Abstract
Grown only in humid tropical conditions, the palm tree provides high-quality oil essential for cooking and personal care or biofuel in the energy sector. After the refining process, this demand could cause numerous oil palm biomass waste management problems. However, the emergence of carbon nanomaterials or CNMs could be a great way to put this waste to a good cause. The composition of the palm waste can be used as a green precursor or starting materials for synthesizing CNMs. Hence, this review paper summarizes the recent progress for the CNMs production for the past 10 years. This review paper extensively discusses the method for processing CNMs, chemical vapor deposition, pyrolysis, and microwave by the current synthesis method. The parameters and conditions of the synthesis are also analyzed. The application of the CNMs from palm oil and future recommendations are also highlighted. Generally, this paper could be a handy guide in assisting the researchers in exploring economic yet simple procedures in synthesizing carbon-based nanostructured materials derived from palm oil that can fulfill the required applications.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Carbon nanomaterials have a crucial role in nanotechnology. It is the most common and feasible material used in various applications, from energy generation to purification of water pollution. To address the increasing global demand for numerous carbon nanomaterial applications, the production of carbon nanomaterials is also being scaled up. Generally, fossil-derived precursors are used in synthesizing carbon nanomaterials. The large-scale usage of fossil-derived precursors can be attributed to the decline of fossil fuel deposits, not to mention its detrimental environmental effects. Hence, the development of sustainable materials is significant. In this regard, several solutions must be considered to develop a low-cost and industrially scalable eco-friendly method while attaining the intended application performance. Renewable natural materials such as an oil palm–based precursor, which is carbon–neutral, low sulfur, and available virtually everywhere, could be the best alternative for fossil-derived precursors.
The oil palm tree, Elaeis Guineensis, originated from Africa [1]. It was introduced to South America and southern Asia in the sixteenth and nineteenth centuries. Palm oil is recognized as one of the edible oils besides soybean oil, rapeseed oil, and sunflower oil. These major oils contributed around 76.54 million metric tonnes compared to other edible oils production, as shown in Fig. 1 [2]. Approximately palm oil was produced around 74.02 million tonnes (31.4%) of the total oils and fats produced globally in 2020. It rose to 76.54 million tonnes in the next year [3, 4]. Countries in Southeast Asia have been recognized as the primary global raw palm oil production. Indonesia is the primary producer of palm oil in Southeast Asia in 2021 with 44.50 million tonnes of production, followed by Malaysia and Thailand with 19.70 million tonnes and 3.12 million tonnes, respectively [4]. Oil palm is grown in around 43 countries worldwide, and if the growth rates do not decline, oil palm plantation.
Production of edible oil in a million metric tonnes worldwide until November 2021. Data retrieved from [2]
After harvesting the fresh fruit bunches (FFB), a massive amount of waste is being produced annually, including empty fruit bunches (EFB), mesocarp fiber (MF), shell fractions or palm kernel shells (PS), oil palm fronds (OPF), oil palm trunks (OPT), and also palm leaves [5, 6]. The expansion of oil palm plantations will face a significant problem in the disposing of the lignocellulosic biomass and other large-scale products from the oil palm industry. Over the years, Malaysia has been striving to minimize the disposal problems by optimizing the use of oil palm in various applications, especially for the sustainability of energy supply. In 2020, Malaysian Palm Oil revealed that the planted area covers approximately 5.90 million hectares of land, with 5.216 million hectares for the mature oil palm and 0.683 for the immature oil palm planted across the country [7]. The fruit bunches (FFB) harvested in 2020, 17.19 tonnes per hectare, was reported by a Malaysia Palm Oil Board, bringing around 101.42 million tonnes in that year. There are no constant values for each oil palm waste, but according to Loh [6], the type of oil palm waste could be estimated based on the standard fresh fruit bunches ratio as shown in Table 1. If 95.38 Mt of FFB is processed, the estimated oil palm biomass production comprises 7.809 million tonnes of EFB dry weight, 8.215 million tonnes of ton mesocarp fibers in dry weight 4.741 million tonnes of palm shells in dry weight [6].
Many residues from the industry create a major problem regarding waste disposal. This is because open burning is discouraged since it may cause the deterioration of the ecosystem and horrible haze. At the same time, the palm waste littered in the mill sites can give unpleasant views and contaminate the underground water due to unwanted chemicals in the soil [8, 9]. Hence, in addressing the issue, Malaysia’s Department of Environment implemented the 1978 Environmental Quality Clean Air Regulation Act to restrict the burning and incineration of wastes. Unfortunately, we should not depend entirely on the act; serious measures should be taken to exploit functional waste materials produced in the oil palm industry. As stated by Nasir et al., the integration of “waste to wealth” and “zero-waste industry” mantras should be applied together in this case [10]. Exploration of various carbon-based nanomaterials (CNMs) derived from oil palm waste has been conducted. It resulted in vertically aligned carbon nanotubes, carbon nanotubes via stacked and non-stacked silicon substrate graphene, few-layer graphene, carbon quantum dots, activated carbon, and carbon nanosphere [11,12,13,14,15,16,17]. The transformation of oil palm waste into CNMs can open up more job opportunities in the cultivation area, especially in the rural area, increasing the social status of the surrounding society [18]. Furthermore, the conversion of oil palm waste to CNMs can be an alternative to the carbon precursor to substitute for the non-renewable precursors such as natural gas, coke, and another coal-based feedstock. This promotes a cleaner and affordable way of synthesizing CNMs.
The present paper will review the production of CNMs, including activated carbon, carbon nanotubes, graphene, reduced graphene oxide, carbon nanospheres, and even nanocellulose derived from the by-products of oil palm trees for the past 10 years back [16, 19,20,21,22]. Nevertheless, some research published a couple of decades ago is included since the current findings are pretty much limited, especially in exploiting the usage of palm oil or crude palm oil as CNMs. This paper will also review the application or prospects of the production of CNMs.
This review paper is organized as follows: (i) the first section is devoted to the introduction of the study; (ii) the second section discusses the current trend in the oil palm industries and its production, especially in Malaysia; (iii) the third section details the carbon-based nanostructured materials, together with the discussion on the production of oil palm by-products using various methods; (iv) the fourth section compiled the application of CNMs derived from palm oil; (v) the fifth section discusses the prospects of converting and challenges of CNMs derived from palm oil; and finally (vi) the sixth section presents the summary or conclusion for this review paper.
2 The current trend in the palm oil industry
2.1 Production of palm oil in Malaysia
Since the coronavirus (COVID-19) was declared as a global pandemic on March 11, 2020, by World Health Organization (WHO), governments from numerous countries implemented mandated restrictions, quarantines, social distancing, and other preventative measures [23]. This situation had a significant impact on the global community, from industrial scale to agricultural fields. Palm oil or Elaeis guineensis is the most used edible oil globally, surpassing other types of edible oil such as rapeseed oil, soybean oil, and sunflower oil. Due to its high yield and reproducibility, the requirement of oil palm trees can be lessened. Each hectare can produce ten times more oil than other types of sources of oil [24]. A high yield of palm oil requires less area, making it a desirable source of income for smallholder farmers and other oil productions. As the second-highest country in palm oil production, this pandemic significantly impacts palm oil productivity in Malaysia. The average production of palm oil in 2020 was 1.595 million tonnes, which is less than palm oil production in 2019, 1.655 million tonnes [25]. As palm oil was considered the main contributor in Malaysia’s agricultural sectors, it is vital to sustain the palm oil industry for the importance of the country’s economy by shifting to high-demand products [26].
Palm oil could be categorized as the second most important Malaysian agriculture since it was introduced in 1870 by planting in the Botanical Gardens in Singapore. Later in 1918, the first commercial oil palm estate was set up at Tennamaran Estate, Selangor. Malaysia’s palm oil industry thrives due to the ideal climatic condition, efficient milling and refining facilities, and efficient management. Malaysians palm oil is accepted globally and exported to many countries such as China, India, and the Netherlands. In 2020, higher export revenue was at MYR 73.25 billion on average. The palm oil production for the country throughout January to December showed some decrement with 19.14 million tonnes from 19.86 million tonnes and 2.203 million tonnes from 2.322 million tonnes both for crude palm oil and crude palm kernel oil [27, 28].
Furthermore, the production of refined palm oil, such as cooking oil, was 0.414 million tonnes from 0.574 million tonnes [29]. Although the number shows some inflation, we cannot deny that palm oil is still the primary agricultural crop grown in Malaysia [30]. Until 2020, Malaysia’s total oil palm planted area was estimated at around 5.87 million hectares, indicating a decrease in the trend of 0.6% compared to 5.90 million hectares in the previous year. Sarawak is the largest state for palm oil plantation with 1.59 million hectares, followed by Sabah with 1.54 million hectares. The oil palm planted in peninsular Malaysia is around 2.74 million [31].
According to Kushairi and colleagues [32], the oil palm industry has the following stages: upstream, midstream, and downstream, as shown in Fig. 2. The upstream area covers the planting and cultivation of oil palm, including producing fresh fruit bunches, crude palm oil, and crude palm kernel oil. The midstream stage comprises refining, processing, and by-products valorisation. Finally, the downstream or the last stage for the industry chain consists of manufacturing the end products of the palm oil, which can be divided into two types of palm oil: (i) crude palm oil (CPO) from the fibrous mesocarp of FFB and (ii) crude palm kernel oil (CPKO) from palm kernel or the seed inside the shell of the endocarp. The oil palm’s average economic lifespan ranges between 25 to 30 years [33]. Palm oil is obtained from the mesocarp; palm kernel oil is obtained from the seed or kernel. These CPO and CPKO are mainly obtained for edible usages [34], cosmetics, personal care [35], skin-based pharmaceutical products [36] as well in biodiesel production [37].
However, palm oil mills and industries generate about 90% of the abundant raw materials and only 10% of the oil produced. The oil palm biomass waste is from the fruit bunches, leaves, and trunks, as shown in Fig. 3. The fresh palm fruits are removed from the fiber-like sacks during palm oil cultivation, leaving the empty oil palm fruit (EFB). The oil from fruits will then produce oil palm mesocarp fibers (MF) and oil palm kernel shells (OPKS). Palm oil mill effluent (POME) is the by-product of CPO production [38, 39]. Other than that, during the replanting and pruning period, the oil palm frond (OPF) and oil palm trunk (OPT) are mainly obtained from the plantation. The combustion of oil palm bunches or palm kernels in the mills can generate palm oil fuel ash (POFA). This high yield of palm oil production will generate a massive amount of biomass waste.
2.2 Problem encountered post palm oil cultivation
Palm oil production often leads to a large generation of residues or wastes, which inherit less economic value. This is very common in forestry and the agricultural sector [40]. For the palm oil industry, burning waste is one of the methods for disposing of it. However, since this method is considered harmful and often leads to the greenhouse effect, some mill operators will abandon the waste at their sites. More often than not, waste disposal gives rise to water and environmental pollution [41].
Furthermore, leaving the waste for an extended period may create a nest for the pests, resulting in more toxic chemicals in the soils. This will also produce a foul odor in the environment [42]. To reduce the causes of pollution while continuing with palm oil cultivation, several ways have been proposed to convert the abundant wastes into useful materials, which can be utilized in the industries [9].
The oil palm waste has been used for combustion and heating processes to exploit its potential fully. Generally, the conversion process will produce several products such as bio-oil or biochar from the pyrolysis method, syngas from gasification, ethanol from fermentation, biogas from anaerobic digestion, and heat from the combustion. These products provide low-carbon energy promoting alternative energy resources other than fossil fuels to a large extent. Pyrolysis is always preferred to dispose of the residue economically and convert it into alternative fuels [40]. Rapid biomass pyrolysis has gained interest as an alternative to biomass disposal. Fuel or bio-oil generation uses the oil palm shell to substitute synthetic phenol and formaldehyde [43]. Gasification of oil palm waste could produce hydrogen when replaced with synthetic precursors such as fossil fuel [44, 45]. Furthermore, many residues, including fiber shells, oil palm trunks, and empty fruit bunches, can be utilized by transforming them into various multipurpose products, including paper-making pulp, woods such as saw-wood, and plywood, briquette, and fillers in thermoplastics. Thermoset composites used as absorbents for removing pollutant gases such as nitrogen oxide and sulfur oxide are also examples of the products [18, 46,47,48,49,50].
It was acknowledged that oil palm waste comprises primary lignin, cellulose, and hemicellulose constituents, indicating the long chain of carbon and high carbon content in the oil palm waste. According to Ayinla et al. [9], empty fruit bunches and palm fibers contain the highest cellulose and hemicellulose content, respectively. Mohan et al. [51] report that the cellulose starts to degrade at 240 °C and ends at 360 °C, resulting in organic acid production and low pH value. On the other hand, hemicellulose degradation started at 200 °C and ended at 260 °C, producing less tar and less char than cellulose [51]. Table 2 shows the ultimate analysis of the typical palm oil by-products, including carbon (C), nitrogen (N), hydrogen (H), oxygen (O), and sulfur (S).
