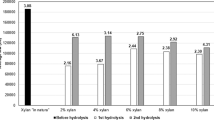
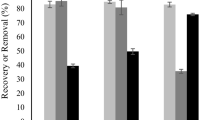
Abstract
Valorization of renewable carbon sources such as lignocellulosic biomass (LCB) or agricultural residues to produce value-added bioproducts has attracted increasing attention. In this way, a sustainable and abundantly present biopolymer xylan can be utilized to produce xylo-oligosaccharide (XOS) which has potential benefits for individuals and the environment as well. Xylan can be enzymatically degraded into XOS via enzymatic hydrolysis (EH), but the inherently recalcitrant xylan limiting the yield of XOS by incomplete hydrolysis. The aim of this study is to identify the factors hindering the efficient EH of extracted xylan from corn stalk (CS). For this purpose, xylan extracted from CS sequentially fractionated into three different fractions followed by primary enzymolysis, acetic acid treatment, and secondary enzymolysis. The results showed that the structural complexity, degree of branching, and/or substituents of the substrate also played a vital role during enzymolysis and therefore the solubility was not the only factor to determine the hydrolysis ratio. Compared with the primary enzymolysis, the yield of XOS from CS extracted xylan increased from 48 to 67% with an additional 10% of xylose obtained in the dual enzymolysis and acid treatment. The total enzymolysis ratio reached up to 77% with only 0.15 xylose to XOS yield.






Similar content being viewed by others
References
Azelee NIW, Jahim JM, Ismail AF, Fuzi SFZM, Rahman RA, Illias RM (2016) High xylooligosaccharides (XOS) production from pretreated kenaf stem by enzyme mixture hydrolysis. Industrial Crops and Products 81:11–19. https://doi.org/10.1016/j.indcrop.2015.11.038
Vazquez MJ, Alonso JL, Domınguez H, Parajo JC (2000) Xylooligosaccharides: manufacture and applications. Trends in Food Science & Technology 11(11):387–393. https://doi.org/10.1016/S0924-2244(01)00031-0
Vegas R, Alonso JL, Domínguez H, Parajó JC (2005) Manufacture and refining of oligosaccharides from industrial solid wastes. Industrial & engineering chemistry research 44(3):614–620. https://doi.org/10.1021/ie049289+
Zhang H, Zhou X, Xu Y, Yu S (2017) Production of xylooligosaccharides from waste xylan, obtained from viscose fiber processing, by selective hydrolysis using concentrated acetic acid. Journal of Wood Chemistry and Technology 37(1):1–9. https://doi.org/10.1080/02773813.2016.1214154
Reddy SS, Krishnan C (2016) Production of high-pure xylooligosaccharides from sugarcane bagasse using crude β-xylosidase-free xylanase of Bacillus subtilis KCX006 and their bifidogenic function. LWT-Food Science and Technology 65:237–245. https://doi.org/10.1016/j.lwt.2015.08.013
Samanta AK, Jayapal N, Kolte AP, Senani S, Sridhar M, Suresh KP, Sampath KT (2012) Enzymatic production of xylooligosaccharides from alkali solubilized xylan of natural grass (Sehima nervosum). Bioresource Technology 112:199–205. https://doi.org/10.1016/j.biortech.2012.02.036
Xiao X, Bian J, Peng XP, Xu H, Xiao B, Sun RC (2013) Autohydrolysis of bamboo (Dendrocalamus giganteus Munro) culm for the production of xylo-oligosaccharides. Bioresource technology 138:63–70. https://doi.org/10.1016/j.biortech.2013.03.160
Shen R, Li HQ, Zhang J, Xu J (2016) Effects of impurities in alkali-extracted xylan on its enzymatic hydrolysis to produce xylo-oligosaccharides. Applied Biochemistry and Biotechnology 179(5):740–752. https://doi.org/10.1007/s12010-016-2028-5
Gopalan N, Rodríguez-Duran LV, Saucedo-Castaneda G, Nampoothiri KM (2015) Review on technological and scientific aspects of feruloyl esterases: a versatile enzyme for biorefining of biomass. Bioresource Technology 193:534–544. https://doi.org/10.1016/j.biortech.2015.06.117
Jayapal N, Samanta AK, Kolte AP, Senani S, Sridhar M, Suresh KP, Sampath KT (2013) Value addition to sugarcane bagasse: xylan extraction and its process optimization for xylooligosaccharides production. Industrial Crops and Products 42:14–24. https://doi.org/10.1016/j.indcrop.2012.05.019
Zhu Y, Kim TH, Lee YY, Chen R, Elander RT (2006) Enzymatic production of xylooligosaccharides from corn stover and corn cobs treated with aqueous ammonia. Applied Biochemistry and Biotechnology 130(1-3):586–598. https://doi.org/10.1385/ABAB:130:1:586
