dried at 45 °C for 24 h. Protein extraction was carried out by incubating 100–300 mg of the solid sample in 4.5 mL 0.1 M NaOH at 80 °C for 3 h. Total protein determination was conducted using a Quick Start™ Bradford protein assay (Bio-Rad, CA, USA) against a calibration curve made with bovine serum albumin (BSA) standard diluted in 0.1 M NaOH.
2.3 Acid hydrolysis of SBP
SBP (66 g dry weight/L) was treated with 30 mL of 0.32 M HCl or 0.64 M H2SO4 in 50 mL Erlenmeyer flasks with magnetic stirring at 80 °C water bath for 2–7 h. One-millilitre samples was taken at regular time intervals for analyses. The larger-scale acid hydrolysis was performed for 8 h using the SBP and acid at same concentrations in 2 L volume in a 2.5 L stirred reactor, equipped with a temperature control at 80 °C with overhead stirring at 100 rpm. Two-millilitre samples was taken every 2 h. At the end of hydrolysis, the SBP suspension was vacuum filtered using a cellulose filter paper (grade 4). The liquid fraction was kept at 4 °C, and the solid fraction was washed with deionised (DI) water and vacuum filtered using the standard Buchner funnel method. After washing, the solid fraction was re-suspended in DI water and pH adjusted to 4.0 with NaOH for subsequent enzymatic hydrolysis.
2.4 Enzymatic hydrolysis of SBP
SBP (66–90 g/L) was treated with commercial pectinase mixtures at ratios ranging between 0.2 and 1.1 mg enzymes per g SBP in 30 mL volume in 50 mL Falcon tubes. Cellic CTEC2 was used at 1.4 mg enzyme (5 FPU) per g SBP. The optimal pH of the enzyme mixtures is between pH 3.5 and 4.5. The initial pH of the SBP suspensions was approximately pH 4, and no pH control was applied during enzymatic hydrolysis. Enzymatic digestion conditions were set at 50 °C for 72 h in a Hybaid Shake ‘n’ Stack rotating oven (Thermo Fisher, Waltham, MA, USA) at 24 rpm/min. One millilitre samples was taken at regular time intervals during the experiment.
The up-scaled experiments were performed in 3 L laboratory bioreactors (Infors HT, Switzerland) with a final working volume of 2 L. The solid fraction from the previous stage of acid hydrolysis, after re-suspension and adjustment to pH 4, was treated using Pectinase ME (67 PECTU/g SBP; 175 rpm stirring) for 72 h. After digestion, the solid and liquid fractions were separated by vacuum filtration. The solid fraction was then washed, re-suspended in DI water and the pH adjusted to 4 using NaOH for the next hydrolysis by Cellic CTEC2 (5 FPU/g SBP) while stirring at 250 rpm and 50 °C for 72 h. Samples of 2 mL were taken at regular intervals. The galacturonic acid-rich liquid fraction was kept at − 20 °C until further analysis.
2.5 Scanning electron microscopy
Scanning electron microscopy (SEM) images were taken using a Hitachi SU3500 instrument (Hitachi High-Technologies Corporation, Tokyo, Japan) with an acceleration voltage of 5 kV. Prior to imaging, oven-dried biomass samples were glued onto aluminium stubs, then sputter-coated with gold (Cesington 108 auto, 65 s, 20 mA).
2.6 Analysis of monomers and oligomers
Liquid samples from acid and enzymatic hydrolyses were centrifuged at 13,000 rpm for 3 min using a MiniSpin centrifuge (Eppendorf AG, Hamburg, Germany). The supernatant was separated from solid particles and filtered (0.2 µm) for compositional analysis of monomers and oligomers.
2.6.1 High-performance liquid chromatography
High-performance liquid chromatography (HPLC; JASCO, Tokyo, Japan) equipped with Aminex Bio-Rad Fast Acid Analysis chromatographic column (Bio-Rad, Richmond, CA, USA) and a Micro-Guard column Cation H (Bio-Rad, Richmond, CA, USA) was used to quantify target monomers, i.e. glucose (Glu), arabinose (Ara) and galacturonic acid (GalU). The mobile phase used for chromatographic separation was 0.5 mM H2SO4 at 0.6 mL/min on the column maintained at 65 °C, and the analytes were detected using a refractive index (RI) detector (JASCO, Tokyo, Japan). Other monosaccharides (galactose, mannose, xylose and fructose) were analysed using high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD; ICS-5000, Thermo Fisher Scientific, Sunnyvale, CA, USA), equipped with a CarboPac PA20 analytical column (150 mm × 9.3 mm; 6 µm particle size) and a guard column (30 mm, 9.3 mm). The mobile phase used was 0.75 mM NaOH at a flow rate of 0.5 mL/min.
2.6.2 Size-exclusion chromatography
A size-exclusion chromatography (SEC) system (Waters Alliance 2695 HPLC System, Waters, Milford, MA), equipped with Waters 2414 Refractive Index Detector, was used to follow the molecular weight distribution of oligosaccharides. Superdex™ 200 Increase and Superdex™ 30 Increase columns (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) were connected in series and the analysis was performed using a mobile phase of 0.1 M NaOH at 0.5 mL/min and 25 °C. Polyethylene glycol (PEG) standards with molecular weights of 200, 400, 1000, 4000, 10,000 and 35,000 Da were used for calibration.
