Abstract
The most considerable solid waste from crude palm oil plants is oil palm empty fruit bunch (OPEFB) which contains cellulose, lignin, and hemicellulose. Hemicellulose can be hydrolyzed to xylose and then converted to furfural via dehydration. Pretreatment is one of the steps in the bioconversion of lignocellulose material to reduce lignin. This study developed a one-pot process to conduct pretreatment and furfural production simultaneously. This process uses a green solvent called ternary deep eutectic solvent (DES). DES was synthesized by mixing choline chloride, oxalic acid, and ethylene glycol with a molar ratio of 1:1:2 (CHOAEG). Simultaneous delignification and furfural production were carried out in a stainless steel reactor. The temperature was varied at 100, 120, and 150 °C, with the various processing time at 30, 60, and 90 min, respectively. The highest furfural concentration reached 9.68 g/L, and the delignification was achieved up to 55.81% at 150 °C for 90 min. The OPEFB pretreated was hydrolyzed by cellulase and achieved 90.79% glucose yield. Overall, the simultaneous delignification and furfural production process by ternary DES CHOAEG demonstrated a novel and efficient process by reducing the number of complex processes stages of biorefinery lignocellulose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Lignocellulosic is the most abundant biomass resource. Lignocellulose can be converted into various products, such as chemicals, materials, and energy sourcing. Lignocellulosic biomass has a complex structure consisting of three main polymer components: cellulose, hemicellulose, and lignin [1]. The concept of biorefinery on lignocellulosic aims to produce several products from lignocellulosic. Cellulose can be utilized as raw material for bioethanol, biofuel, pulp mills, lactic acid, nanocellulose, cellulose acetate, and other biochemical materials [2,3,4]. Lignin is a potential biopolymer as a wood adhesive [5, 6]. Hemicellulose can be converted to obtain various products, such as xylose and xylitol, and can be converted into furfural [7].
The bioconversion of cellulose and hemicellulose may be hampered by the lignin present in oil palm empty fruit bunches (OPEFB). By generating a lignin carbohydrate complex (LCC) with hemicellulose upon binding, the lignin complex structure prevents bioconversion. Hence, it is necessary to perform a delignification or pretreatment procedure to decrease the lignin content when utilizing hemicellulose. The pretreatment of lignocellulosic materials involves the utilization of alkaline or acidic solvents. Alkaline solvents are effective in reducing the lignin content by employing intermolecular saponification of hemicellulose, xylan ester cross-links, and other constituents [8]. In pretreatment, acid solvents destroy the lignocellulosic biomass's carbohydrates, turning them into sugar [9, 10]. Due to their toxicity and corrosiveness, these solvents do have some restrictions. The development of green solvents for the pretreatment process has been the subject of current research.
Furfural can be utilized as a raw material for the formation of other compounds, making it a promising building block material [11]. Furfural is commonly used in petroleum processing as a solvent and finds other applications in industries such as agrochemicals and pharmaceuticals [12]. Furfural is obtained from the hydrolysis of hemicellulose to xylose, followed by a dehydration process [7]. The furfural market continues to increase due to the high use of furfural and its derivative compounds. Around 300–700 tonnes/year of furfural is produced worldwide [13]. However, the constraints of the current furfural production process are the low yield and the long process. Therefore, the various efforts to improve the process efficiency of furfural production are interesting to develop.
Obstacles to the creation of commercial furfural production processes from waste biomass include low yield, toxic solvent that used in process, corrosive catalyst caused this process need specialized equipment and materials and industrial furfural production can be energy-intensive, which can increase production costs and limit the scalability of the process [12, 14, 15]. Ensuring sustainable and environmentally friendly production processes is important. Minimizing energy consumption, water usage, and waste generation, as well as addressing any potential environmental concerns, is crucial for commercial furfural production [14, 16, 17]. Furthermore, developing sustainable technologies for the supply of chemicals from renewable raw materials, increasing product yield, and reducing production costs can enhance the efficiency and sustainability of furfural production processes. Addressing these challenges through technological advancements, process optimization, and ongoing research and development efforts can pave the way for the successful commercialization of furfural production processes using waste biomass.
Some research has been performed to increase furfural production. The amount of hemicellulose, the solvent, the catalyst, and the hydrolysis and dehydration procedure were some variables that affected the furfural production from lignocellulose [18,19,20]. Solvent utilization is one of the challenging factors in producing furfural from OPEFB. Sulfuric acid is a common solvent as well as a catalyst for the manufacture of furfural. Several chemicals have also been used to manufacture furfural, including metal chlorides, organic acids, and hydrochloric acids [18, 21, 22]. However, these materials harm the environment and damage equipment, so various studies on solvents to replace sulfuric acid have been conducted.