The analysis is essential before any conversion method to determine the major elements that existed in the carbon precursor. High fixed carbon and volatile contents of the palm oil by-products are favorable as good carbon precursors. At the same time, the elemental contents can be a good alternative in determining the formation mechanisms of the CNMS by comparing the initial and final percentages of the by-products. High initial carbon content is favorable in forming CNTs, as shown by Maryam, M. et al., whereby palm oil was used as the precursor via aerosol-assisted CVD [56]. The carbon content increase to 84.44% when the synthesis temperature is increased. N.H did a study. Abdul Rani [52] synthesized activated carbon from EFB and found that their fixed carbon content increased from 48.48 to 68.32 wt.% due to the release of volatile matter. Based on the works of literature discussed, most of the palm oil products such as empty fruit bunches [16, 19, 57], oil palm fibers [58,59,60], and even oil palm shells [19, 59, 61,62,63,64] are favorable to synthesized into activated carbons, biochar, and nanofiller epoxy. Later, other CNMs such as reduced graphene oxide [65] or CNT [66] are generated from the activated carbon of palm oil by-products. Meanwhile, CNTs [11, 12, 67, 68], carbon nanospheres [17, 69], and graphene [14, 70, 71] are commonly synthesized using crude palm oil or cooking oil. However, different conditions of synthesis, including high temperature [12, 19, 69, 72], percentage of catalyst used [16, 60, 64], or types of furnaces [11, 16, 20], do have a great impact in dictating the end synthesis products.
The crude palm oil and crude kernel palm oil contain a balanced ratio of unsaturated fatty acids (UFA) and saturated fatty acids (SFA) such as palmitic acid, lauric acid, oleic acid, and capric acid. In crude palm oil, it contains 50% of SPA with a high composition of 44.0%; meanwhile, crude kernel palm oil contains 85% of SFA with a high composition of lauric acid with 47.8%. The long carbon chain derived from the various acid found in palm oil summed up huge carbon content, which can become a potential green precursor in producing carbon-based nanomaterials that can be applied in various situations. Table 3 summarizes the physiochemical properties of cooking palm oil and crude palm oil. More discussion on the synthesis and methodology can be found in the following chapter.
3 Nanotechnology advancements in oil palm industries
3.1 Carbon-based nanostructured materials
Since the success of Taniguchi in manipulating single nanoscale objects in 1974, these miniaturized products have been dominating our daily lives progressively [79]. The advantage of having components in smaller sizes which promotes a high surface-to-volume ratio, is that they can improve their optical and electronic properties [80]. Thus, it offers exciting opportunities in various applications from physics to the life-biology field [81]. Among the nanomaterials, carbon nanomaterials or CNMs have been highlighted and one of the breakthroughs since the first report by Kroto and colleagues in 1985 [82] pursued by Iijima later in 1991 [83]. A wide range of CNMs is reported based on their diverse shape, composition, and orientation. The different allotropes in CNMs are generally based on carbon bonding. The 2 s and 2p play a different role for each hybridization; one 2 s and one 2p electron are mixed, giving rise to a linear chain of carbon atoms. Meanwhile, in sp2, the 2 s and two 2p orbitals form three in-plane covalent bonds that connect 20° evenly to the three carbons in XY-plane, creating two-dimensional planner sheets or honeycomb structures that derived graphene [84]. On the other hand, forming tetrahedrons between the electron will result in sp3, which forms a diamond-like structure [85].
The CNMs existed in different morphologies ranging from spheres, fibers, horns, onions, sheets, and tubes. To conclude, the allocation is fourfold: (i) 0—dimension CNMs such as fullerenes, onion-like carbons, carbon dots, and graphene quantum dots; (ii) 1—dimension CNMs such as carbon nanotubes, carbon nanofibers, and carbon nanohorns; (iii) 2—dimensions CNMs such as graphene, multilayer graphitic nanosheets, graphene nanoribbons, and; (iv) 3—dimensions CNMs such as nanostructured diamond-like carbon. The different dimensions of CNMs are grouped as in Fig. 4.
Molecular models of different types of hybridized carbon nanostructure exhibiting different dimensions: 0D, 1D, 2D, and 3D. Retrieved with permission from [86]
The different structures of CNMs are responsible for their remarkable properties, including their high strength, large surface area to volume, and intensified optical absorption properties to advance carriers for electrons and holes transports [87,88,89]. At the same time, the CNMs offer intrinsic strength about 200 times of steel yet are still malleable [90]. Table 4 compiled the different properties between some selected CNMs. Thus, promoting and exploiting the CNMs into various applications in our daily life such as in sensors [91], catalyst [92], fillers in composites [93], and energy conversion and storage [94, 95] to drug carries in cancer treatment [96]. Owing to the excellent performance of the CNMs, they have received a lot of attention from researchers. In the Web of Science itself, around 4745 related keywords “carbon nanomaterials” and “synthesis” were published from 2016 to 2020. The increasing number of publications in question is shown in Fig. 5.
Generally, the synthesis method has an essential role for the applications as it will affect the condition of the CNMs, including their morphologies and yield. Yi Yang and colleague proposed increasing the yield of carbon nanotubes or CNT due to increasing temperature and the gas flow rate [124]. On the other hand, using a catalyst such as a ferrocene can help speed up the catalytic decomposition of the carbon precursor, rice husk, and the plasma-enhanced in forming CNTs, as demonstrated by M. Asnawi and colleagues [125]. A study forward by Liu and colleagues shows that the length of hydrophilic groups from polymer carbon precursor, pyrrole–aniline polymers, could tune the diameter of the carbon nanospheres, which can be applied in the usage of high-performance supercapacitors [126]. Since the report on “vapour-grown carbon fibre” by Endo et al. (1995) [127], several techniques and scaled-up synthesis have been successfully established, such as high-temperature preparation methods, which include arc discharge and laser ablation. The growth of the temperature can reach 1726.85 °C, forming high crystallinity of CNMs, which leads to good conductivity and mechanical strength [128, 129]. However, Maurice et al. [130] state that the low-temperature techniques (< 800 °C) such as chemical vapor deposition or CVD and pyrolysis had been taken into account as the orientation, alignment, and other properties can be precisely controlled. This synthesis mainly required the decomposition of gases of hydrocarbons, and in certain circumstances, water and liquid nitrogen were also mixed with high-energy sources [131]. Arc discharge, laser ablation, and CVD are considered the primary conventional technique in producing CNMs, as shown in Fig. 6. According to O’Connell (2006) [132], the process is used to synthesize CNMs depends on the nature of the three major items needed, namely (i) carbon sources, (ii) catalyst nanoparticles, and (iii) energy input. Other than that, chemical routes such as chemical oxidation, solvothermal method, and ultrasonic energy were highlighted in producing CNMs on an extensive scale [133,134,135]. A facile yet homogenous reaction from the microwave method was also introduced in the production of CNMs [136].
Schematic diagram of the experimental apparatus for a arc discharge, b laser ablation, and c CVD. Retrieved with permission from [137]
Despite the advancements in synthesizing CNMs, toxic precursors, harsh environment, and post-synthetic steps often complicate the process afterwards and obstruct the possible wide applications [138, 139]. Challenges and drawbacks such as expensive methods, corrosive chemicals, and the formation of non-homogeneous products have been reported [136, 140]. Besides that, producing the CNMs at a large scale for a reasonable price is required to uphold the industrial demand. Unfortunately, it was claimed that not all the CNMs could be used in the market as the production is too costly [141]. Subsequently, the final price will be more expensive, unsuitable for wide usage and only limited to a specific niche of applications [95, 142]. These problems have been lingering in the production of CNMs; thus, the efforts and innovative research have been focusing on exploring renewable starting materials that are cost-efficient and readily available such as renewable plant-based precursors, waste, or low-cost biomass.
Renewable precursors are potential sources of hydrocarbons in supporting green alternatives in producing nanocarbon. Following the principles of green chemistry, the feedstock of any industrial process must be organic or renewable and not one that results in a deficiency of a natural resource [143]. Nishant Tripathi et al. synthesized carbon nanotubes at low temperatures (575 °C) by using green leaves of garden grass, rose, neem, Kaner, and walnut by thermal CVD. The MWNTs range from 8–15 nm with a yield of around 0.0113 gm [144]. Other than solid based–plant precursors, liquid-based plant precursors can be considered in preparing CMNs. Camphor oil, which can mainly be extracted from the wood of camphor trees, can contribute to CNT synthesis. Research carried out by TermehYousefi et al. (2014) was a success in synthesizing CNTs using camphor oil mixed with ferrocene using the CVD method [145]. They discovered that reducing the argon gas flow rate inside the reactor could result in a uniform central layer of CNTs like the previous layer. According to S. M.J and M. Rusop [146], adding the catalyst aluminium isopropoxide to camphor oil and ferrocene can produce single-walled CNTs in the range of 0.63–1.14 nm in diameter. Besides camphor oil, castor oil obtained from castor beans can also be used in nanocarbon syntheses. Raziah et al. successfully synthesized multiwalled carbon nanotubes (MWCNTs) from castor oil at 300–400 °C [147]. Other liquids and oils such as eucalyptus oil, sesame oil, sunflower oil, and turpentine oil can also be carbon precursors. Several works representing the usage of plant, plant extract or plant-based biomass in synthesizing CNMs are available and compiled [138, 148,149,150]. Table 5 shows other potential details of CNMs derived from various low-cost, abundant waste. However, not much research gives enough attention to the usage of specific plants such as palm oil in synthesizing CNMs.
3.2 Synthesis of CNMS from palm oil
The literature on the derivation of CNMs from palm oil is relatively limited. However, the publications on palm oil and its synthesis show an increasing trend. Oil palm by-products, including raw palm oil and wastes, could be categorized as excellent green precursors as several studies claimed that they produce nanocarbon, polymers precursor, and nanoparticles such as gold nanoparticles [12, 172, 173]. The oil palm by-products consisted of long carbon chains and lignocellulosic categorized as polymeric materials that may be suitable precursors for CNMs [174]. Cellulose is the highest (40–50%), a fundamental reinforcement unit for the green walls [175]. When exposed to extreme heat, these partially crystalline linear molecules can produce carbon residue without melting, hence fulfilling the applicable requirement for CNMs precursors [174, 176].
Despite the low-cost and abundant sources, palm oil usage as a carbon source for CNMs is discussed in this section. Oil palm waste is an excellent source of biomass for synthesizing carbon products, including biochar, and recently in the production of carbon nanomaterials [177]. Suriani and co-workers did early research in synthesizing CNMs by reporting the synthesis of CNTs using raw palm oil as the source [11]. Based on the past literature, most of the standard methods in the decomposition of palm oil into CNMs can be categorized in the thermal-based synthesis, such as CVD [66], pyrolysis [17], hydrothermal carbonization [15] as well as microwave [59]. However, another synthesis route that only applies chemical usage with no external heating is available, such as polymerization with POME [16, 22]. The synthesis method for converting oil palm waste into various carbon nanomaterials, including their composites, will be discussed further in the next sections.
3.2.1 Chemical vapor deposition
Carbon nanomaterials, or CNMs, can be synthesized using various methods, including arc discharge, laser ablation, mechanical exfoliation, and chemical reduction [178, 179]. The chemical vapor deposition or CVD method is preferable in producing CNMs as it has been proven capable of mass production of CNMs such as CNTs [180]. CVD has been used in growing carbon fibers since the 1990 s using cyanogen. Until 1993, when Endo and his colleague reported the growth of pyrolytic CNT via CVD, which marked the preliminary study of CNT via CVD [83, 180,181,182]. This chemical vapor deposition method converts some volatile carbon sources into a solid non-volatile carbon product—using catalysts during the catalytic chemical vapor deposition (CCVD) method. According to Hugh [183], CVD is a method that can be categorized as an atomistic vapor transfer process. The deposition’s outcome is atoms, molecules, or sometimes a combination of both. The growth of CNMs is associated with the decompositions of carbon sources with any presence of a catalyst. The carbon sources can exist in liquid, solid, or gas. The molecular structure of carbon could affect the morphology of the CNMs and lead to different CNMs’ outcomes [182].
The typical setup of CVD consists of carbon sources, carrier gas, a furnace, and an outlet tube. Generally, the carbon sources will decompose and form carbon atoms and hydrogen because of the heat or plasma generated in the reactor. Then, the carbon and hydrogen will be collected and adsorbed on the catalyst’s surface. The carbon atoms will form nanomaterials depending on the behavior of the catalyst [184]. Sometimes, the system uses the catalyst to “crack” the carbon sources into carbon and hydrogen by lowering the temperature [137]. The introduction of a catalyst is usually done by adding a “floating” catalyst or placing it in the middle of the quartz reactor [185]. The ability to provide a large scale of products from CVD is one of the critical factors that support the evidence that CVD is the preferable method in producing CNMs. Other than that, CVD has a flexible technology and method, making it uncomplicated and easy to manipulate. At the same time, the method can coat almost any shape and size of the preferable substrate. Thus, the method is economically competitive in sputtering on film or coating applications [137, 183]. The standard carbon sources exist in gas forms, such as carbon monoxide, ethane, methane, ethylene, and benzene [186]. Solid and liquid carbon precursors are gaining popularity in synthesizing CNMs via CVD. Generally, solid carbon precursors will be placed together with the catalyst inside the quartz boat and in the tubular reactor.