2.7 Yield calculation
Yields of Glu, Ara and GalU (YGlu, YAra, YGalU.) in a single treatment stage were calculated using the following equation, with glucose as the example:
where Cg is the measured Glu concentration in the liquid hydrolysate, Cg0 is the initial Glu concentration, \({\varphi }_{G}\) is the molecular weight ratio of glucose/glucan monomer (180/162 = 1.11), Cis0 is the initial concentration of dry biomass and xg0 is the glucan mass fraction of the initial dry biomass.
Monomer yields at multiple fractionation stages were determined using Eq. 2, which is the percentage of the monomer released in the liquid fraction with respect to the original monomer content in the raw material.
where SF is the mass of initial SBP feed on dry weight basis and VLF is the liquid fraction volume after separation.
2.8 Preliminary feasibility analysis
2.8.1 Process simulation
Aspen Plus® V10 software (Aspen Technology, Bedford, MA, USA) was used to simulate the process of SBP fractionation followed by the valorisation of the monomer-enriched streams. Non-random two-liquid (NRTL) thermodynamic property model was used for the simulation. Experimental values obtained from SBP fractionation in this study were used as the basis in designing unit operations, while literature values were used for the valorisation part. The proposed biorefinery process was assumed to have an input of 4000 kg/h of SBP (25% dry weight). The Nordic Sugar processing plant in Örtofta, Sweden, produces about 95,000 kg/h of SBP.
2.8.2 Technical assessment
Mass, energy and environmental indicators for the biorefinery are based on product yields (Yp, Eq. 3), total material consumption (mmat,tot, Eq. 4), mass intensity (MI, Eq. 5), specific energy consumption (SEC, Eq. 6) and E-factor (Eq. 7) [38,39,40]. These indicators and process simulation were used to evaluate the technical feasibility of the proposed process.
where mproduct is the mass flow of product in kg/h, and mSBP is the mass flow of sugar beet pulp feedstock in kg/h.
where miin is the mass flow of material input in kg/h and miout is the mass flow of material output in kg/h.
where Q (MJ/h) and W (MJ/h) are the thermal and power requirements of the biorefinery, respectively.
E-factor or environmental factor represents the amount of waste generated per kg of product [41].
where mwaste is the mass of total waste generated in the process and mproduct is the total mass of the target products.
2.8.3 Economic assessment
The potential economic feasibility of the proposed SBP biorefinery was assessed using the metric for inspecting sales and reactants (MISR). This metric is based on stoichiometric-economic targeting, which correlates the flows of reactants and products with the respective chemical pricing data [42]. MISR is a useful tool in guiding early-stage decisions regarding a suggested process design and is defined as:
where Fp is the annual production rate of product p, Sp is the selling price of product p, Fr is the annual feed rate of reactant r, and Cr is the cost price of reactant r. Data related to cost/selling prices of reactant and products are given in Table S1 in the supplementary material.
3 Results and discussion
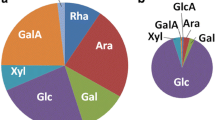
The compositional analysis of SBP obtained from Nordic Sugar A/S showed the presence of more than 75% water. The dry biomass comprised 23.0% glucan, 23.4% arabinan and 18.0% galacturonan and lower concentrations of other sugars, protein, lignin and other components (Fig. S1).
3.1 Screening of enzymes for SBP hydrolysis
Initial studies involved screening of different enzyme preparations from Novozymes A/S for the treatment of dried, milled SBP in 30 mL volume to determine their efficiency and selectivity. The pulp concentration was limited to 66 g/L due to increase in viscosity with increase in concentration that affected the stirring of the suspension. The amount of enzyme used varied between 0.2 and 1.1 g/kg SBP in contrast to the previous studies that used around 0.6–10 g enzyme/kg SBP [8, 9]. Table 1 shows the amount of sugars released after 72 h of the treatment at 50 °C at the respective enzyme concentrations giving the highest yields of the corresponding sugars. Among the pectin hydrolysing enzymes, Pectinase ME (with key activities of polygalacturonase and pectin lyase) yielded the highest release of GalU equivalent to 76.7% (10.1 g/L) and also released 55.3% (10.3 g/L) Ara at the enzyme concentration of 0.3 g/kg SBP; higher enzyme concentrations did not increase the yield of the sugars (not shown). The second highest release of GalU was with Neopectinase (with pectin lyase as key activity) at 69.5% (9.2 g/L), along with 46.7% Ara (8.7 g/L). Pectinex and Pectinase ARA (both with polygalacturonase as main enzyme) had similar GalU yields at 55.5% (7.3 g/L) and 55.9% (7.4 g/L), respectively, although Pectinase ARA possesses tenfold higher polygalacturonase activity than Pectinex. But the Pectinase ARA treatment resulted in higher Ara release at 64.5% compared to 23.4% with Pectinex. SBP treatment with a combination of Pectinase ME and Pectinase ARA led to a minor increase in GalU and Ara release compared to the treatments using single enzyme preparations (Table 1). The commercial enzyme formulations are a blend of different enzymes (such as polygalacturonase, pectin lyase, pectin methyl esterase and perhaps even cellulases and hemicellulases), with one or two enzymes as key components, and the difference in the monomer yields seems to be related to the predominant enzyme activity in the formulation. The release of different sugars also reflects the close association of the polysaccharides in the SBP.
Treatment with Cellic CTEC2 alone led to almost 70% release of Glu from the initial glucan component of the biomass, while GalU and Ara were released in lower but significant amounts. Combination of Pectinase ME and Cellic CTEC2 increased the Glu release to more than 91% but the GalU yield was more than halved to only 36.3% compared to the treatment with only Pectinase ME. It is evident that pectinase treatment helps to make the glucan chains easily accessible for the cellulases for almost unrestricted hydrolysis and also increases the release of other sugars as compared to the case when only Cellic CTEC2 was used for SBP treatment (Table 1).
