Abstract
This research work focuses on the influence of Neodymium (Nd) doping (0–10 at.%) on the structural, optical, electrical, and photo-response properties of Zinc Oxide thin films deposited on glass substrates by cost-effective spray pyrolysis technique. For all the deposited films, the X-ray diffraction peaks matched the hexagonal wurtzite structure of ZnO with the maximum intensity along the (1 0 1) plane. Using XRD data, the crystallite size, dislocation density, and micro-strain of the films were estimated. Compared to other films, Zn0.96Nd0.04O film exhibited higher crystallinity ~ 18 nm. At higher doping concentrations, a fibrous-granular mixed structure was observed. Above 80 percentage of transparency in the visible region and bandgap of 3.42 eV was observed for the Zn0.96Nd0.04O film. The decrease in Urbach energy with increase in the doping concentration indicated the improvement in crystallinity. The peaks related to band edge emission, zinc, and oxygen-related defects were observed in the photoluminescence analysis also increased band edge emission and lesser defects were observed in the Zn0.96Nd0.04O film. The highest charge carrier concentration ~ 1.7 × 1017 cm−3 and mobility ~ 62.8 cm2/Vs were noticed in of Zn0.96Nd0.04O film. When exposed to UV light, Zn0.96Nd0.04O film exhibited the maximum photocurrent ~ 10−4 A. Hence the Zn0.96Nd0.04O film can be used as a UV photodetector.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Transparent conductive oxides (TCOs) are widely used in the manufacturing of optoelectronic devices, due to its exceptional transparency in the visible region and better electrical conductivity. Zinc Oxide (ZnO) has been extensively used as a TCO material due to its direct broad bandgap (3.37 eV). It is a semiconductor with strong electrical conductivity and transmittance [1]. These materials have received significant attention from researchers due to its environmentally acceptable behavior, strong flexibility for easy tuning, nontoxicity, chemical stability, broad bandgap, and exciton binding energy (60 meV) [2, 3]. Due to these upcoming, it has been employed in the field of gas sensors [4], UV sensors [5], light-emitting diodes [6], photovoltaics [7], and the spintronic device [8], etc.
Many researchers are now using rare-earth ions as dopants to modify the characteristics of host materials. These rare-earth-doped semiconductors are crucial in optoelectronic devices, and their features aroused the researcher’s attention, as effective energy transfer from the host to rare-earth ions is thought to be crucial for most applications nowadays. The rare earth doping into the ZnO host matrix is an emerging field and has been extensively used because of its ability to change structural [9], optical [10], electrical [11], optoelectronic [12], and magnetic properties [13]. The surface structure may be modulated and optical characteristics, such as the bandgap and transmittance, can alter when RE components are added to the ZnO host matrix. Additionally, because of their enormous conductivity and electronic transitions happening in 4f energy shells, RE materials are excellent as doping elements. There are many preparation techniques to synthesise ZnO based thin films like spin coating [14], thermal evaporation [15], sol–gel [16], sputtering [17], spray pyrolysis [18]. Compared to other methods, spray pyrolysis is a simple, non-vacuum, and low-cost technique with excellent stoichiometry control and uniformity. The transport characteristics of depositing films are generally sensitive to preparation parameters such as substrate type, doping level, precursor concentration, substrate temperature, nozzle to substrate distance, carrier gas pressure, and solution flow rate. Due to the discrepancy in the published reports, doping concentration, substrate temperature, and solution molarity have gotten a lot of attention among the preparation factors. For example, Divya et al. [19] noticed hexagonal structure with maximum intensity peak along (1 0 1) plane. For 8 at.% Nd-doped ZnO films, secondary phases were observed corresponding to Nd2O3. The introduction of oxygen vacancies with doping was confirmed by Raman spectra analysis. Deeparani et al. [20] deposited pure and Nd-doped ZnO on glass substrates at 400 °C using the chemical spray pyrolysis technique and showed a decrease in the intensity of (0 0 2) peak with an increase in the doping concentration. From the photoluminescence analysis, they observed 3 peaks corresponding to band edge emission, blue emission, and green emission. However, Poulraj et al. [21] noticed a reduction in the bandgap at higher Nd doping concentration, and PL studies showed five luminescence centres corresponding to NBE and DLE emission. 6 at.% Nd-doped ZnO showed an improved value of photocurrent and better optoelectronic properties. Verma et al. [22] observed oxygen-related defects which are responsible for the increase in the photocurrent, and they noted a fast rise time with a slow decay time of photocurrent with doping. An available very few reports on the photoresponse properties of Nd-doped ZnO thin films deposited using the spray pyrolysis technique. In addition, inconsistencies in available reports on structure and transport properties of the deposited films as well as its co-relationship among them. Hence, extensive study needed for clear understanding of these areas is necessary. As a result, the influence of Neodymium doping concentration on the structure, surface morphology, optical, photoluminescence, electrical, and photo-response characteristics of the ZnO host matrix has been extensively investigated in the current work.
