Abstract
The inadequate sanitation, global warming, and dwindling natural oil reserves pose significant threats to global sustainable growth. With this objective, biomass availability for bioenergy production could be ensured alternately by exploring non-conventional plant species and algae. This idea was examined by growing divergent plants and algae species separately in horizontal subsurface flow constructed wetlands and raceway ponds, later collected their biomass for bioethanol determination. Through this approach, sewage was fed to constructed wetlands and raceway ponds as influent and passed through these cultivated systems for wastewater treatment. Although all the four plant and algae species showed efficient results in sewage treatment, however, Ipomoea aquatica and Spirogyra were statistically superior to the others and produced the highest biomass yield of 4.5 and 4.1 kg m−2, respectively. For bioethanol production, alkali–autohydrolysis combined pretreatment followed by enzymatic hydrolysis with two successive incubation periods (12 and 24 h) were employed. Results suggested that Spirogyra biomass rendered greater carbohydrate and ethanol concentration (3.7 and 1.9 mg L−1, respectively) during 24 hours of incubation, whereas Oryza sativa acquired similar carbohydrate concentration as Spirogyra but slightly lower bioethanol yield. This study postulates, Spirogyra is promising for wastewater treatment coupled with bioethanol production.
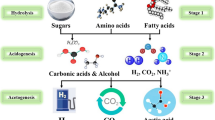
Graphical abstract

Open raceway pond and constructed wetland coupled with wastewater treatment and bio-ethanol production





Similar content being viewed by others
References
Perea-Moreno M-A, Esther Samerón-Manzano A-JP-M (2019) Biomass as renewable energy: worldwide research trends. Sustainability 11:863. https://doi.org/10.3390/su11030863
Saini JK, Saini R, Tewari L (2015) Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. 3 Biotech 5:337–353
Zabed H, Sahu JN, Boyce AN, Faruq G (2016) Fuel ethanol production from lignocellulosic biomass: an overview of feedstocks and technological approaches. Renew Sust Energ Rev 66:751–774
Tahir B, Mezori HA (2020) Bioethanol production from Quercus aegilops using Pichia stipitis and Kluyveromyces marxianus. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00704-2
Isikgor FH, Becer CR (2015) Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym Chem 6:4497–4559. https://doi.org/10.1039/c5py00263j
Kucharska K, Rybarczyk P, Hołowacz I, Łukajtis R, Glinka M, Kamiński M (2018) Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 23
Zhi-Wen W, Ming-Qiang Z, Ming-Fei L et al (2016) Comprehensive evaluation of the liquid fraction during the hydrothermal treatment of rapeseed straw. Biotechnol Biofuels 9:142. https://doi.org/10.1186/s13068-016-0552-8
Zhuang X, Wang W, Yu Q et al (2016) Liquid hot water pretreatment of lignocellulosic biomass for bioethanol production accompanying with high valuable products. Bioresour Technol
Olver B, Van Dyk JS, Beukes N, Pletschke BI (2011) Synergy between EngE, XynA and ManA from Clostridium cellulovorans on corn stalk, grass and pineapple pulp substrates. 3 Biotech. https://doi.org/10.1007/s13205-011-0011-y
Pihlajaniemi V, Sipponen MH, Pastinen O, Lehtomäki I, Laakso S (2015) Yield optimization and rational function modelling of enzymatic hydrolysis of wheat straw pretreated by NaOH-delignification, autohydrolysis and their combination. Green Chemistry 17(3):1683–1691
Chen X, Li H, Sun S, Cao X, Sun R (2016) Effect of hydrothermal pretreatment on the structural changes of alkaline ethanol lignin from wheat straw. Sci Rep 6. https://doi.org/10.1038/srep39354