3.2 Acid treatment of SBP
Parallel to the enzymatic treatment, SBP hydrolysis using H2SO4 and HCl, respectively, was also studied; the acid concentrations chosen were based on an earlier report [43]. As seen in Table 1, 80% release of the original Ara content (14.8 g/L) was observed in 7 h with H2SO4 treatment at 80 °C; the high Ara release being in accordance with that reported earlier [43]. Only 1.6% (0.3 g /L) of Glu and 3.4% (0.4 g/L) of GalU were released, while the monosaccharides present in minor amounts, galactose, xylose and mannose, were also released at 62.0% (3.3 g/L), 37.9% (0.6 g/L) and 8.6% (0.1 g/L), of their respective initial contents. Although treatment with HCl was slightly more effective in hydrolysing the pulp, H2SO4 was deemed more suitable because of its relatively safer handling issues and lower risk for corrosion with sulphate ions than with chloride ions [44]. Also, hydrolysis using HCl breaks down the glucan fraction to some extent [43], and for the purpose of separating monomer-enriched streams, it was considered more desirable to perform hydrolysis with H2SO4.
3.3 Evaluation of different approaches for SBP treatment
Based on the above observations, two approaches for sugar fractionation from SBP were evaluated (Fig. 1). The first approach involved direct treatment of the SBP with enzymes in two steps using Pectinase ME and Cellic CTEC2, respectively, while the second approach involved acid hydrolysis prior to the enzymatic hydrolysis steps.
The latter approach provides an efficient means of recovering Ara in a relatively higher yield. Subsequent treatment with Pectinase ME releases primarily GalU, thus leaving a cellulose-rich solid residue that could be utilised as such or hydrolysed to Glu. Once the two approaches were tested in 30 mL working volume, the process was scaled up to 2 L in a bioreactor while maintaining the concentrations of biomass, enzymes and acid as in the smaller-scale experiments but without milling the SBP in order to avoid the potential costs associated to drying and milling at large scale. Figure 2 shows the release of the target monomers during hydrolysis at each stage of Approach 1 (Fig. 2A1; A2) and Approach 2 (Fig. 2B1; B2; B3). As seen above in the small-scale experiments, pectinase treatment of SBP directly using the Approach 1 released a mixture of sugars (Fig. 2A1). The soluble product contained mainly GalU (78.8%; 10.2 g/L) but also significant amounts of Ara (36.0%; 7.0 g/L) and Glu (29.8%; 8.1 g/L), separation of which would be costly, and also result in further losses. The subsequent hydrolysis with cellulases further released 40.1% Glu (11.2 g/L) along with 8.8% GalU (1.9 g/L) and 12.7% Ara (3.7 g/L) (Table 2; Fig. S3). Various studies have already investigated SBP saccharification using enzymatic mixtures [8, 9, 45, 46]; the highest yield of soluble GalU (79%) and Ara (82%) in a mixture while keeping 83% of cellulose intact has been reported using a mixture of commercial enzyme and experimental pectinase preparations [8].
Concentrations of main monomers Glu, Ara and GalU versus hydrolysis time for each hydrolysis stage in the two approaches used for treatment of SBP. The two-stage Approach 1: Pectinase ME treatment (A1), Cellic CTEC2 treatment (A2) and the three-stage process; Approach 2: acid treatment (B1), Pectinase ME treatment (B2), Cellic CTEC2 treatment (B3). Error bars indicate the standard deviation from duplicate bioreactor experiments. The starting working volumes were 2 L
In comparison, in the three-stage process of Approach 2, the first step of hydrolysis using low concentration of mineral acid released Ara more selectively at 71.3% (10.4 g/L) with only 0.5% Glu (1.1 g/L) and no GalU (Fig. 2B1), most of the sugar release occurring during the first 2 h of treatment. Pectin is known to get decarboxylated at high acid concentration; complete decarboxylation was shown employing 12% HCl within 5 h at boiling temperature [47]. Mass balance-based process analysis of the solid fractions (Table 3) remaining after acid hydrolysis (0.64 M, at 80 °C for 8 h) in this study did not reveal decarboxylation of the GalU to Ara, i.e. 20.5% of the original galacturonan chains in the residual solid fraction after acid hydrolysis remained relatively intact and there was no over-detection of Ara during the fractionation stages.
The subsequent pectinase treatment solubilised 68.5% GalU (20.6 g/L), 1.2% Ara (0.5 g/L) and 0.1% Glu (0.04 g/L); there was no notable increase in soluble sugars beyond 24 h (Fig. 2B2). Lastly, the cellulase treatment released 81.9% Glu (24.9 g/L), 12.7% GalU (3 g/L) and no Ara (Table 3; Fig. 2B3); the maximal release was observed up to 48 h. Hence, the combination of acid and enzymatic hydrolysis was more favourable for releasing the target monomers predominantly in separate stages.
Minor monosaccharides, i.e. galactose, mannose and xylose were also released at lower concentrations (Tables 2 and 3). It is noteworthy that protein and lignin made up to 78.2% of the remaining solid mass after the three-stage process in Approach 2 while carbohydrates constituted the remaining component (Table 3). In comparison, the solid mass after the two-stage process consisted of up to 79.1% carbohydrates while only 16.2% was protein and lignin (Table 2). Scanning electron microscopy images of untreated SBP and the material after treatment via the different approaches show exposure and disruption of the structural components in the biomass (Fig. 3).