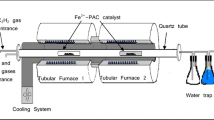
Palm oil–based has been found to thrive in the synthesis of CNMs since Suriani et al. synthesized vertically aligned carbon nanotubes, or VACNTs on 1cm2 areas of silicon substrates by selecting raw materials palm oil as the carbon source [11]. The experiment was conducted using a two-stage tube furnace system’s thermal chemical vapor deposition technique. The synthesis process at 450 °C fundamentally decomposed the ferrocene molecules, which catalyzed the Fe particles. The decomposition of rich hydrocarbons (also referred to as carbon, hydrogen, and oxygen molecules) also occurred throughout the process. Eventually, the hydrocarbon vapors will be mixed with nanosized Fe particles. Thus, the mixture ended up with the silicon substrates where the temperature at the fabrication area remained at 750 °C. Correspondingly, the carbons disintegrated into the Fe particles, leading to the release of hydrogen and oxygen. The dissolved carbons were condensed to nanotubes at nanosized Fe particles with approximately 90% purity with minimal amorphous carbon content.
In 2016, Suriani et al. discovered a way to scale up the carbon nanotube production by modifying the TCVD system [68]. Their study had to use waste cooking palm oil as the carbon precursor. The system had been modified by introducing continuous flow or supply of both catalyst and precursor by attaching them to a peristaltic sprayer. The collection for the CNTs is located at the end of the deposition zone for the CNTs. They successfully synthesized dense and uniform CNTs with a diameter ranging between 19.5 to 36.2 nm. Then, about 433.3 g of CNTs were collected from 945 g of carbon precursor. Suriani et al. claimed that the conversion rate was 56% higher than the volatile organic compound such as toluene, xylene, and benzene, which indicate that waste cooking oil palm is a suitable green alternative to the fossil fuel precursor.
Substrates play an important role in the growth of CNMs. Different CNMs could be grown on the substate due to a chemical reaction between the precursors and the substrate by varying the substrate’s condition or temperature. A study by Robaiah and colleagues investigates the effect of confined spaces in growing the CNTs [12]. They used stacking and non-stacking silicon substrates to test the proposed hypothesis. The experiment was done using 200 g of palm oil with 5 g of ferrocene, synthesized at 750 °C, 850 °C, and 950 °C. It was deduced that CNTs that grew on the stacking substrate had a better quality as they had a low ID/IG ratio, ranging from 0.89–0.94 for 750–950 °C, respectively. The D and G bands (ID/IG ratio) helps in estimating the graphene defects, and a higher ratio ensures more defects on graphene [187]. The CNTs have a better degree of nanotube crystallinity and better graphitization. The best deposition temperature for non-stacking and stacking substrates is 750 °C, as shown in Fig. 7.
Besides substrates, it is also observed that process temperature plays a vital role in producing the CNMs as CNTs favor high temperatures of synthesis ranging from 750–1000 °C. Ismail et al. (2019) evaluated the chemical vapor deposition process to synthesize multiwalled carbon nanotubes using waste cooking oil to develop a microstrip patch antenna [67]. The experiments were performed inside a furnace. The waste cooking oil was injected with a constant flow of 10 ml/H into an alumina ceramic tube at the center of the surface with iron oxide nanoparticles as the catalyst. Figure 7 shows the Field emission scanning electron microscopy (FESEM) and high-resolution transmission electron microscopy (HRTEM), which reveal that the MWCNT’s structure is mixed and consists of straight, spiral, and twisted forms with the average diameters ranging from 80–140 nm for 850 °C. The intensity ratio for ID/IG, on the other hand, is very close to zero, indicating a better degree of graphitization.
Besides CNT, graphene has also been successfully synthesized using palm oil by-products as a CVD method. Generally, in producing graphene, inert condition with high temperature is required. Rahman et al. reported the growth of a few-layer graphene substrate on nickel within the thermal chemical vapor deposition method [14]. They had confirmed the usage of refined cooking palm oil and heated it at 800–1000 °C. The samples were annealed at the set temperature and called the growth time. Later, the samples were cooled down inside the furnace. According to the Raman spectra, synthesized graphene at 1000 °C shows a more symmetrical and sharper 2D band with 80 cm−1 and 3.7 for the 2D band and IG/I2D, respectively. Another research conducted by Salifairus et al. determined a slightly lower deposition temperature than Rahman et al. [70]. This study used palm oil and nickel substrate with deposition’s temperature set at 900 °C and varied from 5 to 60 min. They deduced that high-quality graphene was produced at 15 min with ID/IG and crystallite size of 0.10 and 25.53 nm, respectively. In the same year, Robaiah and her colleague determined the synthesis of graphene by using a low deposition time of 15 min compared to the usual route, which took around 60 min [71]. In their study, different plant oil was used for carbon precursors such as refined palm oil (RPO), refined corn oil (RCO), and waste cooking palm oil (WCPO) [71]. The carbon precursor’s furnace was set at 300 °C in an argon atmosphere. WCPO shows the best result by forming a hexagonal pattern based on the FESEM images and a high-weight percentage of carbon, 1.54%. Meanwhile, the peak in Raman’s spectra shows a formation of amorphous carbon with 1400 cm−1 for D-band and 1580 cm−1 for G-band, respectively.
Mat Tahir et al. made another attempt to synthesize graphene using a different substrate to anneal the graphene [188, 189]. The research was done using oil palm fibers at 1020 °C inside a CVD reactor. The precursor was annealed and grew for 30 and 90 min, respectively. The SEM image shows the presence of ripple on the surface compared to pure copper. Meanwhile, the EDS analysis suggested that the carbon content is high at 61.03%, followed by 38.1% and 0.87% for copper and oxygen. Besides graphene and CNT, carbon nanospheres were also successfully produced using the CVD method by diversifying the temperature growth. A study performed by Zobir, Abdullah et al. produced carbon nanosphere by CVD with temperature between 850 and 1000 °C [72]. A ratio of 30:20 of palm olein (PO) and zinc nitrate solution was used as the carbon and catalyst precursor. As the size of the carbon-based materials depended on the temperature, the formation of carbon nanoparticles was observed at 900 °C with diameters of between 25 and 35 nm. The researcher concluded that secondary growth of carbon nanoparticles on the surface of the CNNS prepared at 850 °C transformed into carbon nanosphere when in the range of 900 °C, which is favorable for the growth temperature of the spheres.
Additionally, a study was done by Mamun, and his colleagues proved the growth of CNT on top of powdered activated carbon (PAC) of palm oil shells. Before impregnating the PAC with a Fe3+ catalyst, the PAC was first dried and calcinated with nitrogen at 350 °C for 1 h. Then, the PAC was reduced with hydrogen gas at the same period and temperature inside the multi-stage system of CVD. The PAC was impregnated with different percentages of Fe3+ for 2 h with N2 gas and then reduced with hydrogen gas for 1 h. The impregnated PAC was then placed inside the reactor and injected with H2 and C2H2 gases at different temperatures and reaction times. The optimum temperature for producing the nanocomposite was 550 °C, with 391 mg of yield produced. At the same time, the specific surface area (SBET) of the nanocomposite increased up to 9.7 times compared to the SBET of the raw PAC with 101.1 m2/g. The FESEM and TEM images showed that CNT ranges from 107–200 nm and an internal planer distance of 3.535 A. CVD methods are widely used and can be considered a preferred method in the production of CNMs since most of the syntheses would exhibit a high yield. The preparation method was more straightforward than other methods. Synthesis parameters, CMNs characteristics, technology readiness level (TRL), and future applications for the synthesized CMNs are illustrated in Table 6. Besides CVD, pyrolysis is also favorable for producing CNMs with different biomass, including palm oil by-products. Further discussion about the process is in the following section.
3.2.2 Pyrolysis
The pyrolysis method can be considered as one of the thermochemical conversions and an endothermic process [190, 191]. Pyrolysis is often a good solution to thermally upgrading biomass and plastics into energy or another valuable process. Pyrolysis can be defined as a process for decomposition of organic matter with the absolute absence of an oxidizing agent and takes place within a temperature range of 280 to 850 °C. Generally, the expected yield of pyrolysis is biochar (solid), biogas (gaseous), and bio-oil (liquid), all of which are relatively dependent on the operating condition, that is, slow, fast, and flash pyrolysis. The char production at low temperature, heating rate, and long vapor residence time can be defined as slow pyrolysis. Fast pyrolysis is a conversion route that involves heating the waste or biomass within the range of temperature between 400 and 600 °C for a short period. Fast pyrolysis often produces bio-oil with the by-product biochar [192]. Flash pyrolysis, on the other hand, results in high yields of bio-oil with low water content because the process involves a high reaction of temperature and high heating rates [193].
Generally, the process takes place inside a reactor that undertakes a crucial matter, especially the concept of heat distribution, heat supply, and volatile residence time in producing an optimum yield [194]. The typical pyrolysis reactor technologies are divided into three main sections; (1) feedstocks, that is, the system where the raw materials are heated and melted; (2) pyrolysis reactor operating at the desired temperature; and (3) products collector where the products are separated, recovered and purified for the next steps [195, 196]. Different reactors can be used for different pyrolyses, such as cylindrical fixed–bed, batch, and packed bed for slow biomass pyrolysis. Meanwhile, bubbling fluidized bed, rotating cone, and vortex for fast pyrolysis of biomass. On the other hand, the entrained microwave could be enhanced and used as the reactor for fast pyrolysis. Several studies have proved that biomass stocks can be used to produce carbon nanomaterials such as carbon nanotubes, carbon nanofibers, and graphene [20, 155]. Usually, for the production of CNMs, the stock precursors for CNMs are generated through pyrolysis, and later on, the precursors are deposited over catalyst or substrates such as nickel foam [197]. Carbon precursors play an essential role in synthesizing CNMs because the morphologies of the products depend on the characteristics of precursors. However, the presence of a catalyst is vital to enhance the reaction and transform the carbon precursor, especially when the carbon precursor has high thermal stability. Arie and the team produced carbon nanospheres sized approximately 200 nm with cooking oil waste mixed with ferrocene as the catalyst [69]. The experiment was conducted within the temperature of 650–750 °C for 20 min using nebulized spray pyrolysis; meanwhile, salacca peel–based activated carbon was used as the substrate. The ID/IG ratio was reduced after the deposition of the nanocarbon, approximately 0.899–0.87 for 650–750 °C, indicating the increase of graphitic carbons.
Another study by Kristianto and his colleague also produced carbon nanospheres (CNNs) using cooking palm oil as the precursor. The synthesis was done by utilizing a simple pyrolysis process under nitrogen ambient for an hour with a temperature of 700 °C [17]. In this research, commercial activated carbon was chosen to support the growth of CNNs with the addition of ferrocene as an iron catalyst. The ferrocene was varied from 2.5, 5.0, and 7.5 mg/100 ml. The optimum dense CNN yields a diameter of 87.5 nm when the catalyst concentration is 7.5 g/100 mL. The FESEM images for all different concentrations are shown in Fig. 8. There was a drop in the surface area due to the reaction.
FESEM images of activated carbon at various catalyst concentration a 2.5, b 5.0, and c 7.5 g/100 ml. Retrieved with permission from [17]
The gas, mainly nitrogen flow during the pyrolysis, is favorable in producing carbon nanospheres. In the research conducted by Ali et al. in 2014, oil palm frond as the carbon precursor in producing porous carbon nanoparticles (PCN) with uniform molecular sizes ranging between 35–45 nm [198]. The pyrolysis was done at the temperature of 500–600 °C for 2 h with a continuous nitrogen flow at 5 °C m−1. The PCN was observed for its electrochemical properties after being cooled down a room temperature. Another research done by Hegde et al. used pyrolyzed oil palm leaves as the carbon precursor in producing microporous carbon nanospheres using single-step pyrolysis under a nitrogen atmosphere at the temperature of 700 °C [199]. After synthesis, the samples were cleaned using 2.5 M NaOH solution followed by deionized waters. The FESEM images suggested that the nanoparticles were spherical with a porous structure with 40–50-nm particle sizes.
Generally, the synthesis of activated carbon involves two steps, namely, the carbonization and the activation process. The condition and parameters during carbonization are important because the initial pores of the char are generated during this process. Heating rate, temperature, and residence time are considered factors in the pyrolysis of the by-products. It is essential to determine the temperature and condition of gas flow during the process to ensure a full devolatilization of the by-products [200].
A pyrolysis study in producing activated carbon from different oil palm by-products such as the empty fruit bunches (EFB), mesocarp fibers (MF), and palm kernel shells (PKS) was conducted by Abd Wafti et al. [19]. The by-products were pyrolyzed and activated inside a tube furnace at 700 °C for 2 h with nitrogen [19]. The physical activation of AC was done with the purging of nitrogen for 15 min, followed by an increasing temperature of 10 °C per minute; when it reached 90 °C, the nitrogen gas was switched to carbon dioxide and kept constant for 30 min. The surface areas, SBET of the oil palm-based were 347.043 m2g−1 for EFB, 217.347 m2g−1 for MF, and 205.859 m2g−1 for PKS. The AC has microporous characteristics with a diameter of pores ranging from 2.0–3.5 nm. The study also found that process temperature was one of the significant factors in producing the AC before physical activation. The temperature varied from 500 to 900 °C. Abd Wafti et al. also found out that 800 °C is the optimum activation temperature as it gave the maximum specific surface area (SBET) of 937 m2 g−1 compared to the other temperature.