Recently, deep eutectic solvents (DES) are becoming popular green solvents for converting biomass. While DES functions similarly to ionic liquids, it is simpler to make, generally less expensive, and less harmful [23, 24]. DES, as a new solvent, is created by combining hydrogen-bond donors and acceptors (HBD and HBA) [25, 26]. DES is frequently used for chemical extraction, lignin isolation, biomass processing, and other applications [27,28,29]. Commonly DES used in this process consists of two components. Several recent studies have used the addition of a third material in the DES synthesis, known as "the ternary DES". The addition of this third component can maintain the stability and viscosity of DES. Using ternary DES can improve the reduction of lignin and hemicellulose in lignocellulosic biomass [30, 31]. However, there are fewer publications related to the utilization of ternary DES for delignification and furfural production from OPEFB conducted simultaneously in one-pot production. OPEFB contains high lignin that requires an effective solvent to remove the lignin. In this research, the delignification process and furfural production carried out in a novel one-pot system using ternary DES can reduce the lengthy process stages of furfural production in general. This process is more efficient due to reduced processing time and equipment. This paper discusses the delignification and furfural production by three-constituent DES containing choline chloride/oxalic acid/ethylene glycol in varied temperatures and processing times. Besides that, the characterization of the biomass resulting from DES pretreatment was also investigated, and an enzymatic hydrolysis process was carried out to produce glucose. This study aims to demonstrated an efficient process to produce high yield of furfural from lignocellulose biomaterial and reduce the complexity process stage of biorefinery from lignocellulose.
2 Materials and Method
2.1 Materials
The empty fruit bunch used in this research was from a palm oil plantation in Sumatra, Indonesia. A Furfural solution for the standard was purchased from Merck. As an analytical grade, the xylose, choline chloride, and oxalic acid were purchased from Sigma-Aldrich. The cellulase complexes of Cellic® Ctec2 from Novozyme were used for enzymatic hydrolysis.
2.2 Deep Eutectic Solvent (DES) Synthesis
The DES was generated by combining two or three components acting as hydrogen bond donors and hydrogen bond acceptors with a specific molar ratio. The mixture was heated for 60 min at 80–100 °C using an oil bath until it reached a clear and homogenous solution [22]. Then, in order to determine the properties of DES, density, viscosity, and pH were measured.
2.3 Simultaneous Delignification and Furfural Production
The delignification and furfural production was conducted in one-pot system. A glass reactor with agitation is used in the experiment, and it is submerged in a thermostatic oil bath. 10% w/w substrate of OPEFB was mixed with DES (solid–liquid ratio 1:5). The reactor was sealed and submerged in a heated oil bath between 100 and 150 °C. The reactor was removed from the oil bath and placed in the ice chamber to cool to room temperature after a series of time reactions in order to prevent undesired reactions. The sample was added with ethanol with ratio DES/ethanol of 1:3 and then filtered. The collected filtrate was analyzing to measured furfural content. The solid fraction was washing with ethanol and finally with aquadest until the neutral pH. The solid fraction was drying in oven 50 °C overnight to get below 10% moisture content. The solid fraction was named as DES pretreated OPEFB (DP-OPEFB). The DP-EBF was then analyzed to measure chemical composition after DES pretreatment.
The intensity of the DES pretreatment was expressed as the severity factor (log Ro), as shown in Eq. 1. The severity factor corresponds to combination of reaction time, t (min), and the process temperature, T (°C), while TRef is the reference temperature of 100 °C, and 14.75 °C is the fitted parameter used to normalize the temperature difference (T − Tref) in the exponential term of the severity factor equation [32].
2.4 Enzymatic Hydrolysis
Enzymatic hydrolysis was processed in 15% DP-OPEFB immersed in sodium citrate buffer pH 4.8. The dose of the cellulase enzyme Cellic® Ctec2 was 30 FPU/g biomass. The enzymatic hydrolysis was conducted in a shaker incubator at 50 °C temperature, 150 rpm for 72 h, and the sample was sampled every 24 h to measure glucose content. Enzymatic hydrolysis was conducted in duplicate experiments.
2.5 Analytical Methods
DES characterization was conducted by measuring pH with a pH meter (Mettler Toledo), density by density meter (DMA 4100 M), and viscosity by viscometer kinematic (Tamson Instrument BV). OPEFB chemical composition was measured according to NREL method [33]. Acid hydrolysis was used to determine the lignin, cellulose, and hemicellulose content of OPEFB. First, 300 mg of OPEFB (dry weight) was subjected to acid hydrolysis. After hydrolysis, the sample was filtered to separate solid fractions and filtrate. Acid-insoluble lignin (AIL) in the solid fraction was weighed using a Sartorius BS224S. Meanwhile, acid-soluble lignin (ASL) in the filtrate was measured using a UV/Vis Spectrophotometer (Optizen 2120 UV) at 205 nm. The sum of AIL and ASL yielded total lignin. On the other hand, filtrate is also used to measure cellulose and hemicellulose content by HPLC (Waters e2695).