Both the enzymatic steps using pectinase and cellulase, respectively, clearly revealed a decrease in the rate of sugar release with time (Fig. 2B2; B3). Although this could be attributed to enzyme inactivation, product inhibition seems to play an important role as observed by the significant decrease in the rate of GalU release with increase in the initial GalU concentration in the reaction medium, reaching almost 50% of the activity in the presence of 20 g/L GalU (Fig. S2).
3.4 Closing the mass balance of sugars
Quantification of the predominant sugars in the soluble and insoluble fractions at each process stage revealed that mass balances closed at 98.1% for Glu, 86.9% for Ara and 99.0% for GalU for Approach 1 (Table 2, Fig. S3). In comparison, the mass balances for Approach 2 closed at 86.5% for Glu, 75.8% for Ara and 70.7% for GalU (Table 3, Fig. S4). In order to find the missing carbohydrate content, SEC analysis of the hydrolysate samples from each treatment step was performed. The analysis indicated the presence of oligomers (> 200 Da) ranging from dimers (~ 400 Da) to pentamers (~ 1 kDa), as illustrated in Fig. 4 for Approach 1 and Fig. 5 for Approach 2.
Size-exclusion chromatograms of the two-stage Approach 1 used for treatment of SBP: Pectinase ME treatment (A1) and Cellic CTEC2 treatment (B1). The arrows point out the molecular weights of the PEG standards used for calibration. Oligomeric content determination in the liquid fractions from each hydrolysis stage was done by measuring the difference in the monomeric concentration in the liquid fraction of each stage before and after total acid hydrolysis: Pectinase ME treatment (A2), Cellic CTEC2 treatment (B2)
Size-exclusion chromatograms of the three-stage process. Approach 2: acid treatment (A1), Pectinase ME treatment (B1) and Cellic CTEC2 treatment (C1). The arrows point out the molecular weights of the PEG standards used for calibration. Oligomeric content in the liquid fractions of each hydrolysis stage was determined by the difference in monomeric concentration before and after total acid hydrolysis: acid treatment (A2), Pectinase ME treatment (B2), Cellic CTEC2 treatment (C2)
The oligosaccharides in the liquid fractions were subjected to total acid hydrolysis to identify and quantify the predominant oligosaccharides present. Galacturonan oligosaccharides were mostly present in the liquid fraction after acid hydrolysis (3.7 g/L) as seen in Fig. 5A1. Galacturonan pentamers (~ 1 kDa) and dimers (~ 400 Da) (Fig. 5A2) accounted for up to 33.9% of the original GalU content of SBP. Similarly, after pectinase treatment, arabinan pentamers (~ 1 kDa) and dimers (~ 400 Da) (7.18 g/L, 17.8% of original content) were solubilised along with GalU monomers. These results are comparable to an earlier study reporting recalcitrant arabinan oligosaccharides and homogalacturonan-derived oligosaccharides with most abundant degree of polymerisation of 4–5 after pectinase treatments [8]. Additionally, the cellulose fraction of SBP, previously thought to have remained intact, was solubilised as glucan oligosaccharides to the extent of 2.9% and 6.6% of the original content after acid and pectinase treatment, respectively (Fig. 5A2; Fig. 5B2).
Taking the yields of the oligomers into account in the Approach 2, the mass balances closed at 96.0% for Glu, 96.6% for Ara and 104.6% for GalU, which is within 5% deviation error. This also confirms that no decarboxylation of GalU to Ara occurred and there was no significant loss of arabinose, e.g. by degradation to furfural [48]. However, it reveals that losses of GalU and Ara occur mainly through their release as oligomers in the liquid fractions from stages 1 to 2, respectively. The need for further separation would depend on how the sugars are to be valorised. For example, for obtaining pure Ara and GalU as the final products from the acid hydrolysis and enzymatic hydrolysis steps, respectively, would require an additional step for separation of the oligomers, which could be based on the charged carboxyl group on the Gal residues, e.g. precipitation, ion exchange adsorption, extraction or electrodialysis.
3.5 Preliminary technical and economic assessment
With the purpose of the valorisation of the liquid streams enriched in the respective sugars, the three-stage process was chosen for preliminary technological and economical assessment with a feed capacity of 1000 kg dry SBP/h. Figure 6 shows a simplified scheme with mass and energy flows during the SBP hydrolysis (based on the data from scaled-up (2 L working volume) experiments in this study) and potential valorisation of Glu, Ara and GalU to levulinic acid, arabitol and mucic acid, respectively. It is important to note that for the development of a biorefinery the layout in Fig. 6 should be expanded to include the heat and power distribution system, treatment of the residues/wastes, product purification and water recirculation. The process flowchart of the hydrolysis and valorisation systems is shown in Fig. S5 and the specifications of the unit operations used are given in Table S2.