Another study by Hidayu and Muda suggests that chemical activation enhances the adsorption properties by impregnating different metal oxides [63]. Palm kernel shells were mixed with ZnCl2, heated to 550 °C, and washed with distilled water for chemical activation. Meanwhile, the palm kernel was only heated up to 300–800 °C without adding a catalyst in physical activation. After the impregnation process of various metal oxide ranging from BaO, MgO, CuO, TiO2, and CeO2, Hidayu and Muda concluded that the AC from chemical activation exhibits a high surface area of 1223 m2g−1 and the loaded activated carbon with BaO is more efficient due to the high BET surface area and high reactivity. Other than that, a pyrolysis study on the effect of different pyrolysis temperatures was conducted by Allwar et al., who had used oil palm shells with ZnCl2 as the catalyst [64]. The oil palm shells and ZnCl2 were mixed and refluxed in the water bath. After filtered and dried, the sample was loaded inside the reactor and heated for 3 h in various temperatures ranging from 400 to 700 °C with a constant nitrogen flow. The second pyrolysis stage was continued with carbon dioxide heated for 90 min to obtain the maximal adsorptive capacities. The study concludes that 500 °C is the optimum temperature for activating oil palm shells-AC with SBET of 1429.71 m2/g. The parameter used in synthesizing the discussed CNMs, characteristics of CMNs, technology readiness level, and future applications are summed in Table 7. Further discussion about pyrolyzing the oil palm by-products will be discussed in the next section. The method is improved with the introduction of microwave heating as the heating element.
3.2.3 Microwave
Microwave heating is different from conventional heating in terms of thermal energy delivery. According to K. Patel and C. Desai (2014) [201], the thermal energy is transferred to the bulk material by the conduction for conventional heating, while the heating of microwaves is via molecular interaction of the electromagnetic field with the surface of the material. Sahoo et al. (2018) state that the microwave-assisted method promotes direct heating of the reaction mixture, thus making it an attractive choice compared to the former conventional heating method [202]. Figure 9 shows the temperature profile of the reaction mixture after a few minutes for both conventional and microwave heating.
In conventional heating, the heat is transferred via convection. The electromagnetic energy will be converted into thermal energy in the microwave, and the heating process starts in the middle of the material. Microwave heating could be considered more efficient in producing high-temperature homogeneity and energy usage, advantageous for conventional heat sources.
Over the past few years, microwave heating has been widely used in chemical-related applications due to the capability of molecular level heating and the homogenous thermal reaction [203]. The ability of fast reactions promotes a new microwave-assisted synthesis used in many studies, including in chemical reactions and synthesis of nanomaterials [204] [205]. According to Y. Li et al. (2013) [206], solvent-free microwave-assisted extraction of bioactive compounds allows mass and heat transfer to move from inside the plant cell to the outside of it, which helps increase the temperature in a much shorter time [206]. The MW-assisted reaction can have high-purity results because fewer unwanted products were observed due to a shorter reaction time [207]. At the same time, the product could end up with a higher yield [206].
Microwave-assisted synthesis is a peculiar but simple technique that provides rapid and effective nanocarbon production with higher reproducibility. During the heating process, the dielectric properties of the receptor affect the maximum temperature of the material. A hot spot rises during the microwave heating of carbon-based materials, which mainly appear as tiny sparks or electric arcs with a local temperature higher than 1100 °C [208]. These hotspots have been recognized as thermal sensitizers due to microwave irradiation in the areas of environmental remediation, carbon nanostructures, organic synthesis, and the preparation of catalysts [209]. It has become an economic tool for reducing particle size and enhancing dissolution. Hence, this technique has been essential in organic materials’ synthesis action, especially nanocarbon [210]. The research on the development of fullerenes was written by T. I. Ikeda et al. (1995). They investigated the microwave-induced naphthalene-nitrogen plasma at atmospheric pressure via a cylindrical coaxial cavity [211]. Later, Kharissova (2004) disclosed an efficient one-step finding on synthesized vertically aligned CNTs from ferrocene using a domestic microwave oven [209]. Microwave-initiated carbon nanotube or the Pop-Tube approach is one of the methods for synthesizing carbon nanotube. Carbon materials and indium tin oxide (ITO) powders have been described as efficient heating sources for the growth of nanocarbon [212].
Microwave heating has been widely used in oil palm production, starting with preparation until after use [213]. Cheng et al. did a study, producing dry and clean crude palm oil extraction under stringent quality control parameters [214]. The use of the microwave completed the solvent extraction and recovery system, which simplified the complicated standard oil palm milling process into three significant steps, which could be a feasible method in addressing the economic aspects.
A study done by González-Navarro et al. suggested synthesizing activated carbon by chemical activation inside a modified commercial microwave furnace [59]. Oil palm shells were dried at 105 °C for 48 h and impregnated with LiOh solution, which was dried in an oven for 4 h at 100 °C. The samples were then activated inside a modified microwave oven with various power and exposure times ranging from 150–800 W and 5–60 min, respectively. ACs of 800 W had the most remarkable development of micropores, reaching 1350 m2/g SBET value. According to González-Navarro et al. [59], this was supported by the results obtained from the textural analysis using nitrogen isotherm, whereby the N2 adsorption capacity increased when the others did not. Another study by Foo and Hameed determines the use of other catalysts for the activated carbon’s chemical activation [58]. In their research, KOH was used as the catalyst with a ratio of 1:0.5 wt.% for char: KOH. The oil palm fibers were carbonized into char at 700 °C under purified nitrogen. After soaking them with KOH, the mixture was put in a modified microwave oven with the power set at 350 W at 5 min with a continuous nitrogen flow. The result was a versatile activated carbon with homogenous, high porosity of honey-combed structures compared to the original char as shown in Fig. 10. The generated AC’s SBET and total pore volume are 707.79 m2/g and 0.3806 m3/g, respectively.
SEM images for a char from oil palm fibers and b activated carbon from oil palm fibers. Retrieved with permission from [58]
Another research was done by Lam et al. had developed activated carbon from palm kernel shells (PKS) with a steam activation with different microwave powers and activation time of 500–700 W and 20–40 min, respectively. The PKSs were pyrolyzed inside a modified microwave oven for 30 min under different microwave power. Purging gas flow is not applicable in this study as a vacuum pump was used to remove the air inside the microwave to create a vacuum condition. Later, the biochar was subjected inside the microwave oven using steam as the activating agent with a heating rate of 10 °C/min. Like microwave pyrolysis, the steam activation does not require any gas flow as a vacuum pump was used to remove the air. The SEM images show AC that contains porous surface, and the SBET shows 257–419 m2/g of surface area. Manipulating gas flows and adding catalysts could affect the behavior or porous structure of the activated carbon. In research conducted by Guo and Lua, the appropriate amount of carbon dioxide could lead to the formation of desired activated carbon from oil palm stones via microwave-induced carbon dioxide or CO2 [215]. Oil palm stones were pyrolyzed inside the vertical tube furnace at 700 °C for 3 h before moving to the activation process. The microwave-induced reaction was conducted inside a microwave oven with different timespans and power of 5–60 min and 80–750 W, respectively. Purified carbon dioxide gas or nitrogen was used continuously for the synthesis. Using CO2, the gas could lower the yield of the AC due to the carbon-CO2 reaction during the microwave process. However, the maximum SBET was obtained at 412.5 m2/g at 750 W of 60 min with a CO2 flow rate. Adding CuO as the catalyst also increased the SBET to 527.6 m2/g, creating more mesoporosity.
The oil palm waste can be documented as a viable source in producing hollow carbon nanofibers via microwave-assisted synthesis. A study by Omoriyekomwan et al. showed an exciting result when producing biochar in their microwave pyrolysis study [20]. They synthesized hollow carbon nanofibers (HCNFs) during microwave pyrolysis of palm kernel shells (PKSs) with pure nitrogen gas passing through the reactor at a 400-ml/min flow rate. The 30-min reaction was conducted at 500 and 600 °C with nitrogen flow. Later, the char was mixed with activated carbon and loaded into a microwave oven with 200 W, and synthesis was run for 3 min with nitrogen flow. The synthesis produced HCNFs with 0.34 nm of layer spacing. The tubular, hollow and bamboo-like shape of HCNFs was estimated to be 50–100 mm in diameter. A higher concentration of carbon layer of HCNFs was detected at 600 °C with a 0.86 ID/IG ratio. Omoriyekomwan et al. proposed a growth mechanism for the HCNFs called “self-extrusion model growth,” which had initiated between the microwave irradiation and the volatiles or bio-liquid from the biomass shown in Fig. 11. Heating and penetration of microwaves could trigger heating and devolatilization of the biomass particles, causing the formation of carbon nanospheres on the surface of the particles. The nanospheres then extended into a cylindrical form due to microwave exposure, which induced heating and electrical arcs throughout the microwave pyrolysis. The liquid tar continuously extruded itself out of the biomass particles forming the growth of HCNFs through the nano-size chamber.
Illustration of the self-extrusion mechanism proposed by J. E. Omoriyekomwan et al. to grow HCNFs. Retrieved with permission from [20]
Hossain et al. demonstrated the microwave pyrolysis of oil palm fiber (OPF) divided into three types of Na-based catalysts, including sodium hydroxide (NaOH), sodium chloride (NaCl), and sodium carbonate (Na2CO3) in the production of biochar [60]. The maximum biochar yield collected after the microwave pyrolysis was 51.42 wt.% using the catalyst NaOH with an N2 flow rate of 200 cm3/min. The biochar had porous holes in the microstructure observed from the SEM and BET analysis and had a BET surface area of 260 m2/g. The researcher had also conducted an ultimate and proximate analysis of biochar-OP to compare the elemental analysis with another research. They could conclude that the carbon percentages of biochar produced from oil palm shell [216] and corn stover [217] were 59.42% and 62.8%, respectively. These results are like biochar-OPF. An investigation conducted by Omar et al. on microwave pyrolysis of empty fruit bunch (EFB) concludes that properties of EFB match those of other biomass feedstocks with dielectric properties and moisture content reaching up to 60% at 2.4Ghz [218].
Other than that, another pertinent research was conducted by Marpongahtun, Gea et al. (2018) [21]. They successfully demonstrated the conversion of oil palm empty fruit bunches to carbon nanodots (CNs). The empty fruit brunches underwent alkaline bleaching and hydrolysis to produce nanocrystal cellulose. The cellulose was then placed inside the microwave at different heating temperatures within 250–400 °C to produce carbon nanodots. The TEM results displayed rod-like forms, all of which are randomly arranged.
Other than microwave, pyrolysis, CVD, or manipulation of both methods, CNMs could be synthesized in other physical or chemical ways. In the next section, other methods of producing CNMs that have not been mentioned will be discussed briefly. The parameters in producing CNMs are summarized in Table 8 based on their synthesis conditions, CMNs characteristics, technology readiness level, and future applications.
3.2.4 Other synthesis methods
Based on the past literature, there are other methods for synthesizing CNMs using oil palm–based carbon precursors. Most methods involve the thermal conversion of the feedstocks or the carbon precursor. Hydrothermal carbonization is one of the popular methods used to convert palm oil. The method involved producing carbonaceous materials (hydro-char) for the most part. These materials are from pressurized low-temperature thermal conversion of feedstocks [219]. The common feedstocks used in hydrothermal carbonization are biomass such as sucrose, glucose, fructose, starch, cyclodextrin, and biomass derivates such as 5-hydroxymethyl-furfural-1-aldehyde (HMF) [220, 221]. According to Inagaki and his colleagues (2014) [220], hydrothermal carbonizations can be divided into two classes, depending on the temperature applied, (i) high temperature (> 400°c) and (ii) low temperature (< 250 °C). The high-temperature hydrothermal carbonization often produces various carbon materials, including multiwalled carbon nanotubes, fullerenes, and carbon spheres with different nanotextures. Often carbons produced in the method do not have enough rigidity and will collapse upon the silica removal. Therefore, the method is often followed by further pyrolysis to stabilize the carbon skeleton [222].
Interestingly, a study done by Mahat and Shamsudin synthesized carbon quantum dots (CQDs) from activated carbon originating from oil palm empty fruit bunches (OPEFBs) [15]. OPEFBs were heated for 3 h in a furnace for carbonization and activation purposes with nitrogen ambient. Next, 6 g of the activated carbon was used in synthesized carbon quantum dots via hydrothermal carbonization with a low-temperature process resulting in an average diameter of 507 nm of spherical shapes of CQDs.
In research conducted by Hendriansyah et al., empty fruit bunches and palm oil mill effluent (POME) were utilized to produce activated carbon via hydrothermal carbonization followed by pyrolysis [16]. The synthesis was carried out in the hydrothermal reactor at 275 °C using ZnCl2. After 40 min, the activation process was done inside the tubular furnace at 800 °C with a flow of carbon dioxide for 2 h. The surface area for activated carbon is 383.748 m2/g with a pore size of 2.14 nm. Based on the SEM images, more pores existed on the biomass surface, forming holes, thus increasing the surface area of the activated carbon. In the same study, Hendriansyah and his colleagues also prepared graphene and nanotube via a combination of carbonization and chemical reaction. For graphene, the biomass is mixed with FeCl3, ZnCl2, and water, and it is dried for 18 h at 105 °C. After that, the mixture was heated inside an atmospheric furnace at 800 °C for 1 h.
Meanwhile, in synthesizing CNT, polymerization of POME was done by mixing it with formaldehyde and NH4OH for 4 h at 95 °C. After drying, the powder form was minced and mixed with ferrocene and ethanol and then pyrolyzed using a tubular furnace at 90 °C for 2 h with the presence of N2. The surface area for the CNMs is 401.17 and 526.420 m2/g for graphene and CNT, respectively.