With the assistance of a secondary graphite monochromator, a Philips PW 1710 X-ray diffractometer, CuK irradiation at 40 kV, and 30 mA, the crystallinity index and structural alterations of OPEFB before and after the pretreatment were examined. Shimadzu FTIR spectroscopy was used to analyze OPEFB's structural alterations. Additionally, the surface morphology of the treated and untreated biomass samples was examined using scanning electron microscopy (SEM) with the JEOL JSM-IT200 apparatus at SE 10 kV and 1000× magnification.
The aniline acetate colorimetric technique was used to evaluate the qualitative furfural concentration [34]. By combining aniline, acetic acid, and 95% ethanol in a ratio of 1:1:8, aniline acetate reagent was created. Dehydration sample was pipetted into a test tube with 0.1 mL, followed by the addition of 5 mL of aniline acetate reagent. The sample was vortex to homogenize the reaction, and the sample's color was changed to a red solution. By employing an HPX-87P (Bio-RAD, CA, USA) column and a RI detector with a mobile phase flow rate of 0.6 mL/min and a five mM H2SO4 solution, the concentrations of glucose and furfural were determined by HPLC (Waters e2695).
3 Result and Discussion
3.1 OPEFB Composition
The OPEFB samples were analyzed using the NREL biomass characterization method to analyze cellulose, hemicellulose, and lignin content. Carbohydrates, specifically cellulose and hemicellulose, were found to be the predominant components of OPEFB, accounting for 46.08% of the biomass. Lignin, on the other hand, made up 39.13% of the biomass. The significant amount of lignin in OPEFB necessitates a delignification process before it can be utilized as a raw material for bio-based chemicals. The cellulose and hemicellulose content can be converted into glucose and furfural, respectively.
3.2 DES Characterization
In this study, ten variations of DES were synthesized using various types of HBA and HBD as constituents to find the appropriate DES, as shown in Table 1. HBA and HBD with a certain number of mole ratios were mixed and heated while stirring at 80 °C for 30 min to form a clear solution. The DES is stable if it remains at room temperature in the clear liquid phase. Due to a drop in the freezing point value and a usually lower freezing point than the ingredient, the DES will remain in the liquid phase [35]. HBD complexes, halide salt anions, and hydrogen bonds all help lower the freezing point of DES. The mole ratio of each DES was determined based on the stability of the DES liquid phase at room temperature. Some DES have a molar ratio of more than one because, in a 1:1 ratio, the DES solution has not been completely formed, indicated by the presence of sediment in the solution. This residue is caused because the ratio with the mole ratio does not match, and the hydrogen bonds that occur are not perfect.
The DES solvent is screened using OPEFB with DES solvent at a solid-to-liquid ratio of 1:5. The process was carried out at 150 °C for 30 min. This is the optimal condition for the OPEFB delignification process using the alkaline solvent NaOH as a result of previous studies [36]. The evaluation was carried out by looking at the delignification ability of DES and also the furfural production in the process. Furfural content was tested qualitatively using an aniline acetate reagent. Aniline acetate can react with furfural to form a red complex [34]. The results of the DES screening are shown in Table 2. Several DES can directly produce furfural from OPEFB subtract with varying concentrations. The higher the furfural content, the darker the red color indicator color will be. If no red color is formed, it indicates no furfural was formed (symbol -); dark red means a high concentration of furfural (symbol + more than one). Delignification capability is performed by measuring the remaining lignin content in the solid fraction after the process. Table 1 shows that some DES have good delignification abilities but cannot produce furfural, and vice versa. The ability of DES in the process of converting biomass is generally specific to the desired compound target. It can be seen that DES containing ethylene glycol tends to produce good delignification, whereas DES which contains oxalic acid and choline chloride can produce furfural.
Table 2 shows that DES choline chloride (ChCl): oxalic acid: ethylene glycol (CHOAEG) produces high delignification and furfural yields production so that in the next process, this research focuses on the utilization of CHOAEG. Choline chloride acts as HBA, while oxalic acid and ethylene glycol act as HBD. The primary catalyst for the synthesis of DES is the creation of hydrogen bonds between the halide choline chloride anions, ethylene glycol, and oxalic acid.
The CHOAEG characterization is shown in Table 2. We can see the pH of DES is 1.93; this indicates that CHOAEG is acidic DES. Besides being a solvent, the acidic DES can act as a Brønsted acid catalyst [23, 35]. The CHOAEG solution has a density of 1.21 g/L. The kinematic viscosity of DES-CHOA is 20.3 cSt, more viscous than water but lower than choline chloride/oxalic acid DES [37]. Low viscosity can increase the interaction of DES with the substrate.