2 Experimental Details
2.1 Film Deposition
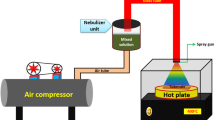
Zn1−XNdXO (x = 0.0–0.1) films were deposited on a chemically cleaned glass substrate (2.5 cm × 2.5 cm), using the spray pyrolysis technique. All films were deposited at 450 °C. A homogeneous precursor was made by dissolving zinc acetate dihydrate (0.1 M) in distilled water and stirring for an hour to obtain pristine ZnO films. Zinc acetate dihydrate and neodymium chloride hexahydrate salts were dissolved in distilled water to prepare a homogeneous, clear solution with a whole solution concentration of 0.1 M. Then the solution stirred for one hour at room temperature to deposit Zn1−xNdxO (x = 0.0–0.1) thin films. The solution was sprayed on the substrate, then maintained at the same temperature for 30 min to eliminate undesirable organic compounds and to improve the crystallinity of the deposited thin films.
2.2 Film Characterization
A surface profilometer was used to calculate the film thickness (Bruker Model no. DXT-18-1715). Rigaku-Miniflex 600 was used for the X-ray diffraction (XRD) study, which used Cu Kα monochromatic radiation (1.5405 Å). SEM (Zeiss EVO 18-15-57) and Energy Dispersive Spectroscopy (EDS) have been used to analyse the surface morphology and elemental studies, respectively. Optical characterization was performed using a Shimadzu-1800 UV–VIS spectrophotometer in the 300 nm to 800 nm range. The room temperature photoluminescence (PL) spectra were recorded using a Jasco FP8300 fluorescence spectrometer equipped with a 150 W Xenon lamp and a high-speed chopper. The electrical characteristics were investigated with a Keithley 6220 source meter and a magnetic field of 0.5 Tesla using the Van der Pauw technique and a Hall Effect measuring setup.
3 Result and Discussions
3.1 Structural Characterization
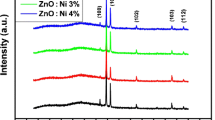
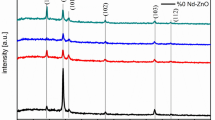
X-ray diffraction (XRD) (Fig. 1a, b) revealed that both undoped and Nd doped ZnO films showed hexagonal wurtzite structure and polycrystalline nature, without any secondary peaks within the XRD detection limit. All the films showed (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), and (1 1 2) peaks with maximum intensity along (1 0 1) plane. The pure ZnO films showed a lesser intensity of (1 0 1) peak and the intensity slightly increased for Zn0.99Nd0.01O film and reached a maximum for the Zn0.96Nd0.04O film. At higher Nd doping concentration the reduction in the peak intensity was observed due to a decrease in the crystallite size. The Zn0.90Nd0.10O film showed lesser (1 0 1) peak intensity. Similar results were reported by Vijaya Prasanth et al. [23] and may be attributed to the change in the crystallinity of the ZnO matrix, because of the larger ionic radius mismatch and the charge imbalance between the host (Zn2+) matrix and the dopant (Nd3+) ion. The Scherrer formula and W–H plot method are used to estimate the crystallite size (D) of the pristine and Nd doped ZnO films [24].
The structural parameters estimated for the deposited films are tabulated (Table 1). The crystallite size (D) of the films is calculated using the formula given below.
where, D is the crystallite size, \(\beta\) is the full width at half maximum, \(\lambda\) is the wavelength of the X-ray used. The reduction in the crystallite size (D) was noticed with an addition of the doping level. The Zn0.96Nd0.04O film showed higher crystallinity of 16 nm and Zn0.90Nd0.10O film showed lesser crystallite size of 8 nm. A similar increase in the crystallite size with the addition of impurity was noticed by Poulraj et al. [21] for Nd–ZnO films deposited using the spin coating technique. In contrast, Poongodi et al. [14] reported reduction in the crystallinity at higher Nd doping concentrations. In the present study, till 4 at.% Nd doping concentration the intensity of (1 0 1) peak increased sharply, which corresponds to the increase in the crystallite size and above 4 at.% Nd doping concentration, decrease in the crystallite size was observed. This can be due to the lattice mismatch produced by the greater ionic radii of Nd3+ ions (0.984 Å), which hinders grain expansion and causes the decrease in the crystallite size [25, 26]. This may be also due to the solubility limit of Nd3+ in ZnO is 4 at.%. Above this limit, the Nd3+ ions occupy the interstitial position in the ZnO host matrix and restricts the crystal growth of ZnO.
The wurtzite ZnO has the lattice parameters ‘a’ and ‘c.’ The lattice parameter ‘a’ and ‘c’ are calculated using the below formula.
The computed lattice parameter and inter-planar spacing values are quite close to a conventional ZnO reference value. The strain induced in the film after doping causes an increase in the values of the lattice parameter of doped films when compared to pristine [27]. It was also noticed that the lattice parameter mainly depends on the doping material and lattice parameter values increases at higher doping concentrations.
Figure 1b confirms that till 7 at.% Nd doping, there is no shift in the peak position and a slightly shift towards lower 2θ values found at higher doping levels. The change of XRD peak positions with an increase in doping concentration confirms the inclusion of doping material into the ZnO matrix.