Another method for synthesizing activated carbon identified by past studies is the carbonization of raw materials followed by char activation. Carbonization can be defined as converting the three-dimensional organic macromolecular systems to a three-dimensional “macro-atomic” network of carbon atoms [223]. In research conducted by Nicholas and his colleague, 5 g of palm kernel shells (PKS) treated with 20% of H3PO4 were carbonized using a tubular furnace at 500–900 °C for 2 h into activated carbon [61]. After cleaning and oven-dried, the sample showed that the carbonization temperature of 500 °C had the highest graphitic content with an IG/ID of 1.166. The FESEM images show a well-developed porous structure. The pores are generally regular in size and shape. Several other nanomaterials, such as nanocellulose and epoxy nanocomposites, were synthesized from oil palm frond leaves, and oil palm empty fruit bunches were reported. The carbon-based nanomaterials were hydrolysed and mechanically stirred into nanocellulose and epoxy.
Other than that, a combination method of carbonization and improved graphene oxide synthesis to produce graphene oxide was done by Nasir et al. [10]. Oil palm leaves, palm kernel shells, and empty fruit bunches were used as the precursors in the study—the carbonization of the sample is done to produce as-carbonized materials. The precursor is heated inside a furnace with a temperature ranging between 400 to 900 °C for 3 h with nitrogen flow. After that, the samples were coagulated with diethyl ether after being washed with deionized water, HCl, and ethanol. The prepared GOs were reduced using low-temperature annealing at 300 °C. The IG/ID obtained ratio are 1.06, 1.14, and 1.20 for RGO from oil palm leaves, palm kernel shells, and empty fruit bunches, providing evidence for the graphitic nature of the sample.
Meanwhile, the RGO produced tiny pores and nano ball-like-structure, distributed heterogeneously because of the different nature of the starting precursors. Table 9 summarized the parameters in producing CNMs based on their synthesis condition, characteristics of the CMNs, technology readiness level, and future applications. Further discussion regarding the possible applications will be discussed in the following section.
4 Application of carbon-based nanostructured materials derived from palm oil
The excellent properties and advantages of the CNMs have led to the expansion of their applications across various industrial sectors throughout the century. These included geometrical complexity, unique properties behavior, and mass customization or manufacturing, which the traditional materials could not have offered. Carbon nanomaterials are widely used in electronics, energy applications, medical, biomedical engineering, aerospace, wastewater management, and automobiles. Whether CNMs can be prepared from various palm oil-based precursors, their available applications are still inadequately reported, with only a few available in the literature [228]. This study said that most of the CNMs ventured into supercapacitors, electrode cells, fillers inside nanocomposite, and absorbers for cationic dyes. The idea of having fossil fuels as the sources of the precursor is worrying due to their being unrenewable and not environmentally friendly. Ergo, the concept of synthesizing CNMs derived from oil palm, should gain more consideration by determining other possible applications based on the CNMs’ properties.
Spherical nanocarbon-shape has been used and tested as electrode materials for energy storage devices. Carbon nanospheres pyrolyzed at 750 °C from waste cooking oil were conducted by Arie et al. They showed a shape like a rectangle with horn peak form on the cut-voltage above 3.5 V, indicating a charging process [69]. At the same time, the specific capacitance of the carbon nanospheres of 97.75 mV/s at 0.1 mV/s was detected with the reduction of the value on the second cycle, indicating the stability of the electrode will be better in the broader scar rate. Porous carbon nanoparticles (PCNs) derived from dried palm fronds showed excellent supercapacitor behavior in KOH due to their porous structure and particle size, 35 nm [198]. The specific capacitance was 343 Fg−1 at 5mVs−1. The PCNs also showed high cyclic stability of 90% and a high coulombic efficiency of 95%. Other than that, the strength and availability of carbon spheres are tested as fillers in the nanocomposites. A study was performed by Hegde et al. in pyrolyzing microporous carbon nanospheres using oil palm leaves and spin-coated into nanocomposites film with poly (3, 4-ethylenedioxythiophene) and poly(4-styrenesulfonate) polymer [199]. The nanocomposite showed a high gauge factor, correlation coefficient, and conductivity of 34.57, 0.86996, and 39.27 Sm−1, respectively, implying a good behavior as a piezoresistive flexible strain sensor.
At the same time, CNTs derived from palm oil were also proven to have good conductivity and can be used in energy storage applications. R. Hendriansyah et al. studied the capacitance of a CNT mixture on the electrode cells [16]. They had reported that the capacitance increased to 2.4137 F/g when CNT was mixed with activated carbon (AC) compared to 1.7554 F/g for AC alone. This happened since CNT has better conductivity that contributed to the increment in capacitance [229]. Other than that, CNT synthesized from cooking palm oil was tested to fabricate CNT/natural rubber latex nanocomposite as the electrode in supercapacitor application. The I–V analysis presented an excellent conductivity value at 2.55 × 10−3 S/cm. Meanwhile, the graph is in rectangular shapes indicating good capacitance behavior of the reaction [68]. Due to the conductivity of the CNT, a study regarding the usage of CNT as an antenna for the microstrip patch was done by synthesizing the CNT from waste cooking oil with the help of iron oxide nanoparticles as the catalyst [67]. After pyrolysis, the CNT paste was printed onto the Kapton substrate with a connector (attached as the patch) and a copper (attached as the ground plane). The performance of the fabricated antenna was examined using a network analyzer in terms of the reflection loss of the sample. The result revealed that the carbon content in the magnetic particles allows for better absorption as the minimum reflection loss was 12.42 dB at 5.46 GHz for the CNT synthesized at 650 °C.
The excellent porosity of activated carbon from oil palm by-products makes it suitable for adsorbent, especially in water treatment. A study carried out by Foo et al. determined the availability of the AC from oil palm fiber in adsorbing methylene blue [58]. The study decided that the BET surface area for the AC was 707.79 m2/g and capable of adsorbing the methylene blue with an adsorption capacity of 312.5 mg/g. Other studies conducted by Wafti et al. [19] and Wirasnita et al. [57] also found out the capability of their ACs in adsorbing methylene blue with the high surface area of 86.62 m2/g and 937 m2/g, respectively. Other than adsorbent for dyes, activated carbon derived from oil palm fibers and shells were used as hydrogen storage based on their N2 adsorption–desorption isotherms. The study conducted by González-Navarro et al. concluded that activated carbon synthesized at 800 W for 15 min in a microwave reactor shows the highest hydrogen storage capacity with an increase in N2 adsorption at low relative pressure [59]. The study conducted by Nicholas et al. also indicates the capability of activated carbon from palm kernel shells as the shape-stabilized phase change materials with encapsulation of n-octadecane into the pores. The resulting nanocomposites show good thermal stability and stand up to 500 melting and freezing cycles for thermal energy storage applications. Hendriansyah et al. prepared activated carbon from empty fruit bunches and POME with the capacitance of 1.7554 F/g, which can be used as a supercapacitor [16].
A study performed by Tahir N.A.M et al. confirmed the tribological effects of graphene growth from oil palm fiber [188]. They concluded that the presence of graphene on top of the substrate could reduce the coefficient of friction. The capability of graphene as capacitance was proved by Hendriansyah et al. when their research team synthesized graphene with 4.3808 F/g capacitance mixed with activated carbon. Other than graphene, synthesis of reduced graphene oxide was performed by Nasir et al. by mixing EFB-derived graphite-like powder with concentrated H2SO4/H3PO4 and KMnO4. The reduced graphene is mixed with empty fruit bunch–derived activated carbon. Later, the mixture is impregnated with n-noncadecane to form shape-stabilized nanocomposites with the latent heat of composites of 82.72 J/g for 37.25 °C and − 622.22 J/g for 25.58 °C, which could be utilized for energy conservation building. Another application of the reduced graphene oxide is electrode materials in supercapacitor application as shown by Ali, G. et al. when they produced RGO from empty fruit bunch, palm kernel shell, and oil palm leaves via carbonation and improved hummers method.
Other studies were also observed for applying CNMs derived from palm oil–based carbon nanofillers, carbon quantum dots, and even cellulose nanocrystals. But unfortunately, only a few were reported. The compilation of various CNMs syntheses from palm oil–based and their application can be found in Table 5.
5 Future prospect and challenges
Oil palm has been considered an essential source for vegetable oils with higher productivity than other crops. Malaysia could generate oil palm waste due to the high production of FFB with high cultivation yield of palm oil in peninsular, Sabah, and Sarawak with 1.82, 1.87, and 1.35 tonnes/hectare, respectively. It goes without saying that when these palm wastes are left unattended, they will cause environmental pollution. It can affect the underground water through leaching, soil pollution due to unwanted chemicals, and unpleasant odor. As a matter of environment is concerned, what are the measures that should be taken to tackle the disposal issue? This brings another question: how will the parties involved transform the unwanted waste into something beneficial? Due to the specialized functional properties of carbon-based nanomaterials in several areas of interest, they should be considered in the end-production, as it is the main objective of this paper.
The derivation of the synthesis process, especially for carbon-based materials, was explored continuously to diminish and limit the disadvantages during the process. Among the criteria required during the process is rapid and high producibility techniques. Thus, the common heat-related conversions of oil palm wastes into CNMs such as CVD, pyrolysis, and microwave were discussed in “Sect. 3.” Interestingly, other synthesis methods such as hydrothermal, carbonization, and polymerization were also identified in producing CNMs such as CNT, graphene, CNS, and activated carbons. The research shows the progress of each conversion method. CVD, pyrolysis, and microwave methods are favored among research to produce CNMs. Some by-product precursors needed high temperatures to crack the long carbon chain and volatilized to convert the materials. The method can also produce high yields of CNMs.
Additionally, the CNMs have grown on top of the substrates, used widely in electronics applications. This can help reduce the workload in growing the CNMs on top of the substrates. There are, however, some drawbacks to the synthesis method. High temperature can lead to high power consumption, which is disadvantageous if the process is continuously conducted on a larger scale. Several research has been done to solve the temperature issue using catalysts, time manipulation, and environmental gas.
Nevertheless, unwanted impurities in the CNMs are possible, especially if the purification is not properly done. On the other hand, activated carbon production when using pyrolysis requires several steps. Thus, char should be prepared by combining the preparation and activation methods and introducing a feasible synthesis method. Other than that, the typical microwave oven will not have any temperature detector. Thus, it would be difficult to control the temperature during the synthesis as a heat curve is sought after to determine the process temperature. Therefore, future research should consider using an infrared temperature camera to use a microwave oven. This is because the reading of the thermocouple rod is not accurate due to its position. Every variable manipulated during the research will have its pros and cons. Hence, more studies should be enhanced to find the optimum parameters or combine the method in producing the CNMs.
The present study also captured different methods for using the CNMs derived from oil palm waste, which has high potential, especially in the energy field. The CNMs is used for energy storage application such as electrodes and capacitance. Other than that, the CNMs are used in fillers and antennas in microstrip. The magnificent properties of the CNMs produced by green precursors allow for various implementations. Thus, more studies should be done on adsorption, capacitance, and thermal properties.
Technology readiness level or TRL is a scale or a method invalidating the maturity of technology from the basic idea generation to commercialization. There were 9 levels allocated for the TRL framework, as summarized in Table 10 [230, 231]. TRL 1–3 were defined as lab scales, meanwhile TRL 4–6 as pilot scales and TRL 7–9 as commercialization. TRL for all the processes synthesizing carbon nanomaterials from oil palm by-product materials is shown in Tables 6, 7, 8, 9. Generally, in Malaysia, the synthesis and production of CNMs from oil palm by-products using common synthesis methods such as CVD, microwave, and pyrolysis are still around TRL 3–5. Suriani et al. [68] found a way to scale up carbon nanotubes production by modifying the TCVD system with a continuous supply of catalyst and precursor by attaching them to a peristaltic sprayer. They claimed that the conversion rate was around 56% higher than the common volatile organic compound.
Meanwhile, Lam et al. [62] combined microwave synthesis with steam activation in producing an 83% yield of highly porous activated carbon. Besides that, Promraksa et al. [232] optimized biochar production from palm kernel shells, empty palm fruit bunches, and oil palm fibers by the Box-Behnken design and RSM analysis. They determined that the highest biochar yield was 44.91 wt% with pyrolysis at 525 °C with nitrogen flow rate. These studies show that the combination and modification of the traditional method can help in boosting the yield production of the CNMs. At the same time, studies claiming low power, synthesis time, and temperature could be the reference for high-scale production as it will save time, energy, and waste of the raw materials [16, 20, 60, 232]. Furthermore, pyrolysis was claimed and reached the commercial scale in Malaysia, but unfortunately, it is limited to producing biocharcoal and bio-oil only.
Regarding the current situation for the synthesis of the CNMs, further research is required to obtain the appropriate technology readiness level for commercial implementation. To maximize the commercialization of the conversion, several measures should be taken, namely (i) efficient supply chain of the oil palm waste by introducing an integrated way of collecting and storing the waste from the palm oil mills; (ii) encouragement of more R&D projects involving the analysis and conversion of the waste in individual or big company’s plantations; (iii) introduction of policies and laws to encourage the synthesis of oil palm waste and; (iv) government should consider in investing and for the start-up for conversion of waste into the CNMs in the palm oil mills.