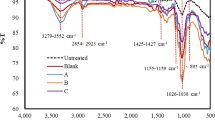
The FTIR spectra and assignment of the functional group of CHOAEG were conducted and are shown in Fig. 1 and Table 3. Table 3 shows the results of the analysis of the FTIR spectra of choline chloride, ethylene glycol, oxalic acid, and DES CHOAEG to determine if hydrogen bonds were formed. The distinctive peaks in the FTIR spectra that are seen at 3279 cm−1, 3393 cm−1, and 3401 cm−1, respectively, correspond to the O–H vibrations of pure choline chloride, ethylene glycol, and oxalic acid. The C-N vibration of choline chloride, on the other hand, is indicated by the vibration that was measured at 1063 cm−1. The O–H vibrations of ethylene glycol were found at 3393 cm−1 in the FT-IR spectra of the DES. The existence of hydrogen bonding between HBDs and choline chloride during the synthesis of the DES is therefore indicated by the change in the O–H vibrations.
3.3 Influence of Three-Constituent DES on Delignification and Furfural Production
The pretreatment process used CHOAEG with temperature and time-variant process parameters based on the severity factor value. Table 4 and Fig. 2 show the severity factor (SV) of variations in temperature and time used and its effect on the OPEFB delignification and biomass recovery using ternary DES. The lowest temperature is 100 °C, with the highest temperature 150 °C, while the variation time is 30–90 min. Severity factor data are made to see the effect of xylose produced and the decrease in lignin content of OPEFB to time and temperature simultaneously.
The pretreated OPEFB (DP-ROPEFB) has a change in composition. The CHOAEG pretreatment process aims to reduce the lignin content, hydrolyze hemicellulose and convert it directly to furfural. Reduction of lignin, hemicellulose, and other chemical compounds will reduce the mass of OPEFB produced. The biomass recovery of OPEFB after the pretreatment process is shown in Fig. 2. The pretreated OPEFB will lose 45–65% of its initial mass [36]. The solubilization of OPEFB has further improved in CHOAEG pretreatment. Li et al. reported that the addition of ethylene glycol in choline chloride/oxalic acid could increase the solubilization of lignin in bamboo [38].
The temperature of the pretreatment process affects biomass recovery. The higher the process temperature, the higher the biomass loss caused by the increasing number of dissolved chemical components. It can be seen from the severity factors 1.78, 2.36, and 3.2, which is the effect of temperature on the pretreatment process, and the biomass recovery is decreasing, 65.1, 58.26, and 46.48%, respectively. The solubility of biomass during the pretreatment process is influenced by processing duration as well. Lignin and hemicellulose are degraded during this process. The lignin in EFB degrades and dissolves in the DES, while the hemicellulose component converts to furfural. As a result of 35% of the EFB being degraded into furfural and lignin that is soluble in the DES. The remaining 65% is solid material with a high cellulose concentration. Based on Fig. 2, the longer the time, the more biomass dissolution is indicated by less biomass recovery at SV 2.94, 3.25, and 3.42. These results align with a study by Hassan et al. (2022), who performed chemical pretreatment with DES on Miscanthus with biomass yields of 46.1–72.7% [39].
Figure 2 also shows a graph of the delignification that occurs in OPEFB. The greater the severity factor value, the higher the OPEFB delignification. The higher temperature and the longer processing time resulted in higher delignification. It may occur because the dissolved biomass component during the pretreatment process is dominated by lignin due to the capacity of DES to selectively disrupt ether bonds without affecting carbon–carbon connections. The breaking of ether bonds may aid in the extraction of lignin from the natural structural body of biomass [40]. The highest delignification was obtained by pretreating with a temperature of 150 °C, 90 min, which was 55.81%. The higher temperature can reduce the viscosity of the DES solvent, thereby increasing the contact between the material and the solvent.
The delignification process using CHOAEG occurs due to the ability of CHOA EG to extract phenolic compounds. Phenolic compounds dominate lignin content. So that CHOAEG can break bonds and remove lignin contained in OPEFB. The mechanism of biomass delignification using DES is due to their ability to selectively cleave the ether bonds between lignin phenyl propane units without affecting the C–C linkages [41]. Ethylene glycol-based DES demonstrates a greater enrichment factor (EF) compared to pure ethylene glycol. This can be attributed to the DES's enhanced hydrogen bonding capability and increased electrostatic interactions with the target analytes, surpassing those of pure ethylene glycol.
Figure 2 also shows the optimal intersection of the process in this condition at a severity value of 3.25. The result indicates a relatively high occurrence of delignification, with a low loss of biomass. Suppose the level of delignification is high, but the biomass yield is minimal. In that case, the resulting product is inefficient because many substrates are wasted. In another scenario, if biomass yield is high with the lignin content still high, the biomass conversion process cannot run well because the high lignin content hinders further processing.
The decrease in lignin content will increase the cellulose content in pretreated OPEFB, as shown in Fig. 3. The cellulose content in OPEFB has risen from 30 to 50–66%. The high cellulose content is due to the very low solubility of cellulose in CHOAEG because the dissolution of highly structured cellulose usually requires harsher conditions [42]. The cellulose content in pretreated OPEFB can be converted into glucose for other chemical raw materials. Hemicellulose levels decreased because hemicellulose converted to xylose. Xylose is then dehydrated to furfural. The presence of hemicellulose in pretreated OPEFB indicates that not all hemicellulose has been converted to xylose or furfural.