Popp J, Lakner Z, Harangi-Rákos M, Fári M (2014) The effect of bioenergy expansion: food, energy, and environment. Renew Sust Energ Rev
Maucieri C, Barbera AC, Vymazal J, Borin M (2017) A review on the main affecting factors of greenhouse gases emission in constructed wetlands. Agric For Meteorol 236:175–193
Avellan CT, Ardakanian R, Gremillion P (2017) The role of constructed wetlands for biomass production within the water-soil-waste nexus. Water Sci Technol 75:2237–2245. https://doi.org/10.2166/wst.2017.106
Cicero Fernandez D, Expósito Camargo JA, Peña Fernandez M, Antizar-Ladislao B (2019) Carex paniculata constructed wetland efficacy for stormwater, sewage and livestock wastewater treatment in rural settlements of mountain areas. Water Sci Technol 79:1338–1347. https://doi.org/10.2166/wst.2019.130
Fahim R, Lu X, Jilani G, Hussain J, Hussain I (2019) Comparison of floating-bed wetland and gravel filter amended with limestone and sawdust for sewage treatment. Environ Sci Pollut Res 26:20400–20410. https://doi.org/10.1007/s11356-019-05325-5
Rehman F, Pervez A, Khattak BN, Ahmad R (2017) Constructed wetlands: perspectives of the oxygen released in the rhizosphere of macrophytes. Clean - Soil, Air, Water
Wu H, Zhang J, Ngo HH, Guo W, Hu Z, Liang S, Fan J, Liu H (2015) A review on the sustainability of constructed wetlands for wastewater treatment: design and operation. Bioresour Technol 175:594–601. https://doi.org/10.1016/j.biortech.2014.10.068
Ahmaruzzaman M, Gupta VK (2011) Rice husk and its ash as low-cost adsorbents in water and wastewater treatment. Ind Eng Chem Res 50:13589–13613. https://doi.org/10.1021/ie201477c
Satlewal A, Agrawal R, Bhagia S et al (2018) Rice straw as a feedstock for biofuels: availability, recalcitrance, and chemical properties. Biofuels Bioprod Biorefin
Mishima D, Kuniki M, Sei K et al (2008) Ethanol production from candidate energy crops: water hyacinth (Eichhornia crassipes) and water lettuce (Pistia stratiotes L.). Bioresour Technol. https://doi.org/10.1016/j.biortech.2007.04.056
He MX, Hu Q, Zhu Q, Pan K, Li Q (2015) The feasibility of using constructed wetlands plants to produce bioethanol. Environ Prog Sustain Energy 34:276–281. https://doi.org/10.1002/ep.11953
Bhatia SK, Mehariya S, Bhatia RK, Kumar M, Pugazhendhi A, Awasthi MK, Atabani AE, Kumar G, Kim W, Seo SO, Yang YH (2021) Wastewater-based microalgal biorefinery for bioenergy production: progress and challenges. Sci Total Environ 751:141599
Jankowska E, Sahu AK, Oleskowicz-Popiel P (2017) Biogas from microalgae: review on microalgae’s cultivation, harvesting and pretreatment for anaerobic digestion. Renew Sust Energ Rev
Saad MG, Dosoky NS, Zoromba MS, Shafik HM (2019) Algal biofuels: current status and key challenges. Energies. 12. https://doi.org/10.3390/en12101920
Fahim R, Lu X, Jilani GA, Mahdi H, Aslam M (2020) Synergistic long-term temperate climate nitrogen removal performance in open raceway pond and horizontal subsurface flow constructed wetland operated under different regimes. Water Air Soil Pollut 231. https://doi.org/10.1007/s11270-020-04632-9
Qu W, Loke Show P, Hasunuma T, Ho SH (2020) Optimizing real swine wastewater treatment efficiency and carbohydrate productivity of newly microalga Chlamydomonas sp. QWY37 used for cell-displayed bioethanol production. Bioresour Technol. https://doi.org/10.1016/j.biortech.2020.123072
Onay M (2018) Bioethanol production from Nannochloropsis gaditana in municipal wastewater. In: Energy Procedia