The crystallite size and strain in the film are also calculated using the W–H plot (Fig. 2) method
where, D is the crystallite size, is the strain and \(\beta_{hkl}\) is the Full-Width Half Maximum (FWHM). The crystallite size matched well with the values calculate from Scherrer equation. The strain values are found to be decreasing with an increase in the doping concentration of Nd in the ZnO host matrix. The sample Zn0.96Nd0.04O film showed a lesser strain value of 2.0 × 10−3 and Zn0.90Nd0.10O sample showed an increased strain value of 3.4 × 10−3. An increase in the strain values is noted at higher doping concentrations (6–10 at.%). The dislocation density is inversely proportional to the crystallite size. The dislocation density showed a similar trend as that of the strain. It increases with a decrease in the crystallite size and values are minimum for the Zn0.96Nd0.04O film. Vijayaprashanth et al. [23] observed a similar increase in the strain and dislocation density values with an increase in the doping concentration.
3.2 Surface Morphology and Compositional Analysis
The surface morphology of the pure ZnO deposited at 450 °C (Fig. 3) showed homogenous fibrous morphology. The decrease in the diameter of the fibrous structure was observed for Zn0.99Nd0.01O and Zn0.98Nd0.02O film. The fibrous structure is due to the strain caused on the film during the drying process. Comparable morphology was noticed by Islam et al. [28] for pristine ZnO films using the spray pyrolysis technique. After 3 at.% Nd doping, the gradual decrease in the fibrous morphology was observed due to the incorporation of Nd ions into the Zn host matrix. The doped films exhibited a homogeneous, continuous, and dense morphology with a smaller nanofiber structure. It was observed that when doping levels increased, the fibrous structure became more noticeable. The arrangement of linked structures including tiny crystallites that extended throughout the whole film surface shows a significant coalescence, which may bear on to develop further due to increased doping concentration. The nucleation is enhanced as concentration increases, resulting in the creation of dense and compact grains. At a higher doping level, due to the incorporation of large number of Nd ions into the ZnO lattice, the morphology of the deposit changed from fibrous structure to granular structure, and the size of the granular structure increased as doping levels increased. The significant change in the morphology indicates the inclusion of higher ionic radii dopant ions in the host matrix. Sindhu et al. [29] noticed a similar morphological change for Mn–ZnO films synthesized by the spray pyrolysis technique.
The elements present in the deposited films are confirmed by EDAX spectra. The EDAX spectra of Zn0.96Nd0.04O sample (Fig. 4) showed peaks related to Zn, O, and Nd. The other small peaks corresponding to Si, Ca and C are due to substrate, which are ignored from EDAX. The spectra showed 42.3 at.% Zn, 53.9 at.% O and 3.8 at.% Nd content. Which is matching with the expected composition of Zn0.96Nd0.04O film. Hence, it is confirmed that the Nd3+ ion is occupying the Zn2+ position. The elemental mapping clearly shows that all the elements are uniformly distributed in both pristine and doped ZnO film.
The X-ray photoelectron spectroscopy (XPS) of pristine and Nd doped ZnO film (Fig. 5) is carried out using a HAXPES beamline PES-BL14 at the Indus-2 synchrotron radiation source. Figure 5a displays the survey spectra of pure ZnO, Zn0.95Nd0.05O and Zn0.92Nd0.08O films deposited at 450 °C. The C 1 s peak (Fig. 5b) (284.6 eV) has been used as a reference to calibrate the binding energies in the XPS spectra [30]. The peaks of Zn 2p3/2 (1022.1 eV) and Zn 2p1/2 (1043.9 eV), respectively, are shown in Fig. 5c, correspond to the zinc lattice in zinc oxide. The Lorentzian-Gaussian model (Fig. 5d and e) can be used to match the O 1 s by using two peaks at 530.3 eV and 532.1 eV. The former, which has lower binding energy, is the inherent O2−, or Zn–O bonds, in ZnO. The peak at 531.8 eV is linked to the surface hydroxyl, chemisorbed oxygen as well as to the absorption of H2O, O2, –CO3, and surface impurity in general. Figure 5f depicts the spectrum of the Nd 3d area. The 981.3 eV and 1009.8 eV peaks correspond to 5/2 and 3/2 spin–orbit double components of Nd 3d core level photoemission, respectively, indicating that Nd ions exist in a trivalent state as compared to Nd ions in Nd2O3. As a result, dopants were successfully inserted into the ZnO lattice, causing a local oxygen deficiency because every two Nd atoms combined with three oxygen atoms.
3.3 Optical Characterization
The transmittance spectra of Zn1−xNdxO (x = 0.0–0.1) deposited at 450 °C is shown in Fig. 6a. The Zn0.96Nd0.04O film showed maximum transmittance above 80% in the visible region. When compared to undoped ZnO films, Nd doped ZnO showed improved transmittance at lower doping concentrations (till 4 at.%), after that with an increase in the doping concentration the transmittance decreased and reached a minimum for 10 at.% Nd doped ZnO films. The charge transfer between the ZnO valence band and the 4f level of Nd might be the main cause of the absorption edge shifting towards the higher wavelength side till 4 at.%. The increase in the transmittance is due to the increased crystallinity of the films and decreased defects. At a higher doping level (5–10 at.%), the shift in the band edge towards the lower wavelength region was observed [14].
The Tauc’s plot (Fig. 6b) relation was used to calculate the optical bandgap [31].