SEM was utilized to examine and illustrate the alterations in the surface morphology of OPEFB. Figure 4 presents the observed changes. Prior to the pretreatment, the untreated OPEFB fiber exhibited a firm surface, which was enveloped by a layer of matrix materials like waxes and silica, extending across the entire fiber surface (Fig. 4a). After CHOAEG pretreatment, there appeared uniform pores in the character of OPEFB. The structure of the OPEFB has been split, and many fibers are formed, as shown in the red circle (Fig. 4b). It indicated that some silica was removed from OPEFB. The pretreatment can reduce lignin content and remove the biomass's silica [43]. According to Fig. 4, CHOAEG changed the surface morphology of OPEFB.
The furfural production process is carried out by mixing OPEFB into CHOAEG solvent. This furfural production process occurs simultaneously, the hydrolysis of hemicellulose in OPEFB into xylose and then dehydration to furfural. This process takes place in a one-pot reaction. In Fig. 3, we can see that the OPEFB's hemicellulose content in OPEFB decreased after being processed with CHOAEG, because the hemicellulose in OPEFB has been converted to xylose and furfural. The higher the temperature, the lower the hemicellulose content, which means more hemicellulose is converted. This is in line with research conducted by Lee et al. [24]. Hemicellulose is composed of different sugar units, such as xylose, mannose, and arabinose. The acidic conditions help to break the bonds between the sugar units, resulting in the release of individual sugar molecules [44]. The DES solvent can act as a reaction medium and catalyst for the hydrolysis and dehydration reactions involved in the conversion process. The process by which furfural is formed from xylose is still a topic of debate and lacks a definitive conclusion. In particular, the mechanism of furfural production can vary depending on the catalyst used and the specific reaction conditions. The protons present in the deep eutectic solvent (DES), which are provided by oxalic acid, proceeded to cleave the β-1,4 glycosidic bonds in the hemicellulose components. This hydrolysis process resulted in the formation of xylose [45]. The acidic DES contain oxalic acid can induced xylose dehydration to form furfural via the xylose isomerization to xylulose [22, 45]. The utilization of DES in furfural production offers several advantages, such as cost-effectiveness, non-toxicity, simplified manufacturing process, and enhanced biodegradability and biocompatibility [46]. Choline chloride-based DES is commonly employed in furfural processes due to the ability of chloride ions in choline chloride to disrupt the hydrogen bonding network of biomass [47]. Furthermore, choline chloride-based DES can produce furfural even without a catalyst by employing dicarboxylic acids such as oxalic acid, succinic acid, and malonic acid [19].
Figure 5 shows the furfural production from OPEFB was conducted in variation temperature and time reactions. Hemicellulose in OPEFB can be hydrolyzed into xylose by using a CHOAEG solvent. The reduced content of hemicellulose in OPEFB can prove it. CHOAEG is an acid DES, so acid hydrolysis occurs in this process. Acid hydrolysis of lignocellulosic acid will convert hemicellulose into xylose [48]. Xylose was converted to furfural through a dehydration reaction. The dehydration reaction in xylose will convert xylose into furfural and release three water molecules [24]. Even without the presence of an additional catalyst, hemicellulose can still be transformed into furfural. Due to DES containing a Brønsted acid group, it can act as both a solvent and a catalyst [23]. The reaction was conducted at 150 °C for 90 min (severity factor 3.43), resulting in the highest furfural concentration of 9.68 g/L, while the reaction at 100 °C for 60 min yielded the lowest furfural concentration. Figure 5 illustrates that the furfural concentration increases as the reaction temperature is raised simultaneously. The maximum furfural content was observed at a temperature of 150 °C. This finding aligns with Zhu's research, which demonstrated that temperature and time can influence the conversion of xylose to furfural when sulfuric acid is used for furfural production [49].
3.4 Influence of Three-Constituent DES on Enzymatic Hydrolysis
The solid fraction that contains high cellulose is then carried out by enzymatic hydrolysis. The complex cellulase enzyme plays a role in saccharification, breaking down long cellulose chains into glucose monomers. Figure 6 shows the glucose concentration for 72 h from OPEFB pretreated with CHOAEG on the severity factor variation. It can be seen in Fig. 6 that the glucose content of the saccharification product increases along with the higher severity factor in the pretreatment process. This aligns with the cellulose content from the pretreatment process shown in Fig. 3. Adding ethylene glycol to DES can reduce the lignin content of biomass [38]. The decrease in lignin levels caused the cellulose content in pretreated OPEFB to increase. It is responsible for increasing the glucose concentration in the enzymatic hydrolysis process. The removal of lignin and the cellulose structure collapse cause increased enzyme performance [50]. The highest glucose concentration in this process was 85.43 g/L.