Roesijadi G, Jones SB, Zhu Y (2010) Macroalgae as a biomass feedstock : a preliminary analysis. Analysis
Liu D, Wu X, Chang J, Gu B, Min Y, Ge Y, Shi Y, Xue H, Peng C, Wu J (2012) Constructed wetlands as biofuel production systems. Nat Clim Chang 2:190–194. https://doi.org/10.1038/nclimate1370
Liu D, Zou C, Xu M (2019) Environmental, ecological, and economic benefits of biofuel production using a constructed wetland: a case study in China. Int J Environ Res Public Health 16. https://doi.org/10.3390/ijerph16050827
Sharma P, Sharma N (2017) Industrial and biotechnological applications of algae: a review. J Adv Plant Biol. https://doi.org/10.14302/issn.2638-4469.japb-17-1534
Rastogi M, Shrivastava S (2017) Recent advances in second generation bioethanol production: an insight to pretreatment, saccharification and fermentation processes. Renew Sust Energ Rev
Sun D, Yang Q, Wang Y, Gao H, He M, Lin X, Lu J, Wang Y, Kang H, Alam A, Tu Y, Xia T, Peng L (2020) Distinct mechanisms of enzymatic saccharification and bioethanol conversion enhancement by three surfactants under steam explosion and mild chemical pretreatments in bioenergy Miscanthus. Ind Crop Prod 153:112559. https://doi.org/10.1016/j.indcrop.2020.112559
Cao S, Aita GM (2013) Enzymatic hydrolysis and ethanol yields of combined surfactant and dilute ammonia treated sugarcane bagasse. Bioresour Technol 131:357–364. https://doi.org/10.1016/j.biortech.2012.12.170
Alencar BRA, Rocha JMTS, Rocha GJM, Gouveia ER (2017) Effect of Tween-80 addition in dilute acid pretreatment of waste office paper on enzymatic hydrolysis for bioethanol production by Shf and Ssf processes. Cellul Chem Technol Cellul Chem Technol
Nababan MYS, Fatriasari W, Wistara NJ (2020) Response surface methodology for enzymatic hydrolysis optimization of jabon alkaline pulp with tween 80 surfactant addition. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00807-w
Yang H, Shi Z, Xu G, Qin Y, Deng J, Yang J (2019) Bioethanol production from bamboo with alkali-catalyzed liquid hot water pretreatment. Bioresour Technol 274:261–266. https://doi.org/10.1016/j.biortech.2018.11.088
Alkasrawi M, Al-Hamamre Z, Al-Shannag M et al (2016) Conversion of paper mill residuals to fermentable sugars. BioResources. https://doi.org/10.15376/biores.11.1.2287-2296
Gurram RN, Al-Shannag M, Lecher NJ et al (2015) Bioconversion of paper mill sludge to bioethanol in the presence of accelerants or hydrogen peroxide pretreatment. Bioresour Technol 192:529–539. https://doi.org/10.1016/j.biortech.2015.06.010
Zhai Y, Yang Q, Hou M (2015) The effects of saline water drip irrigation on tomato yield, quality, and blossom-end rot incidenceâ€"a 3a case study in the south of China. PLoS One 10:e0142204. https://doi.org/10.1371/journal.pone.0142204
APHA, AWWA, WEF (1999) Standard Methods for the Examination of Water and Wastewater Part 4000 INORGANIC NONMETALLIC CONSTITUENTS Standard Methods for the Examination of Water and Wastewater
Kochert G (1978) Carbohydrate determination by phenol-sulfuric acid. Handb Phycol method. https://doi.org/10.1007/978-3-642-67356-6_52
Sluiter A, Hames B, Ruiz RO et al (2011) Determination of structural carbohydrates and lignin in biomass
Tutt M, Olt J (2011) Suitability of various plant species for bioethanol production. Agron Res
Goel A, Wati L (2016) Ethanol production from rice (Oryza sativa) straw by simultaneous saccharification and cofermentation. Indian J Exp Biol
Calinski T, Steel RGD, Torrie JH (1981) Principles and procedures of statistics: a biometrical approach. Biometrics. 37:859. https://doi.org/10.2307/2530180