Mass and energy flows in the biorefinery with an input capacity of 1000 kg/h dry SBP. The blue box ‘Hydrolysis system’ represents Approach 2 designed in this study, with corresponding monomer yields. The green box ‘Valorisation system’ represents the possible valorisation routes of the main monomers. SBP, sugar beet pulp; HP, high pressure steam
Mass and energy indicators are given in Table 4. The yield of arabitol (0.73 g/g Ara) was based on a report by Dien et al. [23]. Utilising yeasts such as Candida auringiensis and Pichia stipitis for the conversion of arabinose to arabitol may eliminate the need for pure sugar substrates [24], hence the arabinose-rich liquid fraction after the acid treatment could most likely be directly subjected to biotransformation. Alternatively, use of a recombinant microorganism with heterologously expressed arabinose reductase could be a possibility for the reduction of arabinose to arabitol [24]. The mucic acid yield (0.63 g/g GalU) was based on our preliminary data (unpublished results) obtained from oxidation of GalU directly in the liquid fraction from the Pectinase ME treatment, using laccase as the biocatalyst, as described elsewhere [30]. The commercial laccase may be costly at present, but it is possible to produce it cheaply by cultivating fungi/mushrooms on lignocellulosic agro-food wastes that secrete large amounts of laccase [49]. The use of laccase can also be made more economical by recycling of the enzyme, e.g. as an immobilised biocatalyst [50]. Lastly, the yield of levulinic acid (0.71 g/g) from glucose using an acidic ion-exchange catalyst was based on our previous report [19]. Formic acid, formed as a co-product in the process for levulinic acid production, was not included in the calculations. Further experimental studies would be needed to investigate the effect of the SBP hydrolysis steps, e.g. formation of inhibitory compounds like furfurals during acid hydrolysis at elevated temperatures, on the efficiency of the routes of valorisation suggested in this study.
Overall productivity per metric tonne of dry SBP feedstock lies at 341.3 kg valorised product per hour. The mass intensity indicator of the biorefinery is at 139.4 kg of input materials per kg of product, of which 86.6% is process water. The high water content of SBP (~ 75%) indicates that the target sugars and valorised products come only from 25% of the original feed undergoing processing. The specific energy consumption of 7.4 MJ per kg dry SBP is predominant in the hydrolysis system (87.3%; 6.4 MJ/kg SBP), while the valorisation system consumes relatively less energy (12.7%; 0.9 MJ/kg SBP) due to lower reactor volume and lower temperature. E-factor was calculated from the information on the input reagents and product outflows provided in Fig. 6 and was estimated to be 127.9 kg waste per kg of product (Table 4), which is significantly reduced to 9.93 considering water recirculation. Recycling of the catalysts used in the valorisation stage can further reduce the E-factor to 6.4, placing the process in the lower E-factor range of the fine chemical industry [51].
Since the value of the products is the incentive for this biorefinery concept, it should outweigh both the energy input and the cost of the total material inputs for the process to be feasible. It is important to highlight that the input and output values shown in Fig. 6 were scaled-up values from the lab-scale experimental data (‘Hydrolysis System’) and literature values (‘Valorisation system’), hence the high values for mass intensity and specific energy consumption.
In order to assess the potential economic viability of the proposed SBP biorefinery, the MISR was used, which accounts for the selling prices of the products, in our case levulinic acid, arabitol and mucic acid, and the purchased cost of the SBP feedstock. Previous studies have used MISR to assess the potential viability of various novel processes and to support further investigation [32, 52, 53]. For targeting purposes, the costs of the SBP feedstock and the products given in Table S1 were used. Figure 7 shows the MISR plot, wherein above the dashed line (MISR > 1) represents potential viability of the biorefinery, which entails a more detailed techno-economic analysis to evaluate process profitability by other metrics, such as net present value and payback period. In general, the higher the MISR value is from 1, the more profitable the biorefinery is expected to be. On the other hand, the values under the dashed line (MISR < 1) render the process undesirable from an economic standpoint, implying that it costs more to purchase the SBP than that earned by selling the pure products.
Effect of the cost of SBP on MISR in the proposed biorefinery process. The purification cost was accounted in the range of 10–50% of the lower-end selling prices of the final products. The dashed line indicates the values at which MISR is equal to 1. SBP, sugar beet pulp; MISR, metric for inspecting sales and reactants
Separation and purification operations can account for up to 50% of the total capital and operating costs in biorefinery systems [54]. An approximation for the purification costs of the final products is presented as percentages of the selling prices of the products, given that the process concept is at its early stage. A range of 10–50% of the selling prices of the products was accounted to the purification cost when deriving the MISR profiles. At 10% purification cost, the process becomes economically unfeasible at SBP cost of 2276 US$/t, while at 50%, SBP cost above 1140 US$/t renders the process unfeasible. This is far from the cost price of SBP sold as animal feed in Sweden (150–200 US$/t) [55], which indicates a sizeable margin of potential feasibility of the process. It should be noted, however, that there is no guarantee of profitability for MISR values greater than 1. Further evaluations of the SBP biorefinery process proposed in this study will require a detailed techno-economic and life-cycle analyses to determine profitability, overall feasibility and environmental footprint. A recent study on a comprehensive techno-economic and energy assessment on levulinic acid production alone from banana residues provided positive insights on the energy performance and economic aspects of biomass valorisation at an industrial scale [35].
4 Conclusions and outlook
SBP, a by-product of the sugar industry, has been studied herein for its valorisable potential. A stepwise treatment of the SBP by acid and enzymatic hydrolyses under mild conditions yields separated sugar-enriched streams. Although the cellulose fraction of SBP was hydrolysed to glucose, it may be used as such for material production [56]. A preliminary assessment of the economic viability indicated a promising potential for the proposed biorefinery; hence, a more detailed techno-economic analysis is encouraged.