Figure 7 shows the glucose levels produced by the two and three constituents of DES, respectively. CHOAEG has glucose levels up to 3–4 times higher than DES, which consists of CH/OA or OA/EG. The result indicated the use of CHOAEG can increase glucose levels. It happens because the three constituents of DES have a lower viscosity, making it possible to improve mass transfer in the process and damage the biomass structure [38, 51].
Table 5 shows the furfural production after the simultaneous delignification and furfural production processes. These results indicate that this process can directly produce furfural, with the highest yield of 57.34% (based on hemicellulose). This process has furfural yield from the OPEFB biomass up to 6.46%; this shows that almost half of the hemicellulose can be converted into furfural. Table 5 also shows the glucose production and yield after the enzymatic hydrolysis process. The results showed that pretreated OPEFB, could be hydrolyzed by cellulase enzymes. It can be seen that the DES pretreatment process produced higher glucose than OPEFB without pretreatment. The yields of untreated glucose based on cellulose and OPEFB substrate only reached 26.93% and 9.25%, respectively. In contrast, the OPEFB after the DES pretreatment process at 150 °C for 90 min (SV 3.43) can reach 90.79% (based on cellulose).
Figure 8 shows the mass balance of the simultaneous delignification and furfural production processes at a severity factor of 3.43. Untreated OPEFB (100 g) contains 31.21 g of cellulose, 14.87 g of hemicellulose, and 39.13 g of lignin. After processing, approximately 44.91 g of solid fractions was collected, which contained high cellulose (29.94 g), low hemicellulose (0.90 g), and lignin (7.76 g). This process has succeeded in reducing the lignin levels in OPEFB. DES pretreatment has been proven successful in reducing lignin levels in lignocellulosic materials [48, 52, 53].
Additionally, this process achieves a relatively high recovery of cellulose, reaching 95.93%. The liquid fraction contains 9.68 g of furfural, which is produced from 14.97 g of hemicellulose in OPEFB. Some hemicellulose has not been converted and remains in the solid fraction. Furthermore, it can be observed that this process also yields 31.36 g of lignin in the liquid fraction. While lignin is typically considered a waste product, it holds potential value as a renewable feedstock for various applications, including the production of bio-based chemicals, materials, and energy [54,55,56].
The CHOAEG solvent can play a crucial role in facilitating these conversions and allowing for the selective extraction of these valuable components. Therefore, the DES process provides an opportunity to extract furfural from hemicellulose, glucose from cellulose, and recover lignin from palm biomass, enabling the beneficial recovery of these valuable components and contributing to a more comprehensive utilization of the biomass feedstock.
Several studies using DES in the lignocellulosic biorefinery process are shown in Table 6. It can be seen that several DES are explicitly used for the desired end product. DES containing carboxylic acids such as oxalic acid and citric acid is used for the furfural conversion process [24, 35, 47, 57]. Lee et al. [24] used DES choline chloride and citric acid on palm oil substrates to produce a furfural yield of 26%. A higher furfural yield of 51% was produced by Zhang dan Hu using xylan [35]. DES containing ethylene glycol is used for the delignification process of lignocellulosic biomass. The resulting delignification is around 40–50% [39]. This study used the CHOAEG ternary to produce delignification of approximately 55.81%; this result was slightly smaller than the results of the Duan et al. study, which yielded 65.6% [58]. It happens because the lignin on the OPEFB was higher than the lignin in the Eucommia ulmoides seed shells. The CHOAEG process also produces glucose yields that are almost the same as in previous studies. The advantage of using the ternary DES is that it can produce several target compounds, such as furfural and glucose, with high yields.
The process of converting hemicellulose to furfural is already available on a commercial scale. The Quaker oats process is a widely used method for the conversion of hemicellulose to furfural from biomass, especially using corncob. However, the current process utilizes a toxic acidic solvent. Yield from the process reaches 40–52%. Several technologies have also been developed and applied commercially such as Microthermal Conversion (MTC), Suprayield, Biofine with a yield of 60–80% [13]. This process produces a yield of 57.34%. This process shows significant promise for commercial application. However, some developments can still be improved to be applied. The materials used in the production of DES, such as choline chloride, oxalic acid, and ethylene glycol, are commonly produced and used in the industry at a relatively affordable cost. Although this cost is still higher than conventional solvents, DES has the advantage of being less toxic.
4 Conclusion
This research successfully conducted delignification and furfural production from OPEFB using three-constituent deep eutectic solvents in one-pot process. After the process at a temperature 150 °C for 90 min, the delignification of OPEFB reached up to 55.81% and also produce furfural in value of 9.68 g/L. The pretreated OPEFB was then converted to glucose at 85.43 g/L. This result was 3–4 times more than using two-constituent DES. Increasing the temperature process can increase delignification and furfural production due to more lignin and hemicellulose reduction. The furfural yield in this method can achieve 57.34%. This process has an advantage over others since it can carry out the delignification process in a single stage, resulting in OPEFB pulp with low lignin concentration and high cellulose content that can be converted to glucose. Utilization of three-constituent DES promised to increasing efficiency process of biorefinery lignocellulosic such as OPEFB.