A rise in the optical bandgap was observed for doped films till 4 at.% Nd doping and reduced at higher doping levels. The increase in the bandgap can be explained using the Brunstein–Moss shift (B–M shift). The reduction in the bandgap is due to the increase in the lattice parameter when Nd is added to the ZnO host matrix, which causing decreased interaction between the atoms because of the increased defects in the ZnO host matrix upon doping.
The refractive index (n) (Fig. 6c) is calculated using the formula
The refractive index of all the films was found to be maximum in the UV region or band edge region and the n value decreases with an increase in the wavelength.
The extinction coefficient (k) (Fig. 6d) is calculated using the formula
The extinction coefficient (k) of the films depends on the incident radiation. Near the absorption edge, the extinction coefficient was maximum in UV region and almost constant in the visible region. The value of k decreases initially and reaches a minimum value for Zn0.96Nd0.04O film and again increases for higher Nd doping concentration, this depicts crystallite degradation and validates our XRD findings.
The tailing of the absorption edge caused by the localized states in the bandgap of ZnO is the Urbach tail and is given in Table 2. It is calculated using the formula
The pure ZnO film showed an Urbach energy (Fig. 6e) of 106.5 meV whereas the Zn0.96Nd0.04O film showed lesser energy of 81.4 meV. The band tailing is impacted by local electric fields that are stimulated by ZnO imperfections. Following that, it was observed that the Urbach energy decreased with increasing concentration till 4 at.% Nd doping and increased at higher doping concentrations [32]
3.4 Photoluminescence study
Figure 7 depicts the PL spectra of Zn1−xNdxO (x = 0.0–0.1) films synthesised at 450 °C using a 325 nm excitation source. All the films showed 8 peaks corresponding to near band edge emission (NBE) and deep level emission (DLE). The electron–hole recombination causes the peak in the near band edge emission. Deep-level emissions are typically thought to be directly related to structural imperfections in the ZnO matrix, such as oxygen vacancies (Vo) or zinc interstitials (Zni) [33]. When the dopant was introduced into the host material, the band edge of the ZnO sample experienced a redshift at lower concentrations and then shifted back to the higher energies at increased doping levels, may be due to the Nd 4f electrons generating new, unoccupied states close to the conduction band edge of the ZnO energy levels [34]. The Nd doping also considerably improved the band edge peak intensity. Doping ZnO with Nd strengthened radiative recombination, which resulted in significant recombination of photo-generated charge carriers, as evidenced by the increased intensities of Nd-doped ZnO. The intensity of NBE emission of doped films raised gradually up to 4 at.% Nd doping and decreased at higher doping concentrations. The Zn0.96Nd0.04O film (Fig. 7f) showed lesser FWHM and increased intensity of NBE emission, which corresponds to the increased crystallinity of the films confirmed from the XRD analysis. However, when the doping concentration increased from 0 to 4 at.%, the emission caused by impurities reduced. The defects are found to be lesser for Zn0.97Nd0.03O film (Fig. 7e) after that slight increase in the intensity of defect-related peaks is noted and compared to other films, Zn0.90Nd0.10O film (Fig. 7l) showed increased defects. However, all the Nd-doped ZnO films showed higher NBE emissions and decreased DLE emissions when compared to pure ZnO. Hence, Nd doping into the ZnO host matrix will increase the recombination of electron–hole pairs by decreasing the defects which were also confirmed by XRD results. It is evident from the deconvoluted graph that all the films emit violet, blue, green, and yellow–orange radiation. The observed pattern of violet emission at 421 nm might be caused by lattice sites occupied by zinc and series of blue emission at 447 nm and 464 nm is due to the extended zinc interstitial sites. The yellow-orange emission is generally triggered by singly ionized oxygen vacancies or other defects, while green emission at 532 nm is mostly caused by defect centres produced by oxygen vacancies or oxygen interstitials. When Nd doping concentration increases to 3 at.%, the green emission intensity gradually decreased and increased again with an increase in doping concentration (4–10 at.%). The possible reason is that, with an increase in the Nd doping concentration, the oxygen vacancies in ZnO thin films are greatly decreased to 3 at.%, thus, the opportunity of electron transition from the energy level of oxygen vacancies to the valence band is less. The yellow-orange emission peak is due to the singly ionized Vo or other defects [24]. These results are matching with some of the reports based on Nd-doped ZnO [21, 23]. However, Kumar et al. [35] observed only two peaks at 384 nm and 564 nm corresponding to band edge emission and strong visible emission. The Nd3+ doping can induce defect energy bands in the bandgap of the host matrix and increases its UV and visible light sensitivity, which can be advantageous for optoelectronic device applications. Also, the ZnO-based semiconductors are more preferred than other semiconductors because their whole luminescence spectrum ranges from violet to orange and encompasses the majority of the wavelengths in the white light spectrum.
3.5 NIR Photoluminescence
To study the near-infrared (NIR) photoluminescence properties of Zn1−xNdxO (x = 0.0–0.1) film, laser-excited spectra were measured under 325 nm excitation at room temperature. The NIR emission of pristine and doped films at various doping percentages is shown in Fig. 8. The radiative relaxation from 4F5/2 to 4I9/2 results in peaks at 836 nm and 861 nm, peaks at 883 nm and 904 nm corresponding to the transition from 4F3/2 to 4I9/2, and radiative relaxations of Nd3+ ions from 4F3/2 to 4I11/2 responsible for the peaks at 1054 nm and 1113 nm. At higher concentrations, there is a reduction in PL intensity, which is induced by the concentration quenching effect. When Nd3+ concentrations rise over a certain range because they tend to cluster, nonradiative de-excitation processes are produced as ion–ion contact of Nd3+ ions increase. This loss process causes a drop in PL intensity as the concentration of Nd3+ increases.