There are several areas of improvement in this biorefinery concept, such as further increase in the yields of the sugar monomers, e.g. by in-situ product recovery using a membrane reactor that may also enable enzymes recycling. A possible integration of the SBP biorefinery into the vicinity of the sugar-processing plant could offer opportunities for energy integration and additional (economic) improvements. More value could be extracted from SBP, such as by recovering the protein stream and phenolic compounds including ferulic acid for use as antioxidants or further transformed to other chemical building blocks [14].
References
Ness B (2011) An intergrated sustainability assessment of the Swedish sugar production system from a life-cycle perspective 2003–2015. Interdisciplin Descrip Complex Systems 9:23–28
Puligundla P, Mok C (2021) Valorization of sugar beet pulp through biotechnological approaches: recent developments. Biotechnol Lett 43:1253–1263. https://doi.org/10.1007/s10529-021-03146-6
EC (2020) EU agricultural outlook for markets, income and environment, 2020-2030. European Commission, DG Agriculture and Rural Development, Brussels. https://doi.org/10.2762/252413
Jordbruksverket (2020) Jordbruksstatistik Sammanställning 2020. Sveriges Officiella Statistik.
Zheng Y, Yu C, Cheng Y-S, Lee C, Simmons CW, Dooley TM, Zhang R, Jenkins BM, VanderGheynst JS (2012) Integrating sugar beet pulp storage, hydrolysis and fermentation for fuel ethanol production. Appl Energy 93:168–175. https://doi.org/10.1016/j.apenergy.2011.12.084
Kelly P (1983) Sugar beet pulp - a review. Animal Feed Sci Technol 8:1–18. https://doi.org/10.1016/0377-8401(83)90038-X
Kuhnel S, Schols HA, Gruppen H (2011) Aiming for the complete utilization of sugar-beet pulp: examination of the effects of mild acid and hydrothermal pretreatment followed by enzymatic digestion. Biotechnol Biofuels 4:14. https://doi.org/10.1186/1754-6834-4-14
Leijdekkers AG, Bink JP, Geutjes S, Schols HA, Gruppen H (2013) Enzymatic saccharification of sugar beet pulp for the production of galacturonic acid and arabinose; a study on the impact of the formation of recalcitrant oligosaccharides. Bioresour Technol 128:518–525. https://doi.org/10.1016/j.biortech.2012.10.126
Micard V, Renard CMGC, Thibault J-F (1996) Enzymatic saccharification of sugar beet pulp. Enzyme Microb Technol 19:162–170. https://doi.org/10.1016/0141-0229(95)00224-3
Zieminski K, Romanowska I, Kowalska-Wentel M, Cyran M (2014) Effects of hydrothermal pretreatment of sugar beet pulp for methane production. Bioresour Technol 166:187–193. https://doi.org/10.1016/j.biortech.2014.05.021
Hamley-Bennett C, Lye GJ, Leak DJ (2016) Selective fractionation of sugar beet pulp for release of fermentation and chemical feedstocks; optimisation of thermo-chemical pre-treatment. Bioresour Technol 209:259–264. https://doi.org/10.1016/j.biortech.2016.02.131
Bellido C, Infante C, Coca M, Gonzalez-Benito G, Lucas S, Garcia-Cubero MT (2015) Efficient acetone-butanol-ethanol production by Clostridium beijerinckii from sugar beet pulp. Bioresour Technol 190:332–338. https://doi.org/10.1016/j.biortech.2015.04.082
Berlowska J, Cieciura W, Borowski S, Dudkiewicz M, Binczarski M, Witonska I, Otlewska A, Kregiel D (2016) Simultaneous saccharification and fermentation of sugar beet pulp with mixed bacterial cultures for lactic acid and propylene glycol production. Molecules 21:1380. https://doi.org/10.3390/molecules21101380
Alexandri M, Schneider R, Papapostolou H, Ladakis D, Koutinas A, Venus J (2019) Restructuring the conventional sugar beet industry into a novel biorefinery: fractionation and bioconversion of sugar beet pulp into succinic acid and value-added coproducts. ACS Sustain Chem Eng 7:6569–6579. https://doi.org/10.1021/acssuschemeng.8b04874
Raj K, Partow S, Correia K, Khusnutdinova AN, Yakunin AF, Mahadevan R (2018) Biocatalytic production of adipic acid from glucose using engineered Saccharomyces cerevisiae. Metab Eng Commun 6:28–32. https://doi.org/10.1016/j.meteno.2018.02.001
Yu Q, Cui Z, Zheng Y, Huo H, Meng L, Xu J, Gao C (2018) Exploring succinic acid production by engineered Yarrowia lipolytica strains using glucose at low pH. Biochem Eng J 139:51–56. https://doi.org/10.1016/j.bej.2018.08.001
Yahiro K, Takahama T, Park YS, Okabe M (1995) Breeding of Aspergillus terreus mutant TN-484 for itaconic acid production with high yield. J Ferm Bioeng 79:506–508. https://doi.org/10.1016/0922-338X(95)91272-7
Sayed M, Warlin N, Hulteberg C, Munslow I, Lundmark S, Pajalic O, Tunå P, Zhang B, Pyo S-H, Hatti-Kaul R (2020) 5-Hydroxymethylfurfural from fructose: an efficient continuous process in a water-dimethyl carbonate biphasic system with high yield product recovery. Green Chem 22:5402–5413. https://doi.org/10.1039/D0GC01422B
Pyo SH, Glaser SJ, Rehnberg N, Hatti-Kaul R (2020) Clean production of levulinic acid from fructose and glucose in salt water by heterogeneous catalytic dehydration. ACS Omega 5:14275–14282. https://doi.org/10.1021/acsomega.9b04406