Xu B, Wang X, Liu J, Wu J, Zhao Y, Cao W (2017) Improving urban stormwater runoff quality by nutrient removal through floating treatment wetlands and vegetation harvest. Sci Rep 7:7000. https://doi.org/10.1038/s41598-017-07439-7
Wang B, Wang Z, Jiang Y, Tan G, Xu N, Xu Y (2017) Enhanced power generation and wastewater treatment in sustainable biochar electrodes based bioelectrochemical system. Bioresour Technol 241:841–848. https://doi.org/10.1016/j.biortech.2017.05.155
Ji MK, Abou-Shanab RAI, Kim SH, Salama ES, Lee SH, Kabra AN, Lee YS, Hong S, Jeon BH (2013) Cultivation of microalgae species in tertiary municipal wastewater supplemented with CO 2 for nutrient removal and biomass production. Ecol Eng 58:142–148. https://doi.org/10.1016/j.ecoleng.2013.06.020
Gani P, Sunar NM, Matias-Peralta H, Jamaian SS, Latiff AAA (2016) Effects of different culture conditions on the phycoremediation efficiency of domestic wastewater. J Environ Chem Eng 4:4744–4753. https://doi.org/10.1016/j.jece.2016.11.008
Al-Qodah Z, Al-Shannag M, Amro A et al (2017) Impact of surface modification of green algal biomass by phosphorylation on the removal of copper (II) ions from water. Turk J Chem 41:190–208. https://doi.org/10.3906/kim-1605-38
Al-Shannag M, Al-Qodah Z, Nawasreh M et al (2017) On the performance of Ballota undulata biomass for the removal of cadmium (II) ions from water. Desalin Water Treat 67:223–230. https://doi.org/10.5004/dwt.2017.20379
Vymazal J (2011) Long-term performance of constructed wetlands with horizontal sub-surface flow: ten case studies from the Czech Republic. Ecol Eng 37:54–63. https://doi.org/10.1016/j.ecoleng.2009.11.028
Abou-Elela SI, Golinielli G, Abou-Taleb EM, Hellal MS (2013) Municipal wastewater treatment in horizontal and vertical flows constructed wetlands. Ecol Eng 61:460–468. https://doi.org/10.1016/j.ecoleng.2013.10.010
Mendoza Martinez CL, Alves Rocha EP, de Cassia Oliveira Carneiro A et al (2019) Characterization of residual biomasses from the coffee production chain and assessment the potential for energy purposes. Biomass Bioenergy 120:68–76. https://doi.org/10.1016/j.biombioe.2018.11.003
Miranda JR, Passarinho PC, Gouveia L (2012) Bioethanol production from Scenedesmus obliquus sugars: the influence of photobioreactors and culture conditions on biomass production. Appl Microbiol Biotechnol 96:555–564. https://doi.org/10.1007/s00253-012-4338-z
Chapple C, Ladisch M, Meilan R (2007) Loosening lignin’s grip on biofuel production. Nat Biotechnol 25:746–748. https://doi.org/10.1038/nbt0707-746
Huang Y, Qin X, Luo XM, Nong Q, Yang Q, Zhang Z, Gao Y, Lv F, Chen Y, Yu Z, Liu JL, Feng JX (2015) Efficient enzymatic hydrolysis and simultaneous saccharification and fermentation of sugarcane bagasse pulp for ethanol production by cellulase from Penicillium oxalicum EU2106 and thermotolerant Saccharomyces cerevisiae ZM1-5. Biomass Bioenergy 77:53–63. https://doi.org/10.1016/j.biombioe.2015.03.020
Jönsson LJ, Martín C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112
Nascimento VM, Manrich A, Tardioli PW, de Campos Giordano R, de Moraes Rocha GJ, Giordano RLC (2016) Alkaline pretreatment for practicable production of ethanol and xylooligosaccharides. Bioethanol. 2. https://doi.org/10.1515/bioeth-2016-0008
Kumari D, Singh R (2018) Pretreatment of lignocellulosic wastes for biofuel production: a critical review. Renew Sust Energ Rev
Sun S, Cao X, Sun S, Xu F, Song X, Sun RC, Jones GL (2014) Improving the enzymatic hydrolysis of thermo-mechanical fiber from Eucalyptus urophylla by a combination of hydrothermal pretreatment and alkali fractionation. Biotechnol Biofuels 7:116. https://doi.org/10.1186/s13068-014-0116-8