3.6 Electrical Characterization
The Hall measurement (Fig. 9) and I–V techniques were used to measure electrical parameters using silver as a contact material and the obtained results are tabulated in Table 3. All the films showed ohmic nature and 4 at.% Nd doped ZnO film showed increased current (I) of 10−7 A, due to the increase in the crystallinity of the films so that the electrons flow easily without getting trapped. The decrease in the current at higher doping concentrations is due to the difficulty in the electron movement, which may be due to increased defects and decreased crystallinity as confirmed by XRD. The carrier concentration steadily increases up to 4 at.% Nd doping, then gradually decreases and reaches the lowest value for Zn0.90Nd0.10O film. The Zn0.96Nd0.04O film showed the maximum carrier concentration (η) of 1.74 × 1017 may be due to the presence of Nd3+ ions at interstitials or the substitution of Nd3+ ions at the Zn2+ cation position. The reduction in the carrier concentration may be due to the grain boundary defects noticed at higher doping levels [36]. On the other hand, ionized impurity scattering of the conduction electrons might account for the reduced mobility. The variation in the mobility with doping is consistent with the variation in the crystallite size determined from the XRD data (Table 1). The number of grain boundaries increases as the grain size is reduced or vice versa. When the doping concentration increases, the resistivity (ρ) values fall till 4 at.% (Table 3). At higher doping concentrations (6–10 at.%) the resistivity increased abruptly may be due to the decrease in crystal quality as observed in XRD analysis. Rohini devi et al. [37] reported a similar reduction in resistivity at higher doping concentrations. In general, TCO materials produce high transmittance and low electrical resistivity for good optoelectronic devices. In the present study, Zn0.96Nd0.04O film showed better optical and electrical properties. Hence, it has the potential to be utilized in the production of semiconductor applications.
3.7 Photo-Response Study
The ohmic behavior with n-type conductivity was noticed in all the films. An I–V measurement system was used to measure the photocurrent of all the films in the dark (Fig. 10a), visible light (Fig. 10b), and UV light (Fig. 10c). In the dark condition, a minimum current of the order 10−7 A was observed in all the films. Compared to the pure ZnO, Nd-doped ZnO films showed increased photocurrent as a result of enhanced carrier concentration and mobility. The Zn0.96Nd0.04O film showed increased photocurrent in the range of 10−6 A, due to the increased crystallinity of the films as confirmed by the XRD analysis. Then the films are illuminated with visible light and a slight increase in the photocurrent values are observed. However, compared to other films Zn0.96Nd0.04O film showed increased photocurrent value. Under UV illumination, a large increment in the value of photocurrent was observed. All the Nd-doped ZnO films showed photocurrent in the range of 10−4 A. This increase of photocurrent in Nd doped films is due to the increase in the defect trapping density with an increase in the Nd doping up to a certain concentration. Compare to dark condition the photocurrent increased by 10−3 orders under UV illumination. This is because, in dark conditions, a significant number of carriers were trapped, and these carriers could be activated under UV illumination. Thus, Nd doping could result in higher defect trapping density and increased photocurrent.
Figure 11 illustrates the variation in photo resistance of pure and Nd doped ZnO films with time. A Xenon 150 W lamp was used as a source to measure the variation of photo resistance. From the graph, the response is stable, with good reproducibility. The photo resistance reached a minimum value of 103 Ω when the light illuminated the sample and there was no further drop in the photo resistance. The photo resistance starts increasing slowly when the light is turned off and reached a maximum resistance in the range of 106 Ω for all the films (Table 4). This slow recovery process is controlled by surface effects such as adsorption and desorption of oxygen. This persistence of photo resistance may be due to large lattice relaxation between the ionized and neutral states of oxygen vacancy (Vo) creates an energy barrier for the decay of metastable state [38]. Also, with an increase in the oxygen vacancy the persistence of photo resistance increases and decreases with a decrease in the oxygen vacancy which can be correlated with the photoluminescence analysis. When Nd is doped into ZnO, it creates an extra electron in the lattice which helps in the conduction process. Compare to other films Zn0.96Nd0.04O film showed a faster fall time of 6 s and rise time of 112 s. Zn0.90Nd0.10O film showed a higher fall time of 23 s and rise time of 334 s. The rise and fall times are quite slower in the higher Nd doped ZnO films. This is because the higher Nd doped films have more grain-boundary-related defects which probably make both the rise and fall time slower. This result shows that Nd doping into ZnO gives a faster response with an increase in the photocurrent value.