Werpy T, Petersen G et al (2004) Top value added chemicals from biomass. Pacific Northwest National Laboratory, National Renewable Energy Laboratory and Department of Energy, Washington, DC. https://doi.org/10.2172/15008859
Pol K, de Graaf K, Diepeveen-de Bruin M, Balvers M, Mars M (2020) The effect of replacing sucrose with L-arabinose in drinks and cereal foods on blood glucose and plasma insulin responses in healthy adults. J Func Foods 73:104114. https://doi.org/10.1016/j.jff.2020.104114
Sifontes Herrera VA, Saleem F, Kusema B, Eränen K, Salmi T (2012) Hydrogenation of L-arabinose and d-galactose mixtures over a heterogeneous Ru/C catalyst. Topics Catal 55:550–555. https://doi.org/10.1007/s11244-012-9833-z
Dien BS, Kurtzman CP, Saha BC, Bothast RJ (1996) Screening for L-arabinose fermentation. Appl Biochem Biotechnol 57–58:233–242
Kordowska-Wiater M (2015) Production of arabitol by yeasts: current status and future prospects. J Appl Microbiol 119:303–314. https://doi.org/10.1111/jam.12807
Leung LMH, Gibson V, Linclau B (2005) Improved synthesis of enantiopure pseudo-C2-symmetric 1,4-bis-epoxide building blocks from arabitol. Tetrahedron Asymmetry 16:2449–2453. https://doi.org/10.1016/j.tetasy.2005.05.043
Subrizi F, Cárdenas-Fernández M, Lye GJ, Ward JM, Dalby PA, Sheppard TD, Hailes HC (2016) Transketolase catalysed upgrading of L-arabinose: the one-step stereoselective synthesis of L-gluco-heptulose. Green Chem 18:3158–3165. https://doi.org/10.1039/C5GC02660A
Cardenas-Fernandez M, Bawn M, Hamley-Bennett C, Bharat PKV, Subrizi F, Suhaili N, Ward DP, Bourdin S, Dalby PA, Hailes HC et al (2017) An integrated biorefinery concept for conversion of sugar beet pulp into value-added chemicals and pharmaceutical intermediates. Faraday Discuss 202:415–431. https://doi.org/10.1039/C7FD00094D
Zhang H, Li X, Su X, Ang EL, Zhang Y, Zhao H (2016) Production of adipic acid from sugar beet residue by combined biological and chemical catalysis. ChemCatChem 8:1500–1506. https://doi.org/10.1002/cctc.201600069
Purushothaman RKP, Fvd K, Frissen AE, Jv H, Mayoral A, van der Bent A, van Es DS (2018) Base-free selective oxidation of pectin derived galacturonic acid to galactaric acid using supported gold catalysts. Green Chem 20:2763–2774. https://doi.org/10.1039/C8GC00103K
Vastano M, P A, Botelho Machado C, Simister R, McQueen-Mason SJ, Farmer TJ, Gomez LD (2019) Sustainable galactarate-based polymers: multi-enzymatic production of pectin-derived polyesters. Macromol Rapid Commun 40:e1900361. https://doi.org/10.1002/marc.201900361
Ward DP, Hewitson P, Cardenas-Fernandez M, Hamley-Bennett C, Diaz-Rodriguez A, Douillet N, Adams JP, Leak DJ, Ignatova S, Lye GJ (2017) Centrifugal partition chromatography in a biorefinery context: optimisation and scale-up of monosaccharide fractionation from hydrolysed sugar beet pulp. J Chromatogr A 1497:56–63. https://doi.org/10.1016/j.chroma.2017.03.003
Abdelaziz OY, Al-Rabiah AA, El-Halwagi MM, Hulteberg CP (2020) Conceptual design of a Kraft lignin biorefinery for the production of valuable chemicals via oxidative depolymerization. ACS Sustain Chem Eng 8:8823–8829. https://doi.org/10.1021/acssuschemeng.0c02945
Kapanji KK, Haigh KF, Görgens JF (2021) Techno-economics of lignocellulose biorefineries at South African sugar mills using the biofine process to co-produce levulinic acid, furfural and electricity along with gamma valeractone. Biomass Bioenergy 146:106008. https://doi.org/10.1016/j.biombioe.2021.106008
Joelsson E, Erdei B, Galbe M, Wallberg O (2016) Techno-economic evaluation of integrated first- and second-generation ethanol production from grain and straw. Biotechnol Biofuels 9:1. https://doi.org/10.1186/s13068-015-0423-8
Hurtado SIM, Puello P, Cabarcas A (2021) Technical evaluation of a levulinic acid plant based on biomass transformation under techno-economic and exergy analysis. ACS Omega 6:5627–5641. https://doi.org/10.1021/acsomega.0c06088
Sluiter A, Ruiz R, Scarlata C, Sluiter J, Templeton D (2005) Determination of extractives in biomass; NREL/TP-510–42619, US National Renewable Energy Laboratory, Golden, Colorado
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Structural carbohydrates and lignin in biomass; NREL/TP-510–42618, US National Renewable Energy Laboratory, Golden, Colorado
Ortiz-Sanchez M, Solarte-Toro J-C, González-Aguirre J-A, Peltonen KE, Richard P, Cardona Alzate CA (2020) Pre-feasibility analysis of the production of mucic acid from orange peel waste under the biorefinery concept. Biochem Eng J 161:107680. https://doi.org/10.1016/j.bej.2020.107680
Alonso-Gómez LA, Solarte-Toro JC, Bello-Pérez LA, Cardona-Alzate CA (2020) Performance evaluation and economic analysis of the bioethanol and flour production using rejected unripe plantain fruits (Musa paradisiaca L.) as raw material. Food Bioprod Process 121:29–42. https://doi.org/10.1016/j.fbp.2020.01.005