Bolker HI, Wang PY (1969) Acid-and alkali-labile bonds in lignin-carbohydrate complexes. Tappi 52(5):920–923
Peng P, She D (2014) Isolation, structural characterization, and potential applications of hemicelluloses from bamboo: a review. Carbohydrate Polymers 112:701–720. https://doi.org/10.1016/j.carbpol.2014.06.068
Sun SL, Wen JL, Ma MG, Sun RC (2013) Successive alkali extraction and structural characterization of hemicelluloses from sweet sorghum stem. Carbohydrate Polymers 92(2):2224–2231
Pouvreau L, Jonathan MC, Kabel MA, Hinz SWA, Gruppen H, Schols HA (2011) Characterization and mode of action of two acetyl xylan esterases from Chrysosporium lucknowense C1 active towards acetylated xylans. Enzyme and microbial technology 49(3):312–320. https://doi.org/10.1016/j.enzmictec.2011.05.010
Moreira LRS (2016) Insights into the mechanism of enzymatic hydrolysis of xylan. Applied Microbiology and Biotechnology 100(12):5205–5214. https://doi.org/10.1007/s00253-016-7555-z
Liu KX, Li HQ, Zhang J, Zhang ZG, Xu J (2016) The effect of non-structural components and lignin on hemicellulose extraction. Bioresource Technology 214:755–760. https://doi.org/10.1016/j.biortech.2016.05.036
Li J, Huang C, Li H, Xu J (2015) Effects of NF membranes MWCO on desalination performance of corn stover xylan extract. J. Cell. Sci. Technol 23(4):1–9. https://doi.org/10.16561/j.cnki.xws.2015.04.09
Li HQ, Xu J (2013) A new correction method for determination on carbohydrates in lignocellulosic biomass. Bioresource technology 138:373–376. https://doi.org/10.1016/j.biortech.2013.03.148
Van Dongen FEM, Van Eylen D, Kabel MA (2011) Characterization of substituents in xylans from corn cobs and stover. Carbohydrate Polymers 86(2):722–731. https://doi.org/10.1016/j.carbpol.2011.05.007
Zhang W, Johnson AM, Barone JR, Renneckar S (2016) Reducing the heterogeneity of xylan through processing. Carbohydrate polymers 150:250–258. https://doi.org/10.1016/j.carbpol.2016.05.013
Ebringerova A, Hromadkova Z, Alföldi J, Berth G (1992) Structural and solution properties of corn cob heteroxylans. Carbohydrate Polymers 19(2):99–105. https://doi.org/10.1016/0144-8617(92)90119-B
Bowman MJ, Dien BS, O'Bryan PJ, Sarath G, Cotta MA (2012) Comparative analysis of end point enzymatic digests of arabino-xylan isolated from switchgrass (Panicum virgatum L) of varying maturities using LC-MSn. Metabolites 2(4):959–982. https://doi.org/10.3390/metabo2040959
Liab K, Azadi P, Collins R, Tolan J, Kim JS, Eriksson KEL (2000) Relationships between activities of xylanases and xylan structures. Enzyme and Microbial Technology 27(1-2):89–94. https://doi.org/10.1016/S0141-0229(00)00190-3
Gübitz GM, Stebbing DW, Johansson CI, Saddler JN (1998) Lignin-hemicellulose complexes restrict enzymatic solubilization of mannan and xylan from dissolving pulp. Applied Microbiology and Biotechnology 50(3):390–395. https://doi.org/10.1007/s002530051310
Brienzo M, Siqueira AF, Milagres AMF (2009) Search for optimum conditions of sugarcane bagasse hemicellulose extraction. Biochemical Engineering Journal 46(2):199–204. https://doi.org/10.1016/j.bej.2009.05.012
Mittal A, Vinzant TB, Brunecky R, Black SK, Pilath HM, Himmel ME, Johnson DK (2015) Investigation of the role of lignin in biphasic xylan hydrolysis during dilute acid and organosolv pretreatment of corn stover. Green Chemistry 17(3):1546–1558. https://doi.org/10.1039/C4GC02258K
Shi P, Chen X, Meng K, Huang H, Bai Y, Luo H, Yang P, Yao B (2013) Distinct actions by Paenibacillus sp. strain E18 α-L-arabinofuranosidases and xylanase in xylan degradation. Applied and environmental microbiology 79(6):1990–1995. https://doi.org/10.1128/AEM.03276-12
Poutanen K, Tenkanen M, Korte H, Puls J (1991) Accessory enzymes involved in the hydrolysis of xylans. https://doi.org/10.1021/bk-1991-0460.ch033
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shakeel, U., Wu, H., Shen, R. et al. Improving xylo-oligosaccharides yield from corn stalk with stepwise enzymolysis. Biomass Conv. Bioref. 13, 3863–3869 (2023). https://doi.org/10.1007/s13399-021-01538-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01538-2