Figure 12 shows the variation of normalized photocurrent versus wavelength of the deposited pristine and Nd doped ZnO films. Between the two electrodes, the variation of photocurrent with change in wavelength was recorded. The maximum sensitivity curve and the reduction in resistance in this study were both close to the band absorption domain. The films with lower Nd concentrations have shown excellent photosensitivity. This behavior might be induced by enhanced crystallinity and a lower defect ratio in less Nd doped films, as corroborated by XRD data. This increased crystallinity also results in a well-connected network, which improves electronic flow along the crystallite axis. From the SEM images, it was clear that there was no drastic change in the continuous fibrous morphology of the films till 4 at.%. This continuous and well-inter-connected network helps to improve the transport properties of the films by allowing the electrons to flow continuously throughout the surface. Under illumination, unpaired electrons that are left in the films because of photo-generated holes traveling to the surface and being trapped contribute to the photocurrent. Another way to represent the photosensitivity of nanocrystals is the photocurrent gain, which is the number of charge carriers that flow between the films for each photon absorbed per second that creating an electron–hole pair. The Zn0.96Nd0.04O film showed the highest photocurrent value due to the increased surface area and the crystallinity of the films. Furthermore, electrons encounter high resistance for higher Nd doped films, resulting in a reduction in light responsiveness. The Zn0.96Nd0.04O film showed the highest photosensitivity and it could be used in the UV photodetectors (Table 5).
3.8 Conclusion
The spray pyrolysis methodology was used to deposit the homogenous and uniform Zn1−xNdxO (x = 0.0–0.1) thin films on the glass substrates at 450 °C. All the films showed hexagonal wurtzite structure with a favoured orientation along the (1 0 1) plane, without any secondary phase formation. As the doping level increased, the crystallite size (~ 18 nm) increased till 4 at.% Nd doping and decreased further. At higher Nd doping concentrations, mixed morphology of granular and fibrous structure was noted in all the films. The composition of the deposited films was studied using EDAX and XPS. The incorporation of Nd3+ ions into ZnO lattice was confirmed by XPS. The bandgap energy and transmittance increased till 4 at.% Nd doping and decreased at higher doping concentrations due to increased defects. In addition to near band edge emission, photoluminescence spectra also revealed violet, green, blue, and orange emissions corresponding to zinc and oxygen related defects. The NIR photoluminescence spectra showed six peaks corresponding to radiative relaxation. The intensity of the peaks decreased at higher Nd doping concentrations due to an increase in the interaction between Nd3+ ions. Electrical parameter values showed systematic variation and Zn0.96Nd0.04O film showed maximum carrier concentration (η) and mobility (μ) are ~ 1.7 × 1017 cm−3 and ~ 62.8 cm2/Vs respectively. The Zn0.96Nd0.04O film showed the highest photosensitivity in the ultraviolet region and photosensitivity showed strong dependence on the structure, optical and electrical properties. Hence it can be used in the fabrication of ultraviolet sensors.
Data availability
Providing transparency on re-use of material and mentioning any unpublished material (e.g., manuscripts in press) included in the manuscript in a cover letter to the editor.
References
Mishra, S.K., Srivastava, R.K., Prakash, S.G.: Photoluminescence and photoconductivity studies of ZnO nanoparticles prepared by solid state reaction method. J. Mater. Sci. Mater. Electron. 24(1), 125–134 (2013). https://doi.org/10.1007/s10854-012-0950-z
Shrisha, B.V., Bhat, S., Kushavah, D., Gopalakrishna Naik, K.: Hydrothermal growth and characterization of Al-doped ZnO nanorods. Mater. Today Proc. 3(6), 1693–1701 (2016). https://doi.org/10.1016/j.matpr.2016.04.061
Nasr, B., Dasgupta, S., Wang, D., Mechau, N., Kruk, R., Hahn, H.: Electrical resistivity of nanocrystalline Al-doped zinc oxide films as a function of Al content and the degree of its segregation at the grain boundaries. J. Appl. Phys. (2010). https://doi.org/10.1063/1.3511346
Keis, K., Magnusson, E., Lindström, H., Lindquist, S.E., Hagfeldt, A.: A 5% efficient photoelectrochemical solar cell based on nanostructured ZnO electrodes. Sol. Energy Mater. Sol. Cells 73(1), 51–58 (2002). https://doi.org/10.1016/S0927-0248(01)00110-6
Cheng, J., Zhang, Y., Guo, R.: ZnO microtube ultraviolet detectors. J. Cryst. Growth 310(1), 57–61 (2008). https://doi.org/10.1016/j.jcrysgro.2007.08.034