6 Conclusion
Nanotechnology and the derivation of nanomaterials could solve the issues in the palm oil industry. As the palm oil industry is expanding and developing, disposing of oil palm waste will continue to become a major drawback, as presented in “Sect. 2.” By combining various synthesis methods, the wastes could be feasible and renewable resources. Heat-related conversions of palm oil by-products such as CVD, pyrolysis, microwave, and other methods were discussed in “Sect. 3.” Thus, by-products can be transformed into beneficial CNMs, explored in various applications ranging from energy storage and wastewater treatment, as discussed in “Sect. 4.” We are aware there are some drawbacks regarding the industry, especially in the development of commercialization of the CNMs and the synthesis methods itself; hence, the prospects and challenges faced by the palm oil industry are discussed in “Sect. 5.” Based on the studies and possible applications of CNMs, we believe that the conversion of oil palm wastes can contribute to the clean carbon-based production of nanomaterials in the future.
References
Sapey E et al (2012) Collection of oil palm (Elaeis guineensis Jacq.) Germplasm in the Northern Regions of Ghana. Asian J Agric Sci 4(5):325-328
Department of Agriculture, U.S. Production, supply and distribution: reports & data 2021 [cited 2021 26 November 2021]; Available from: https://doi.org/https://apps.fas.usda.gov/psdonline/app/index.html#/app/downloads. Accessed 26 Nov 2021
Ritchie H, Roser M (2021) Forests and deforestation. OurWorldInData.org
Department of Agriculture, U.S. Palm oil explorer. Palm Oil 2021: World Production 2021 [cited 2021 26 November 2021]; Available from: https://ipad.fas.usda.gov/cropexplorer/cropview/commodityView.aspx?cropid=4243000&sel_year=2021&rankby=Production. Accessed 26 Nov 2021
Ahmad A, Buang A, Bhat A (2016) Renewable and sustainable bioenergy production from microalgal co-cultivation with palm oil mill effluent (POME): a review. Renew Sustain Energy Rev 65:214–234
Loh SK (2016) The potential of the Malaysian oil palm biomass as a renewable energy source. Energy Conversion and Management 141:285–298
MPOB. Oil Palm planted area as at December 2019 (Hectars) bepi.mpob.gov.my 2020; Available from: https://bepi.mpob.gov.my/images/area/2019/Area_summary.pdf. Accessed 4 Aug 2021
Kurnia JC et al (2016) Advances in biofuel production from oil palm and palm oil processing wastes: a review. Biofuel Res J 3(1):332–346
Ayinla RT et al (2019) A review of technical advances of recent palm bio-waste conversion to activated carbon for energy storage. J Clean Prod 229:1427–1442
Nasir S et al (2017) Oil Palm Waste-Based Precursors as a Renewable and Economical Carbon Sources for the Preparation of Reduced Graphene Oxide from Graphene Oxide. Nanomaterials (Basel) 7(7):18
Suriani AB et al (2009) Synthesis of vertically aligned carbon nanotubes using natural palm oil as carbon precursor. Mater Lett 63(30):2704–2706
Robaiah M et al (2018) Synthesis of carbon nanotubes from palm oil on stacking and non-stacking substrate by thermal-CVD method. AIP Conf Proc 1963(1):020027
Salifairus MJ et al (2016) The effect of synthesis time on graphene growth from palm oil as green carbon precursor. AIP Conference Proceedings 1733(1)
Rahman S, Mahmood M, Hashim A (2014) Growth of graphene on nickel using a natural carbon source by thermal chemical vapor deposition. Sains Malaysiana 43:1205–1211
Mahat NA, Shamsudin SA (2020) Transformation of oil palm biomass to optical carbon quantum dots by carbonisation-activation and low temperature hydrothermal processes. Diamond Relat Mater 102:107660
Hendriansyah R et al (2017) Nano carbon materials from palm oil wastes for supercapacitor applications. in 2017 4th International Conference on Electric Vehicular Technology (ICEVT).
Kristianto H et al (2015) Synthesis and characterization of carbon nanospheres using cooking palm oil as natural precursors onto activated carbon support. Procedia Chem 16:328–333
Shuit SH et al (2009) Oil palm biomass as a sustainable energy source: a Malaysian case study. Energy 34(9):1225–1235
Abd Wafti NS et al (2017) Activated carbon from oil palm biomass as potential adsorbent for palm oil mill effluent treatment. J Oil Palm Res 29:278–290
Omoriyekomwan JE et al (2017) Formation of hollow carbon nanofibers on bio-char during microwave pyrolysis of palm kernel shell. Energy Convers Manage 148:583–592
Marpongahtun et al (2018) Synthesis of carbon nanodots from cellulose nanocrystals oil palm empty fruit by pyrolysis method. J Phys Conf Ser 1120:012071
Elias N et al (2017) Structure and properties of oil palm-based nanocellulose reinforced chitosan nanocomposite for efficient synthesis of butyl butyrate. Carbohyd Polym 176:281–292
Organization, W.H., WHO Director-General’s opening remarks at the media briefing on COVID-19-11 March 2020. 2020, Geneva, Switzerland
Darby S (2014) Palm oil facts and figures. Sime Darby Plantation: Profile and Fact Sheets, Sime Darby: Kuala Lumpur, Malaysia. p. 1–8
MPOB. Monthly production trend of crude palm oil 2019/2020. 2020 [cited 2021; Available from: https://bepi.mpob.gov.my/index.php/en/production/production-2020/production-trend-2020. Accessed 2 Aug 2021
Department of Statistics, M., Selected agricultural indicators, Malaysia, 2020. 2020
MPOB (2020) Production of crude palm oil for the month of January-December 2019 & 2020 (Tonnes). [cited 2021; Available from: https://doi.org/https://bepi.mpob.gov.my/index.php/en/production/production-2020/production-of-crude-oil-palm-2020. Accessed 3 Aug 2021
MPOB (2020) Production of crude palm kernel oil for the month of January-December 2019&2020 (Tonnes). Available from: https://doi.org/https://bepi.mpob.gov.my/index.php/en/production/production-2020/production-of-crude-palm-kernel-oil-2020. Accessed 3 Aug 2021
MPOB (2020) Monthly production of selected processed palm oil for the month of January-December 2019&2020 (Tonnes). [cited 2021; Available from: https://doi.org/https://bepi.mpob.gov.my/index.php/en/production/production-2020/production-of-processed-palm-oil-2020. Accessed 3 Aug 2021
Ahmad AL, Ismail S, Bhatia S (2003) Water recycling from palm oil mill effluent (POME) using membrane technology. Desalination 157(1–3):87–95
MPOB (2020) Oil Palm planted area 2020. Available from: https://doi.org/https://bepi.mpob.gov.my/images/area/2020/Area_summary.pdf. Accessed 13 Aug 2021
Kushairi A et al (2017) Oil palm economic performance in Malaysia adn R&D progress in 2017 - Review Article. J Oil Palm Res 2018:163–195
Lim KO, Zainal Alauddin ZA, Quadir GA, Abdullah MZ (1999) Energy productivity of some plantation crops in Malaysian and the status of bio energy utilization. Proceeding of WREC
Pande G, Akoh CC, Lai OM (2012) Food uses of palm oil and its components. Palm OIl
Dawe D et al (2019) Farming systems in southeast Asia, Ferranti P, Berry EM, Anderson JR (Eds.). Elsevier
Singh I et al (2019) An evaluation of crude palm oil (CPO) and tocotrienol rich fraction (TRF) of palm oil as percutaneous permeation enhancers using full-thickness human skin. Pharm Dev Technology
Mekhilef S, Siga S, Saidur R (2011) A review on palm oil biodiesel as a source of renewable fuel. Renewable and Sustainable Energy Reviews
Rudi Dungani PA, Aprilia S, Yuniarti K, Karliati T, Suwandhi I, Sumardi I (2018) Biomaterial from oil palm waste: properties, characterization and applications
Maluin FN, Hussein MZ, Idr AS (2020) An overview of the oil palm industry: challenges and some emerging opportunities for nanotechnology development. Agronomy
Abdullah N, Sulaiman F (2013) The oil palm wastes in Malaysia. Biomass Now: Sustainable Growth and Use, 75-93
Seyed Ehsan H, Mazlan Abdul W (2015) Pollutant in palm oil production process. Journal of the Air & Waste Management Association 65(7):773–781
Umar HA et al (2021) Assessing the implementation levels of oil palm waste conversion methods in Malaysia and the challenges of commercialisation: towards sustainable energy production. Biomass Bioenergy 151:106179
Md Kawser J, Farid Nasir A (2000) Oil Palm Shell As A Source of Phenol. Journal of Oil Palm Research 12:86–94
Azali A et al (2005) Development of gasification system fuelled with oil palm fibres and shells. American Journal of Applied Sciences 2(2):72–75
Kelly-Yong TL et al (2007) Potential of hydrogen from oil palm biomass as a source of renewable energy worldwide. Energy Policy 35(11):5692–5701
Bernama TMNN (2001) Long term benefit from oil palm biomass. 16th August
Council PAMPO (2006) Palm oil industry- a learning experient
Husin M et al (2002) Research and development of oil palm biomass utilization in wood-based industries. Palm Oil Dev 36:1–5
Sumathi S, Chai SP, Mohamed AR (2008) Utilization of oil palm as a source of renewable energy in Malaysia. Renew Sustain Energy Rev 12(9):2404–2421
Saifuddin NM, Palanisamy K (2005) Removal of heavy metal from industrial wastewater using chitosan coated oil palm shell charcoal. Electron J Biotechnol (ISSN: 0717–3458):8 Num 1, 8
Mohan D, Pittman CU Jr, Steele PH (2006) Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuels 20(3):848–889
Abdul Rani NH (2013) Characterization of activated carbon prepared from oil palm empty fruit bunch using BET and FT-IR techniques. Procedia Eng 68:379–384
Abdul Aziz M, Uemura Y, Sabil K (2011) Characterization of oil palm biomass as feed for torrefaction process. 1–6
Aminu Aliyu S, Nurhayati A, Fauziah S (2018) Potential application of oil palm wastes charcoal briquettes for coal replacement
Guangul FM, Sulaiman SA, Raghavan VR (2012) Elemental and thermo-chemical analysis of oil palm fronds for biomass energy conversion. AIP Conf Proc 1440(1):1197–1205
Maryam M et al (2013) Synthesis and characterization of carbon nanotubes from palm oil by aerosol-assisted CVD. Adv Mater Res 667:218–223
Wirasnita R et al (2015) Preparation and characterization of activated carbon from oil palm empty fruit bunch wastes using zinc chloride. J Teknol 74(11)
Foo KY, Hameed BH (2011) Microwave-assisted preparation of oil palm fiber activated carbon for methylene blue adsorption. Chem Eng J 166(2):792–795
González-Navarro MF, Giraldo L, Moreno-Piraján JC (2014) Preparation and characterization of activated carbon for hydrogen storage from waste African oil-palm by microwave-induced LiOH basic activation. J Anal Appl Pyrol 107:82–86
Hossain MA et al (2017) Catalytic microwave pyrolysis of oil palm fiber (OPF) for the biochar production. Environ Sci Pollut Res 24(34):26521–26533
Nicholas AF et al (2018) Palm kernel shell activated carbon as an inorganic framework for shape-stabilized phase change material. Nanomaterials 8(9):14
Lam SS et al (2019) Microwave pyrolysis with steam activation in producing activated carbon for removal of herbicides in agricultural surface water. Ind Eng Chem Res 58(2):695–703
Hidayu AR, Muda N (2016) Preparation and characterization of impregnated activated carbon from palm kernel shell and coconut shell for CO2 capture. Procedia Eng 148:106–113
Allwar A, Md Noor A (2008) Nawi M (2008) Textural Characteristics of Activated Carbons Prepared from Oil Palm Shells Activated with ZnCl2from Oil Palm Shells Activated with ZnCl2 and Pyrolysis Under Nitrogen and Carbon Dioxide. Journal of Physical Science 19(1):93–104
Nasir S et al (2021) Preparation of Shape-stabilized phase change material by the valorization of oil palm waste: reduced graphene oxide-activated carbon derived carbon matrix for thermal energy storage. BioResources 16:96–117
Mamun AA et al (2018) Carbon nanotubes grown on oil palm shell powdered activated carbon as less hazardous and cheap substrate. Appl Nanosci 8(7):1767–1779
Ismail I et al (2019) Synthesis of multiwalled Carbon Nanotubes (MWCNTs) from waste cooking oil catalyzed by mill-scale waste for development of microstrip patch antenna (MPA)
Suriani AB et al (2016) Scaled-up prototype of carbon nanotube production system utilizing waste cooking palm oil precursor and its nanocomposite application as supercapacitor electrodes. J Mater Sci Mater Electron 27(11):11599–11605
Arie A et al (2017) Synthesis of carbon nano materials originated from waste cooking oil using a nebulized spray pyrolysis. J Phys Conf Ser 877:012020
Salifairus MJ et al (2018) The synthesis of graphene at different deposition time from palm oil via thermal chemical vapor deposition. AIP Conf Proc 1963(1):020007
Robaiah M et al (2018) Morphology and topography study of graphene synthesized from plant oil. AIP Conf Proc 1963:020045
Zobir SAM et al (2012) Synthesis of carbon nano- and microspheres using palm olein as the carbon source. Mater Lett 78:205–208
Yahya S, Razali FH, Harun FW (2019) Physicochemical properties of refined palm cooking oil and used palm cooking oil. Mater Today Proc 19:1166–1172
Choi H, Lee E, Lee KG (2014) Quality evaluation of noble mixed oil blended with palm and canola oil. J Oleo Sci 63(7):653–660
de Almeida ES et al (2021) Thermal and physical properties of crude palm oil with higher oleic content. Appl Sci 11(15):7094
Esteban B et al (2012) Temperature dependence of density and viscosity of vegetable oils. Biomass Bioenerg 42:164–171
Devi A, Khatkar BS (2017) Thermo-physical properties of fats and oils. Int J Eng Tech Res (IJETR) 7:45–50
Firestone D (2006) Physical and chemical characteristics of oils, fats, and waxes
Taniguchi N (1974) On the basic concept of nanotechnology. Proceeding of the ICPE
Nasrollahzadeh M et al (2019) Applications of Nanotechnology in Daily Life, in Interface Science and Technology 113-143.