Availability of Data and Materials
Not applicable.
References
Sluiter, J.B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, A.D.; Templeton, D.W.: Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. J Agric Food Chem 58, 9043–9053 (2010). https://doi.org/10.1021/jf1008023
Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K.: Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: overview and limits. J. Biotechnol. 156, 286–301 (2011). https://doi.org/10.1016/j.jbiotec.2011.06.017
Amaral, H.R.; Cipriano, D.F.; Santos, M.S.; Schettino, M.A., Jr.; Ferreti, J.V.T.; Meirelles, C.S.; Pereira, V.S.; Cunha, A.G.; Emmerich, F.G.; Freitas, J.C.C.: Production of high-purity cellulose, cellulose acetate and cellulose-silica composite from babassu coconut shells. Carbohydr. Polym. 210, 127–134 (2019)
Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Ivančić Šantek, M.; Komes, D.; Novak, S.; Šantek, B.: Bioethanol production from renewable raw materials and its separation and purification: a review. Food Technol. Biotechnol. 56, 289–311 (2018)
Karunakaran, V.; Abd-Talib, N.; Yong, T.-L.K.: Lignin from oil palm empty fruit bunches (EFB) under subcritical phenol conditions as a precursor for carbon fiber production. Mater. Today Proc. 31, 100–105 (2020)
Hermiati, E.; Lubis, M.A.R.; Risanto, L.; Laksana, R.P.B.; Zaini, L.H.: Characteristics and bond performance of wood adhesive made from natural rubber latex and alkaline pretreatment lignin. Proc. Chem. 16, 376–383 (2015). https://doi.org/10.1016/j.proche.2015.12.067
Gozan, M.; Panjaitan, J.R.H.; Tristantini, D.; Alamsyah, R.; Yoo Y.J.: Evaluation of separate and simultaneous kinetic parameters for levulinic acid and furfural production from pretreated palm oil empty fruit bunches. Int J Chem Eng 2018 (2018)
Sivanarutselvi, S.; Poornima, P.; Muthukumar, K.; Velan, M.: Studies on effect of alkali pretreatment of banana pseudostem for fermentable sugar production for biobutanol production. J. Environ. Biol. 40, 393–399 (2019). https://doi.org/10.22438/jeb/40/3/MRN-721
Kristiani, A.; Abimanyu, H.; Setiawan, A.H.; Aulia, F.: Effect of pretreatment process by using diluted acid to characteristic of oil palm’s frond. Phys. Proc. 32, 183–189 (2013). https://doi.org/10.1016/j.egypro.2013.05.024
Solarte-Toro, J.C.; Romero-García, J.M.; Martínez-Patiño, J.C.; Ruiz-Ramos, E.; Castro-Galiano, E.; Cardona-Alzate, C.A.: Acid pretreatment of lignocellulosic biomass for energy vectors production: a review focused on operational conditions and techno-economic assessment for bioethanol production. Renew. Sustain. Energy Rev. 107, 587–601 (2019)
Lee, C.B.T.L.; Wu, T.Y.: A review on solvent systems for furfural production from lignocellulosic biomass. Renew. Sustain. Energy Rev. (2021). https://doi.org/10.1016/j.rser.2020.110172
Dashtban, M.; Gilbert, A.; Fatehi, P.: Production of furfural: overview and challenges. J. Sci. Technol. For. Prod. Process. 2, 44–53 (2012)
Cousin, E.; Namhaed, K.; Pérès, Y.; Cognet, P.; Delmas, M.; Hermansyah, H.; Gozan, M.; Alaba, P.A.; Aroua, M.K.: Towards efficient and greener processes for furfural production from biomass: a review of the recent trends. Sci. Total Environ. 847, 157599 (2022)
Naga Sai, M.S.; De, D.; Satyavathi, B.: Sustainable production and purification of furfural from waste agricultural residue: an insight into integrated biorefinery. J. Clean. Prod. 327, 129467 (2021). https://doi.org/10.1016/j.jclepro.2021.129467
Yong, K.J.; Wu, T.Y.; Lee, C.B.T.L.; Lee, Z.J.; Liu, Q.; Jahim, J.M.; Zhou, Q.; Zhang, L.: Furfural production from biomass residues: current technologies, challenges and future prospects. Biomass Bioenergy 161, 106 (2022). https://doi.org/10.1016/j.biombioe.2022.106458
Granados, M.L.; Alonso, D.M.: Furfural: an entry point of lignocellulose in biorefineries to produce renewable chemicals, polymers, and biofuels. World Scientific, Singpore (2018)
Wiranarongkorn, K.; Im-orb, K.; Panpranot, J.; Maréchal, F.; Arpornwichanop, A.: Exergy and exergoeconomic analyses of sustainable furfural production via reactive distillation. Energy 226, 120339 (2021)