Khan, A., Ali Abbasi, M., Hussain, M., Hussain Ibupoto, Z., Wissting, J., Nur, O., Willander, M.: Piezoelectric nano-generator based on zinc oxide nanorods grown on textile cotton fabric. Appl. Phys. Lett. (2012). https://doi.org/10.1063/1.4766921
Ahn, M.W., Park, K.S., Heo, J.H., Park, J.G., Kim, D.W., Choi, K.J., Lee, J.H., Hong, S.H.: Gas sensing properties of defect-controlled ZnO-nanowire gas sensor. Appl. Phys. Lett. 93(26), 263103 (2008). https://doi.org/10.1063/1.3046726t
Zheng, J.H., Song, J.L., Zhao, Z., Jiang, Q., Lian, J.S.: Optical and magnetic properties of Nd-doped ZnO nanoparticles. Cryst. Res. Technol. 47(7), 713–718 (2012). https://doi.org/10.1002/crat.201200026
Bao, D., Gu, H., Kuang, A.: Sol-gel-derived c-axis oriented ZnO thin films. Thin Solid Films 312(1–2), 37–39 (1998). https://doi.org/10.1016/s0040-6090(97)00302-7
Tan, S.T., Chen, B.J., Sun, X.W., Fan, W.J., Kwok, H.S., Zhang, X.H., Chua, S.J.: Blueshift of optical band gap in ZnO thin films grown by metal-organic chemical-vapor deposition. J. Appl. Phys. (2005). https://doi.org/10.1063/1.1940137
Lu, Y.M., Hwang, W.S., Liu, W.Y., Yang, J.S.: Effect of RF power on optical and electrical properties of ZnO thin film by magnetron sputtering. Mater. Chem. Phys. 72(2), 269–272 (2001). https://doi.org/10.1016/S0254-0584(01)00450-3
Zhao, L., Lian, J., Liu, Y., Jiang, Q.: Structural and optical properties of ZnO thin films deposited on quartz glass by pulsed laser deposition. Appl. Surf. Sci. 252(24), 8451–8455 (2006). https://doi.org/10.1016/j.apsusc.2005.11.054
Ayouchi, R., Martin, F., Leinen, D., Ramos-Barrado, J.R.: Growth of pure ZnO thin films prepared by chemical spray pyrolysis on silicon. J. Cryst. Growth 247(3–4), 497–504 (2003). https://doi.org/10.1016/S0022-0248(02)01917-6
Poongodi, G., Kumar, R.M., Jayavel, R.: Structural, optical and visible light photocatalytic properties of nanocrystalline Nd doped ZnO thin films prepared by spin coating method. Ceram. Int. 41(3), 4169–4175 (2015). https://doi.org/10.1016/j.ceramint.2014.12.098
Yao, B.D., Chan, Y.F., Wang, N.: Formation of ZnO nanostructures by a simple way of thermal evaporation. Appl. Phys. Lett. 81(4), 757–759 (2002). https://doi.org/10.1063/1.1495878
Hasnidawani, J.N., Azlina, H.N., Norita, H., Bonnia, N.N., Ratim, S., Ali, E.S.: Synthesis of ZnO Nanostructures Using Sol-Gel Method. Procedia Chem. 19, 211–216 (2016). https://doi.org/10.1016/j.proche.2016.03.095
Water, W., Chu, S.Y.: Physical and structural properties of ZnO sputtered films. Mater. Lett. 55(1–2), 67–72 (2002). https://doi.org/10.1016/S0167-577X(01)00621-8
Ashour, A., Kaid, M.A., El-Sayed, N.Z., Ibrahim, A.A.: Physical properties of ZnO thin films deposited by spray pyrolysis technique. Appl. Surf. Sci. 252(22), 7844–7848 (2006). https://doi.org/10.1016/j.apsusc.2005.09.048
Divya, N.K., Pradyumnan, P.P.: Enhancement of photocatalytic activity in Nd doped ZnO with an increase in dielectric constant. J. Mater. Sci. Mater. Electron. 28(2), 2147–2156 (2017). https://doi.org/10.1007/s10854-016-5779-4
Rani, T.D., Tamilarasan, K., Thangaraj, K., Elamurugu, E., Ramamurthi, K., Leela, S.: Structural and optical properties of Nd3+ doped zinc oxide thin films deposited by spray pyrolysis. Optik (Stuttg) 127(1), 72–75 (2016). https://doi.org/10.1016/j.ijleo.2015.10.007
Poul Raj, I.L., Valanarasu, S., Hariprasad, K., Ponraj, J.S., Chidhambaram, N., Ganesh, V., Ali, H.E., Khairy, Y.: Enhancement of optoelectronic parameters of Nd-doped ZnO nanowires for photodetector applications. Opt. Mater. (Amst.) (2020). https://doi.org/10.1016/j.optmat.2020.110396
Verma, K.C., Goyal, N., Kotnala, R.K.: Lattice defect-formulated ferromagnetism and UV photo-response in pure and Nd, Sm substituted ZnO thin films. Phys. Chem. Chem. Phys. 21(23), 12540–12554 (2019). https://doi.org/10.1039/c9cp02285f
Vijayaprasath, G., Murugan, R., Mahalingam, T., Hayakawa, Y., Ravi, G.: Enhancement of ferromagnetic property in rare earth neodymium doped ZnO nanoparticles. Ceram. Int. 41(9), 10607–10615 (2015). https://doi.org/10.1016/j.ceramint.2015.04.160
Ayana, A., Gummagol, N.B., Patil, P.S., Sharma, P., Rajendra, B.V.: Enhancement of optical limiting performance in nanocrystalline La3+ doped ZnO film. Mater. Sci. Semicond. Process. (2021). https://doi.org/10.1016/j.mssp.2021.105931
Huang, Z.Y., Luo, P., Chen, M., Pan, S.R., Chen, D.H.: Microstructure and hemocompatibility of neodymium doped zinc oxide thin films. Mater. Lett. 65(15–16), 2345–2347 (2011). https://doi.org/10.1016/j.matlet.2011.05.031
Xian, F., Li, X.: Effect of Nd doping level on optical and structural properties of ZnO: Nd thin films synthesized by the sol-gel route. Opt. Laser Technol. 45(1), 508–512 (2013). https://doi.org/10.1016/j.optlastec.2012.06.002