Saliev T (2019) The advances in biomedical applications of carbon nanotubes. C 5(2):29
Kroto HW et al (1985) C60: Buckminsterfullerene. Nature 318(6042):162–163
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354(6348):56–58
Mohamed E (2017) Nanotechnology: future of environmental air pollution control. Envirol Manag Sustain Dev 6:429
Georgakilas V et al (2015) Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem Rev 115(11):4744–4822
Terrones M et al (2010) Graphene and graphite nanoribbons: morphology, properties, synthesis, defects and applications. Nano Today 5(4):351–372
Marathe U, Padhan M, Bijwe J (2021) Carbon nanotubes- a powerful nano-filler for enhancing the performance properties of polyetherketoneketone composites and adhesives. Compos Sci Technol 210:108813
Han L et al (2021) Nitrogen-containing carbon nano-onions-like and graphene-like materials derived from biomass and the adsorption and visible photocatalytic performance. Appl Surface Sci 543:148752
Gspann TS et al (2017) High thermal conductivities of carbon nanotube films and micro-fibres and their dependence on morphology. Carbon 114:160–168
Lee C et al (2008) Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321(5887):385–388
Liu C, Lafdi K, Chinesta F (2020) Durability sensor using low concentration carbon nano additives. Compos Sci Technol 195:108200
Esteves LM, Oliveira HA, Passos FB (2018) Carbon nanotubes as catalyst support in chemical vapor deposition reaction: a review. J Ind Eng Chem 65:1–12
Bai Q-Q et al (2017) Dispersion and network formation of graphene platelets in polystyrene composites and the resultant conductive properties. Compos A Appl Sci Manuf 96:89–98
Choi J et al (2019) Waste coffee management: deriving high-performance supercapacitors using nitrogen-doped coffee-derived carbon. C 5(3):44
Kumar S et al (2018) Carbon nanotubes: a potential material for energy conversion and storage. Prog Energy Combust Sci 64:219–253
Chen Z et al (2017) The Advances of carbon nanotubes in cancer diagnostics and therapeutics. J Nanomater 2017:3418932
Horky P et al (2018) Nanoparticles as a solution for eliminating the risk of mycotoxins. Nanomaterials (Basel): 8(9)
Chen L, Wang X, Kumar S (2015) Thermal transport in fullerene derivatives using molecular dynamics simulations. Sci Rep 5(1):12763
Raccichini R et al (2015) The role of graphene for electrochemical energy storage. Nat Mater 14(3):271–279
Konno T et al (2018) A dramatic improvement in the tensile strength of fullerene needle-like crystals. New Carbon Mater 33:310–315
Zeiger M et al (2015) Review carbon onions for electrochemical energy storage. J Mater Chem A 4
Wei X et al (2021) Carbon nano-onions as a nanofiller for enhancing thermal conductivity of epoxy composites. Appl Nanosci
Portet C, Yushin GN, Gogotsi Y (2007) Electrochemical performance of carbon onions, nanodiamonds, carbon black and multiwalled nanotubes in electrical double layer capacitors. Carbon 45:2511–2518
Mamidi N (2020) Carbon nano-onions reinforced multilayered thin film system for stimuli-responsive drug release. Pharmaceutics 12:1208
Baskakova KI et al (2021) Effect of toluene addition in an electric arc on morphology, surface modification, and oxidation behavior of carbon nanohorns and their sedimentation in water. Nanomaterials 11(4):992
Kim JH et al (2020) Enhanced thermoelectric properties of WS2/single-walled carbon nanohorn nanocomposites. Curr Comput-Aided Drug Des 10(2):140
Khoerunnisa F et al (2011) Electronically modified single wall carbon nanohorns with iodine adsorption. Chem Phys Lett 501(4):485–490
Nan Y et al (2017) Probing the mechanical properties of carbon nanohorns subjected to uniaxial compression and hydrostatic pressure. Carbon 125:236–244
Lekawa-Raus A et al (2014) Electrical properties of carbon nanotube based fibers and their future use in electrical wiring. Adv Func Mater 24(24):3661–3682
Kausar A, Ilyas H (2017) Current Research Status and Application of Polymer/Carbon Nanofiller Buckypaper: A Review. Polymer-Plastics Technology and Engineering 56(16):1780–1800
Campos-Delgado J et al (2008) Bulk production of a new form of sp2 carbon: crystalline graphene nanoribbons. Nano Lett 8(9):2773–2778
Murali R et al (2009) Breakdown current density of graphene nanoribbons. Appl Phys Lett 94(24):243114
Arjmand M et al (2019) Carbon Nanotube versus graphene nanoribbon: impact of nanofiller geometry on electromagnetic interference shielding of polyvinylidene fluoride nanocomposites. Polymers 11(6):1064
Mazilova TI, Sadanov EV, Mikhailovskij IM (2019) Tensile strength of graphene nanoribbons: an experimental approach. Mater Lett 242:17–19
Sang M et al (2019) Electronic and thermal properties of graphene and recent advances in graphene based electronics applications. Nanomaterials (Basel, Switzerland) 9(3):374
Yemata TA et al (2017) 6 - Conducting polymer-based thermoelectric composites: principles, processing, and applications. In Hybrid polymer composite materials, V.K. Thakur, M.K. Thakur, and A. Pappu, Editors. Woodhead Publishing, 169–195
Sykam N, Rao GM (2018) Lightweight flexible graphite sheet for high-performance electromagnetic interference shielding. Mater Lett 233:59–62
Inagaki M, Qiu J, Guo Q (2015) Carbon foam: preparation and application. Carbon 87:128–152
Gallego N, Klett J (2003) Carbon foams for thermal management. Carbon 41:1461–1466
Nufer S et al (2018) Carbon nanofoam supercapacitor electrodes with enhanced performance using a water-transfer process. ACS Omega 3(11):15134–15139
Seifert G, Kuc A, Heine T (2011) Hexagon preserving carbon nanofoams
Röcker D et al (2021) Design of 3D Carbon nanotube monoliths for potential-controlled adsorption. Appl Sci 11(20):9390
Chen J et al (2012) Superlow thermal conductivity 3D carbon nanotube network for thermoelectric applications. ACS Appl Mater Interfaces 4(1):81–86
Yang Y, Zhang H, Yan Y (2019) Synthesis of CNTs on stainless steel microfibrous composite by CVD: effect of synthesis condition on carbon nanotube growth and structure. Compos B Eng 160:369–383
Asnawi M et al (2018) Synthesis of carbon nanomaterials from rice husk via microwave oven. J Nanomater 2018:2898326
Liu Z et al (2018) Controlled synthesis of carbon nanospheres via the modulation of the hydrophilic length of the assembled surfactant micelles. Langmuir 34(35):10389–10396
Endo M et al (1995) Pyrolytic carbon nanotubes from vapor-grown carbon fibers. Carbon 33(7):873–881
Chrzanowska J et al (2015) Synthesis of carbon nanotubes by the laser ablation method: effect of laser wavelength. Phys Status Solidi (B) 252(8):1860–1867
Sugai T et al (2003) New synthesis of high-quality double-walled carbon nanotubes by high-temperature pulsed arc discharge. Nano Lett 3(6):769–773
He Z et al (2014) Growth mechanisms of carbon nanostructures with branched carbon nanofibers synthesized by plasma-enhanced chemical vapour deposition. CrystEngComm 16(14):2990–2995
Arora N, Sharma NN (2014) Arc discharge synthesis of carbon nanotubes: comprehensive review. Diam Relat Mater 50:135–150
O'Connell MJ (2006) Carbon nanotubes: properties and applications. Taylor & Francis Group, Boca Rton
Hu S et al (2013) Chemical regulation of carbon quantum dots from synthesis to photocatalytic activity. Chem Asian J 8(5):1035–1041
Shams HR et al (2013) Solvothermal synthesis of carbon nanostructure and its influence on thermal stability of poly styrene. Compos B Eng 55:362–367
Krishnaveni M, Asiri AM, Anandan S (2020) Ultrasound-assisted synthesis of unzipped multiwalled carbon nanotubes/titanium dioxide nanocomposite as a promising next-generation energy storage material. Ultrason Sonochem 66:105105
Liu Y et al (2019) Facile growth of carbon nanotubes using microwave ovens: the emerging application of highly efficient domestic plasma reactors. Nanoscale Adv 1(12):4546–4559
Mubarak NM et al (2014) An overview on methods for the production of carbon nanotubes. J Ind Eng Chem 20(4):1186–1197
Kurian M, Paul A (2021) Recent trends in the use of green sources for carbon dot synthesis–a short review. Carbon Trends 3:100032
Farshbaf M et al (2017) Carbon quantum dots: recent progresses on synthesis, surface modification and applications. Artif Cells Nanomed Biotechnol 46:1–18
Martindale BCM et al (2015) Solar hydrogen production using carbon quantum dots and a molecular nickel catalyst. J Am Chem Soc 137(18):6018–6025
Charitidis CA et al (2014) Manufacturing nanomaterials: from research to industry. Manuf Rev 1:11
Chen Z-G et al (2012) Nanostructured thermoelectric materials: current research and future challenge. Prog Nat Sci Mater Int 22(6):535–549
Kharissova OV et al (2019) Greener synthesis of chemical compounds and materials. R Soc Open Sci 6(11):191378–191378
Tripathi N, Pavelyev V, Islam SS (2017) Synthesis of carbon nanotubes using green plant extract as catalyst: unconventional concept and its realization. Appl Nanosci 7(8):557–566
TermehYousefi A et al (2014) Fast synthesis of multilayer carbon nanotubes from camphor oil as an energy storage material. BioMed Res Int 2014:691537
Salifairus MJ, Rusop M (2013) Synthesis of carbon nanotubes by chemical vapour deposition of camphor oil over ferrocene and aluminum isopropoxide catalyst. Adv Mater Res 667:213–217
Raziah AZ, Junizah AR, Saifuddin N (2012) Synthesis of carbon nanotubes using natural carbon precursor: castor oil. AIP Conf Proc 1482(1):564–567
Kumar R, Singh RK, Singh DP (2016) Natural and waste hydrocarbon precursors for the synthesis of carbon based nanomaterials: graphene and CNTs. Renew Sustain Energy Rev 58:976–1006
Mugadza K et al (2020) Synthesis of carbon nanomaterials from biomass utilizing ionic liquids for potential application in solar energy conversion and storage. Materials 13:18
Janas D (2020) From bio to nano: a review of sustainable methods of synthesis of carbon nanotubes. Sustainability 12(10):4115
Shams SS et al (2015) Synthesis of graphene from biomass: a green chemistry approach. Mater Lett 161:476–479
Chen F et al (2016) Facile synthesis of few-layer graphene from biomass waste and its application in lithium ion batteries. J Electroanal Chem 768:18–26
Qu J, Luo C, Cong Q (2011) Synthesis of multi-walled carbon nanotubes/ZnO nanocomposites using absorbent cotton. Nano-Micro Lett 3(2):115–120
Bernd MGS et al (2017) Synthesis of carbon nanostructures by the pyrolysis of wood sawdust in a tubular reactor. J Market Res 6(2):171–177
Shi K et al (2014) Catalyst-free synthesis of multiwalled carbon nanotubes via microwave-induced processing of biomass. Ind Eng Chem Res 53(39):15012–15019
Zhu J et al (2012) Synthesis of multiwalled carbon nanotubes from bamboo charcoal and the roles of minerals on their growth. Biomass Bioenerg 36:12–19
Fan Y et al (2014) Micro-mesoporous carbon spheres derived from carrageenan as electrode material for supercapacitors. J Power Sources 268:584–590
Pari G, Darmawan S, Prihandoko B (2014) Porous carbon spheres from hydrothermal carbonization and KOH activation on cassava and tapioca flour raw material. Procedia Environ Sci 20:342–351
Ari-Wahjoedi B, Razali R, Narahari M (2012) International conference on fundamental and applied sciences 2012:(ICFAS2012). in American Institute of Physics Conference Series
Suriani AB et al (2013) Vertically aligned carbon nanotubes synthesized from waste chicken fat. Mater Lett 101:61–64
Suriani AB et al (2014) Quasi-aligned carbon nanotubes synthesised from waste engine oil. Mater Lett 139
Quan C, Li A, Gao N (2010) Synthesis of carbon nanotubes and porous carbons from printed circuit board waste pyrolysis oil. J Hazard Mater 179(1–3):911–917
Purkait T et al (2017) Large area few-layer graphene with scalable preparation from waste biomass for high-performance supercapacitor. Sci Rep 7(1):15239
Jacob MV et al (2015) Catalyst-free plasma enhanced growth of graphene from sustainable sources. Nano Lett 15(9):5702–5708
Sankar S et al (2017) Ultrathin graphene nanosheets derived from rice husks for sustainable supercapacitor electrodes. New J Chem 41(22):13792–13797