Zhao X, Elliston A, Collins SRA, Moates GK, Coleman MJ, Waldron KW (2014) Enzymatic saccharification of duckweed (Lemna minor) biomass without thermophysical pretreatment. Biomass Bioenergy 47:354–361. https://doi.org/10.1016/j.biombioe.2012.09.025
Wu FC, Wu JY, Liao YJ, Wang MY, Shih IL (2014) Sequential acid and enzymatic hydrolysis in situ and bioethanol production from Gracilaria biomass. Bioresour Technol 156:123–131. https://doi.org/10.1016/j.biortech.2014.01.024
Li K, Liu S, Liu X (2014) An overview of algae bioethanol production. Int J Energy Res
Park KC, Whitney CGE, Kozera C, O'Leary SJB, McGinn PJ (2015) Seasonal isolation of microalgae from municipal wastewater for remediation and biofuel applications. J Appl Microbiol 119:76–87. https://doi.org/10.1111/jam.12818
Dimos K, Paschos T, Louloudi A, Kalogiannis KG, Lappas AA, Papayannakos N, Kekos D, Mamma D (2019) Effect of various pretreatment methods on bioethanol production from cotton stalks. Fermentation. 5. https://doi.org/10.3390/fermentation5010005
Méndez J, de França Passo D, Wischral D et al (2019) Second-generation ethanol production by separate hydrolysis and fermentation from sugarcane bagasse with cellulose hydrolysis using a customized enzyme cocktail. Biofuels.:1–7. https://doi.org/10.1080/17597269.2019.1608034
Rodrigues B, Lima-Costa ME, Constantino A, Raposo S, Felizardo C, Gonçalves D, Fernandes T, Dionísio L, Peinado JM (2016) Growth kinetics and physiological behavior of co-cultures of Saccharomyces cerevisiae and Kluyveromyces lactis, fermenting carob sugars extracted with whey. Enzym Microb Technol 92:41–48. https://doi.org/10.1016/j.enzmictec.2016.06.012
Chang YH, Chang KS, Chen CY, Hsu CL, Chang TC, Jang HD (2018) Enhancement of the efficiency of bioethanol production by Saccharomyces cerevisiae via gradually batch-wise and fed-batch increasing the glucose concentration. Fermentation. 4. https://doi.org/10.3390/fermentation4020045
Zhang Y, Xu H, Chen H, Wang F, Huai H (2014) Diversity of wetland plants used traditionally in China: a literature review. J Ethnobiol Ethnomed 10:72. https://doi.org/10.1186/1746-4269-10-72
Leong W-H, Lim J-W, Lam M-K, Uemura Y, Ho C-D, Ho Y-C (2018) Co-cultivation of activated sludge and microalgae for the simultaneous enhancements of nitrogen-rich wastewater bioremediation and lipid production. Journal of the Taiwan Institute of Chemical Engineers 87:216–224
Acknowledgments
The principal author is grateful to the government of the People’s Republic of China for financing my postgraduate studies at Southeast University, Nanjing, China. Further, the authors acknowledge the funding under the project “Major Science and Technology Projects of Water Pollution Control and Management in the Peoples’ Republic of China” through Grant No. 2017ZX07202004-002.
Author information
Authors and Affiliations
Contributions
Raana Fahim and Xiwu Lu designed and conceived this project and arranged the experiment materials and analysis instruments. Raana Fahim performed experimental and analysis work, while Xiwu Lu supervised during the study. All authors have equal contribution to the data processing and write-up of this manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fahim, R., Xiwu, L., Jilani, G. et al. An integrated approach to quantifying the efficiency of plants and algae in water purification and bioethanol production. Biomass Conv. Bioref. 13, 1199–1211 (2023). https://doi.org/10.1007/s13399-020-01214-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01214-x