Anastas P, Eghbali N (2010) Green chemistry: principles and practice. Chem Soc Rev 39:301–312. https://doi.org/10.1039/B918763B
Sheldon RA (2008) E factors, green chemistry and catalysis: an odyssey. Chem Commun (Camb) 2008(29):3352–3365. https://doi.org/10.1039/B803584A
El-Halwagi MM (2012) Benchmarking process performance through overall mass targeting. In: El-Halwagi MM (ed) Sustainable Design Through Process Integration. Butterworth-Heinemann, Oxford, pp 63–88
Chamy R, Illanes A, Aroca G, Nufiez L (1994) Acid hydrolysis of sugar beet pulp as pretreatment for fermentation. Bioresour Technol 50:149–152. https://doi.org/10.1016/0960-8524(94)90067-1
Trabanelli G (1991) Whitney Award lecture: inhibitors—an old remedy for a new challenge. Corrosion 47:410–419
Coughlan MP, Mehra RK, Considine PJ (1985) Saccharification of agricultural residues by combined cellulolytic and pectinolytic enzyme systems. Biotechnol Bioeng Symp 15:447–458
Spagnuolo M, Crecchio C, Pizzigallo MDR, Ruggiero P (1999) Fractionation of sugar beet pulp into pectin, cellulose, and arabinose by arabinases combined with ultrafiltration. Biotechnol Bioeng 64:685–691
Conrad CM (1931) Decarboxylation studies on pectins and calcium pectates. J Amer Chem Soc 53:1999–2003. https://doi.org/10.1021/JA01356A059
Kootstra AMJ, Mosier NS, Scott EL, Beeftink HH, Sanders JPM (2009) Differential effects of mineral and organic acids on the kinetics of arabinose degradation under lignocellulose pretreatment conditions. Biochem Eng J 43:92–97. https://doi.org/10.1016/j.bej.2008.09.004
Mayolo-Deloisa K, González-González M, Rito-Palomares (2020) Laccases in food industry: bioprocessing, potential industrial and biotechnological applications. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2020.00222
Wang Q, Yuan T, Liu S, Yang G, Chen J, Li W, Yang R, Fatehi P (2017) Recycling immobilized laccase for lignin removal from prehydrolysis liquor of kraft-based dissolving pulp. BioResources 12:8838–8847
Sheldon RA (2017) The E factor 25 years on: the rise of green chemistry and sustainability. Green Chem 19:18–43. https://doi.org/10.1039/C6GC02157C
Agarwal A, Sengupta D, El-Halwagi M (2018) Sustainable process design approach for on-purpose propylene production and intensification. ACS Sustain Chem Eng 6:2407–2421. https://doi.org/10.1021/acssuschemeng.7b03854
Guerras LS, Sengupta D, Martín M, El-Halwagi MM (2021) Multilayer approach for product portfolio optimization: waste to added-value products. ACS Sustainable Chem Eng 9:6410–6426. https://doi.org/10.1021/acssuschemeng.1c01284
Ramaswamy S, Huang H-J, Ramarao BV (2013) Separation and purification technologies in biorefineries. United Kingdom: John Wiley & Sons, Ltd.
HP-Massa® – ökar lönsamheten med en femma per ko och dag. Nordic Sugar. https://fodernordicsugar.se/content/uploads/2021/03/HP-Massa-prislista-och-bestallningsinformation-2021.pdf. Accessed: May 5 2021
Li M, Wang L-j, Li D, Cheng Y-L (2014) Preparation and characterization of cellulose nanofibers from de-pectinated sugar beet pulp. Carbohyd Polym 102:136–143. https://doi.org/10.1016/j.carbpol.2013.11.021
Acknowledgements
This work was financed by the Swedish Research Council FORMAS in the framework of the Surplus Agricultural Residues to Furanics project Farm2Furan (942-2016-33). O.Y.A., S.-H.P. and R.H.-K. also acknowledge the support of the Swedish Foundation for Strategic Environmental Research MISTRA (F2019/1822) for the research programme, Sustainable Plastics and Transition Pathways (STEPS) at Lund University. We thank Dr. Henrik Almqvist (Department of Chemical Engineering, Lund University) for support with SEC analysis, Dr. Pär Tufvesson (Novozymes A/S) for providing the commercial enzymes and useful discussions and Dr. Oleg Pajalic for critical review of the manuscript.
Funding
Open access funding provided by Lund University.
Author information
Authors and Affiliations
Contributions
SJG: Conceptualisation, methodology, software, validation, formal analysis, investigation, data curation, writing-original draft and visualisation. OYA: Conceptualisation, methodology, formal analysis, investigation, writing-review/editing and visualisation. CD: Conceptualisation, formal analysis and investigation. MG: writing-review/editing and supervision. S-HP: Conceptualisation, writing-review/editing and supervision. JPJ: Resources. RH-K: Conceptualisation, resources, writing-review/editing, supervision, project administration and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Glaser, S.J., Abdelaziz, O.Y., Demoitié, C. et al. Fractionation of sugar beet pulp polysaccharides into component sugars and pre-feasibility analysis for further valorisation. Biomass Conv. Bioref. 14, 3575–3588 (2024). https://doi.org/10.1007/s13399-022-02699-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02699-4