Mahmoud ME, Fekry NA, Abdelfattah AM (2020) A novel nanobiosorbent of functionalized graphene quantum dots from rice husk with barium hydroxide for microwave enhanced removal of lead (II) and lanthanum (III). Bioresource Technol 298:122514
Yong W (2015) Hydrothermal preparation of highly porous carbon spheres from hemp (Cannabis sativa L.) stem hemicellulose for use in energy-related applications. Ind Crops Products 65:216-226–2015 v.65
Shukla K et al (2020) A novel approach to utilize used disposable paper cups for the development of adsorbent and its application for the malachite green and rhodamine-B dyes removal from aqueous solutions. Nat Environ Pollut Technol 19(1):57–70
El Essawy NA et al (2017) Green synthesis of graphene from recycled PET bottle wastes for use in the adsorption of dyes in aqueous solution. Ecotoxicol Environ Saf 145:57–68
Sujiono EH et al (2020) Graphene oxide based coconut shell waste: synthesis by modified Hummers method and characterization. Heliyon 6(8):e04568
Hashmi A et al (2020) Muffle atmosphere promoted fabrication of graphene oxide nanoparticle by agricultural waste. Fullerenes Nanotubes Carbon Nanostruct 28(8):627–636
Tajau R et al (2017) Palm oil-based precursors for development of polymeric delivery system. Malaysian J Anal Sci 21:496–511
Ahmad T et al (2016) Effect of reaction time on green synthesis of gold nanoparticles by using aqueous extract of elaise guineensis (oil palm leaves). Procedia Eng 148:467–472
Watt, W. and B.V.e. Perov, Strong fibres. Vol. 1. 1985: Elsevier Science Limited
Demirbas A, Arin G (2002) An overview of biomass pyrolysis. Energy Sources 24(5):471–482
Karimi K, Taherzadeh MJ (2016) A critical review of analytical methods in pretreatment of lignocelluloses: composition, imaging, and crystallinity. Biores Technol 200:1008–1018
Abdul Khalil HPS et al (2013) Dynamic mechanical properties of activated carbon–filled epoxy nanocomposites. Int J Polymer Anal Charact 18:247–256
Nessim GD (2010) Properties, synthesis, and growth mechanisms of carbon nanotubes with special focus on thermal chemical vapor deposition. Nanoscale 2(8):1306–1323
Mittal G et al (2015) A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J Ind Eng Chem 21:11–25
Colomer JF et al (2000) Large-scale synthesis of single-wall carbon nanotubes by catalytic chemical vapor deposition (CCVD) method. Chem Phys Lett 317(1):83–89
Endo M et al (1993) The production and structure of pyrolytic carbon nanotubes (PCNTs). J Phys Chem Solids 54(12):1841–1848
Ghaemi F et al (2019) Chapter 1 - Synthesis of carbon nanomaterials using catalytic chemical vapor deposition technique, in Synthesis, technology and applications of carbon nanomaterials, S.A. Rashid, R.N.I. Raja Othman, and M.Z. Hussein, Editors. Elsevier, 1–27
Hugh OP (1999) Handbook of chemical vapor deposition (CVD) principles, Technology, and Applications. Second EditionWilliam Andrew Publishing, llc Norwich, New York, USA
Baker RTK et al (1972) Nucleation and growth of carbon deposits from the nickel catalyzed decomposition of acetylene. J Catal 26(1):51–62
Nyamori VO, Mhlanga SD, Coville NJ (2008) The use of organometallic transition metal complexes in the synthesis of shaped carbon nanomaterials. J Organomet Chem 693(13):2205–2222
Kumar M, Ando Y (2010) Chemical vapor deposition of carbon nanotubes: a review on growth mechanism and mass production. J Nanosci Nanotechnol 10(6):3739–3758
Palaniselvam T, Aiyappa HB, Kurungot S (2012) An efficient oxygen reduction electrocatalyst from graphene by simultaneously generating pores and nitrogen doped active sites. J Mater Chem 22(45):23799–23805
Tahir NAM et al (2020) Effect of hydrogen on graphene growth from solid waste products by chemical vapour deposition: friction coefficient properties. Ind Lubr Tribol 72(2):181–188
Mat tahir N et al (2017) Potential of growing graphene from solid waste products
Singh R et al (2015) Chapter 10 - Hydrothermal liquefaction of biomass. In: Pandey A et al (eds) Recent advances in thermo-chemical conversion of biomass. Elsevier, Boston, pp 269–291
Sabzoi N et al (2017) Advanced nanomaterials synthesis from pyrolysis and hydrothermal carbonization: A review. Current Organic Chemistry 21(5):446–461
Bruckman VJ, Pumpanen J (2019) Chapter 17 - Biochar use in global forests: opportunities and challenges, in Developments in soil science, M. Busse, et al., Editors. Elsevier, 427–453
Li L et al (2013) Chapter 8 - An introduction to pyrolysis and catalytic pyrolysis: versatile techniques for biomass conversion. In: Suib SL (ed) New and future developments in catalysis. Elsevier, Amsterdam, pp 173–208
Collard FX, Carrier M, Görgens JF (2016) Chapter 4 - Fractionation of lignocellulosic material with pyrolysis processing. In: Mussatto SI (ed) Biomass fractionation technologies for a lignocellulosic feedstock based biorefinery. Elsevier, Amsterdam, pp 81–101
Khan MU et al (2020) Chapter 14 - Valorization of municipal solid waste in biorefineries for the creation of a circular economy: role of emerging technologies, in Current developments in biotechnology and bioengineering, R. Kataki, et al., Editors. Elsevier, 323–347
Gao F (2010) Pyrolysis of waste plastics into fuels, University of Canterbury
Zhang S et al (2020) Sustainable production of value-added carbon nanomaterials from biomass pyrolysis. Nat Sustain 3(9):753–760
Ali G et al (2014) High performance supercapacitor using catalysis free porous carbon nanoparticles. J Phys D Appl Phys 47:495307
Hegde R et al (2019) Synthesis of carbon nanospheres and piezoresistive study of carbon nanospheres-PEDOT:PSS nanocomposite flexible thin film for strain sensing applications. Mater Res Express 6(7):9
Rafatullah M et al (2013) Oil palm biomass as a precursor of activated carbons: a review. Crit Rev Environ Sci Technol 43(11):1117–1161
Patel K, Desai C (2014) Comparison between Joule heating, Microwave heating and Combined heating. International Journal of Engineering Research & Technology (IJERT) 3(6):303–306
Sahoo BM, Banik B, Panda J (2018) Microwave-assisted green chemistry approach, 475–508
Brunetti FG et al (2007) Reversible microwave-assisted cycloaddition of aziridines to carbon nanotubes. J Am Chem Soc 129(47):14580–14581
Chen W-X, Lee J, Liu Z (2004) Preparation of Pt and PtRu nanoparticles supported on carbon nanotubes by microwave-assisted heating polyol process. Mater Lett - MATER LETT 58:3166–3169
Xu Q-C et al (2007) Microwave-assisted synthesis of MgO–CNTs supported ruthenium catalysts for ammonia synthesis. Catal Commun 8(12):1881–1885
Li Y et al (2013) Solvent-free microwave extraction of bioactive compounds provides a tool for green analytical chemistry. TrAC Trends Anal Chem 47:1–11
Muñoz TE et al (2004) Microwave-assisted immunostaining: a new approach yields fast and consistent results. J Neurosci Methods 137(2):133–139
Nizamuddin S et al (2019) Chapter 5 - Microwave-assisted synthesis for carbon nanomaterials. In Nanomaterials Synthesis, Beeran Pottathara Y et al (Eds.). Elsevier, 121–147
Kharissova O (2004) Vertically aligned carbon nanotubes fabricated by microwaves. Adv Mater Sci 7:50–54
Gupta D et al (2018) 26 - Microwave synthesized nanocomposites for enhancing oral bioavailability of drugs. In Applications of nanocomposite materials in drug delivery, Inamuddin A, Asiri M, Mohammad A (Eds.). Woodhead Publishing, 619–632
Ikeda T, Kamo T, Danno M (1995) New synthesis method of fullerenes using microwave-induced naphthalene-nitrogen plasma at atmospheric pressure. Appl Phys Lett 67(7):900–902
Zhang X, Liu Z (2012) Recent advances in microwave initiated synthesis of nanocarbon materials. Nanoscale 4(3):707–714
Nelson SO, Trabelsi S (2002) Sensing grain moisture content through dielectric properties. in IEEE Antennas and Propagation Society International Symposium (IEEE Cat. No.02CH37313)
Cheng SF, Nor LM, Chuah CH (2011) Microwave pretreatment: a clean and dry method for palm oil production. Ind Crops Products 34(1):967–971
Guo J, Lua AC (2000) Preparation of activated carbons from oil-palm-stone chars by microwave-induced carbon dioxide activation. Carbon 38(14):1985–1993
Tripathi M et al (2016) Thermophysical characterization of oil palm shell (OPS) and OPS char synthesized by the microwave pyrolysis of OPS. Appl Therm Eng 105:605–612
Brewer CE et al (2009) Characterization of biochar from fast pyrolysis and gasification systems. Environ Prog Sustain Energy 28(3):386–396
Omar R et al (2011) Characterization of empty fruit bunch for microwave-assisted pyrolysis. Fuel 90(4):1536–1544
Cai J et al (2016) Hydrothermal carbonization of tobacco stalk for fuel application. Biores Technol 220:305–311
Inagaki M et al (2014) Chapter 4 - Carbonization under pressure. In: Inagaki M et al (eds) advanced materials science and engineering of carbon. Butterworth-Heinemann, Boston, pp 67–85
Deliyanni EA (2019) Chapter 5 - Low-cost activated carbon from rice wastes in liquid-phase adsorption, in Interface Science and Technology, G.Z. Kyzas and A.C. Mitropoulos, Editors. Elsevier, 101–123
García-Bordejé E, Pires E, Fraile JM (2021) Chapter 8 - Carbon materials functionalized with sulfonic groups as acid catalysts, in Emerging Carbon materials for catalysis, S. Sadjadi, Editor. Elsevier, 255–298
Marsh H, Rodríguez-Reinoso F (2006) CHAPTER 2 - Activated carbon (origins). In: Marsh H, Rodríguez-Reinoso F (eds) Activated carbon. Elsevier Science Ltd, Oxford, pp 13–86
Budhi Y et al (2018) Preparation of cellulose nanocrystals from empty fruit bunch of palm oil by using phosphotungstic acid. IOP Conf Ser Earth Environ Sci 105:012063
Lani NS et al (2014) Isolation, characterization, and application of nanocellulose from oil palm empty fruit bunch fiber as nanocomposites. J Nanomater 2014:702538
Saba N et al (2016) Fabrication of epoxy nanocomposites from oil palm nano filler: mechanical and morphological properties. BioResources 11(3):7721–7736
Nasir S et al (2019) Development of new carbon-based electrode material from oil palm waste-derived reduced graphene oxide and its capacitive performance evaluation. J Nanomater 2019:1970365
Nasir S et al (2018) Potential Valorization of By-product Materials from Oil Palm: A review of Alternative and Sustainable Carbon Sources for Carbon-based Nanomaterials Synthesis. Bioresources 14(1):2352–2388
Khomenko V, Raymundo-Piñero E, Béguin F (2006) Hybrid supercapacitors based on α-MnO2/carbon nanotubes composites. in New Carbon Based Materials for Electrochemical Energy Storage Systems: Batteries, Supercapacitors and Fuel Cells. Springer Netherlands, Dordrecht
Armstrong K (2015) Chapter 13 - Emerging industrial applications. In: Styring P, Quadrelli EA, Armstrong K (eds) Carbon dioxide utilisation. Elsevier, Amsterdam, pp 237–251
Neill SP, Hashemi MR (2018) Chapter 1 - Introduction, in Fundamentals of ocean renewable energy, Neill SP, Hashemi MR (Eds.). Academic Press, 1–30
Kristianto H et al (2016) The effect of activated carbon support surface modification on characteristics of carbon nanospheres prepared by deposition precipitation of Fe-catalyst. In Second International Conference on Chemical Engineering
Funding
The Ministry of Higher Education Malaysia funds this research under Fundamental Research Grant no. FRGS/1/2018/TK05/UM/02/11 (ID:12965)—FP005-2018A and University of Malaya research grant no. GPF032A-2018.
Author information
Authors and Affiliations
Contributions
Nurul Zariah Jakaria Zakaria: writing—original draft preparation; Shaifulazuar Razali: supervision, project administration and writing—review and editing; Nabisab Mujawar Mubarak: supervision, writing—review and editing; Suriani Ibrahim: supervision, and review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zakaria, N.Z.J., Rozali, S., Mubarak, N.M. et al. A review of the recent trend in the synthesis of carbon nanomaterials derived from oil palm by-product materials. Biomass Conv. Bioref. 14, 13–44 (2024). https://doi.org/10.1007/s13399-022-02430-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02430-3