Manikandan, A., Manikandan, E., Meenatchi, B., Vadivel, S., Jaganathan, S.K., Ladchumananandasivam, R., Henini, M., Maaza, M., Aanand, J.S.: Rare earth element (REE) lanthanum doped zinc oxide (La: ZnO) nanomaterials: synthesis structural optical and antibacterial studies. J. Alloys Compd. 723, 1155–1161 (2017). https://doi.org/10.1016/j.jallcom.2017.06.336
Islam, M.R., Podder, J.: Optical properties of ZnO nano fiber thin films grown by spray pyrolysis of zinc acetate precursor. Cryst. Res. Technol. 44(3), 286–292 (2009). https://doi.org/10.1002/crat.200800326
Sindhu, H.S., Rajendra, B.V., Kulkarni, S.D., Babu, P.D.: Optical, electrical and magnetic properties of fiber structure Zn0.99Mn0.01O films prepared by spray pyrolysis. Thin Solid Films 655, 83–94 (2018). https://doi.org/10.1016/j.tsf.2018.04.013
Goutam, U.K., Sharma, R.K., Singh, J., Dutta, K., Sule, U.S., Gadkari, S.C.: HAXPES beamline PES-BL14 at the Indus-2 synchrotron radiation source. J. Synchrotron Radiat. 25(5), 1541–1547 (2018). https://doi.org/10.1107/S1600577518008408
Ayana, A., Patil, P.S., Gummagol, N.B., Goutam, U.K., Sharma, P., Rajendra, B.V.: Microstructural, linear and nonlinear optical study of spray pyrolysed nanostructured La–ZnO thin film: an effect of deposition temperature. Opt. Mater. (Amst.) (2021). https://doi.org/10.1016/j.optmat.2021.111742
Rayes, R.M.S., Kumar, Y., Jácome, M.A.C., Antonio, J.A.T., Mathew, X., Mathews, N.R.: Effect of Eu doping on the physical, photoluminescence, and photocatalytic characteristics of ZnO thin films grown by sol-gel method. Phys. Status Solidi (A) Appl. Mater. Sci. (2017). https://doi.org/10.1002/pssa.201700229
Subramanian, M., Senthilkumar, K., Tanemura, M., Soga, T., Hihara, T.: Raman spectra and magnetic property analysis of Nd-doped ZnO thin films. Jpn. J. Appl. Phys. (2013). https://doi.org/10.7567/JJAP.52.01AC14
Srivathsa, M., Rajendra, B.V.: A study on structure, surface morphology, optical and electrical properties of spray pyrolyzed ZnO and Zn0.97Nd0.03O thin films. Mater. Today Proc. 55, 87–93 (2022). https://doi.org/10.1016/J.MATPR.2021.12.302
Kumar, S., Sahare, P.D.: Nd-doped ZnO as a multifunctional nanomaterial. J. Rare Earths 30(8), 761–768 (2012). https://doi.org/10.1016/S1002-0721(12)60126-4
Asikuzun, E., Ozturk, O., Arda, L., Terzioglu, C.: Microstructural and electrical characterizations of transparent Er-doped ZnO nano thin films prepared by sol–gel process. J. Mater. Sci. Mater. Electron. 28(19), 14314–14322 (2017). https://doi.org/10.1007/s10854-017-7291-x
Rohini Devi, A., Jegatha Christy, A., Arun Kumar, K.D., Valanarasu, S., Hamdy, M.S., Al-Namshah, K.S., Alhanash, A.M., Vikraman, D., Kim, H.S.: Physical properties evaluation of nebulized spray pyrolysis prepared Nd doped ZnO thin films for opto-electronic applications. J. Mater. Sci. Mater. Electron. 30(8), 7257–7267 (2019). https://doi.org/10.1007/s10854-019-01039-z
Srivathsa, M., Kumar, P., Rajendra, B.V.: Ultraviolet photoconductivity and photoluminescence properties of spray pyrolyzed ZnO nanostructure: Effect of deposition temperature. Opt. Mater. (Amst.) 131, 112726 (2022). https://doi.org/10.1016/j.optmat.2022.112726
Acknowledgements
The authors would like to thank Manipal Academy of Higher Education (MAHE) for providing a research facility. One of the authors Manu Srivathsa would like to thank UGC-DAE CSR Mumbai Centre for financial assistance (UDCSR/MUM/AO/CRS-M-315/2020/813). RRCAT Indore center for providing XPS facility. Dr. Sudha D Kamath, Department of Physics, MIT Manipal for providing the photoluminescence facility. Dr. Gowrish Rao K, Department of Physics, MIT Manipal for providing the photo-response facility.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. No financial or other considerations from authors or reviewers have the potential to compromise or bias objectivity or professional judgment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ensuring disclosures, declarations and transparency on data statements from all authors are included in the manuscript as appropriate.
Ethical approval
All listed authors have approved the manuscript before submission, including the names and order of authors.
Consent for publication
Managing all communication between the journal and all co-authors before and after publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Srivathsa, M., Kumar, P., Goutam, U.K. et al. Enhancement in the Transport and Optoelectrical Properties of Spray Coated ZnO Thin Films by Nd Dopant. Electron. Mater. Lett. 19, 138–160 (2023). https://doi.org/10.1007/s13391-022-00381-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13391-022-00381-5