Yemiş, O.; Mazza, G.: Acid-catalyzed conversion of xylose, xylan and straw into furfural by microwave-assisted reaction. Bioresour. Technol. 102, 7371–7378 (2011). https://doi.org/10.1016/j.biortech.2011.04.050
Lee, C.B.T.L.; Wu, T.Y.: A review on solvent systems for furfural production from lignocellulosic biomass. Renew. Sustain. Energy Rev. 137, 110172 (2021). https://doi.org/10.1016/j.rser.2020.110172
Kim, J.H.; Cho, S.M.; Choi, J.H.; Jeong, H.; Lee, S.M.; Koo, B.; Choi, I.G.: A simultaneous conversion and extraction of furfural from pentose in dilute acid hydrolysate of quercus mongolica using an aqueous biphasic system. Appl. Sci. 11, 1–12 (2021). https://doi.org/10.3390/app11010163
Kim, E.S.; Liu, S.; Abu-Omar, M.M.; Mosier, N.S.: Selective conversion of biomass hemicellulose to furfural using maleic acid with microwave heating. Energy Fuels 26, 1298–1304 (2012)
Zhang, L.X.; Yu, H.; Yu, H.B.; Chen, Z.; Yang, L.: Conversion of xylose and xylan into furfural in biorenewable choline chloride-oxalic acid deep eutectic solvent with the addition of metal chloride. Chin. Chem. Lett. 25, 1132–1136 (2014). https://doi.org/10.1016/j.cclet.2014.03.029
Yu, Q.; Bai, R.; Wang, F.; Zhang, Q.; Sun, Y.; Zhang, Y.; Qin, L.; Wang, Z.; Yuan, Z.: A sustainable system for maleic acid synthesis from biomass-derived sugar. J. Chem. Technol. Biotechnol. 95, 751–757 (2020). https://doi.org/10.1002/jctb.6260
Lee, C.B.T.L.; Wu, T.Y.; Ting, C.H.; Tan, J.K.; Siow, L.F.; Cheng, C.K.; Md. Jahim J, Mohammad AW,: One-pot furfural production using choline chloride-dicarboxylic acid based deep eutectic solvents under mild conditions. Bioresour. Technol. 278, 486–489 (2019). https://doi.org/10.1016/j.biortech.2018.12.034
Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.L.; Rasheed, R.K.; Tambyrajah, V.: Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains Electronic supplementary information (ESI) available: plot of conductivity vs. temperature for the ionic liquid formed from z. Chem. Commun. 2010–2011 (2001)
Smith, E.L.; Abbott, A.P.; Ryder, K.S.: Deep eutectic solvents (DESs) and their applications. Chem. Rev. 114, 11060–11082 (2014). https://doi.org/10.1021/cr300162p
Ai, B.; Li, W.; Woomer, J.; Li, M.; Pu, Y.; Sheng, Z.; Zheng, L.; Adedeji, A.; Ragauskas, A.J.; Shi, J.: Natural deep eutectic solvent mediated extrusion for continuous high-solid pretreatment of lignocellulosic biomass. Green Chem. 22, 6372–6383 (2020). https://doi.org/10.1039/d0gc01560a
Di Marino, D.; Stöckmann, D.; Kriescher, S.; Stiefel, S.; Wessling, M.: Electrochemical depolymerisation of lignin in a deep eutectic solvent. Green Chem. 18, 6021–6028 (2016). https://doi.org/10.1039/c6gc01353h
Mulia, K.; Masanari, E.; Zahrina, I.; Susanto, B.; Krisanti, E.A.: Optimization of palmitic acid extraction from palm oil with betaine-based natural deep eutectic solvent using response surface methodology. AIP Conf. Proc. (2019). https://doi.org/10.1063/1.5134605
Chen, Z.; Reznicek, W.D.; Wan, C.: Deep eutectic solvent pretreatment enabling full utilization of switchgrass. Bioresour. Technol. 263, 40–48 (2018)
Xia, Q.; Liu, Y.; Meng, J.; Cheng, W.; Chen, W.; Liu, S.; Liu, Y.; Li, J.; Yu, H.: Multiple hydrogen bond coordination in three-constituent deep eutectic solvents enhances lignin fractionation from biomass. Green Chem. 20, 2711–2721 (2018). https://doi.org/10.1039/c8gc00900g
Overend, R.; Chornet, E.: Steam and aqueous pretreatments: are they a prehydrolysis? In: Wood processing and utilization. Elsevier, New York, pp 395–400 (1989)
Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Nrel, D.C.: Determination of structural carbohydrates and lignin in biomass determination of structural carbohydrates and lignin in biomass. 2011 (2012)
Barbosa, B.M.; Colodette, J.L.; Longue Júnior, D.; Gomes, F.J.B.; Martino, D.C.: Preliminary studies on furfural production from lignocellulosics. J. Wood Chem. Technol. 34, 178–190 (2014). https://doi.org/10.1080/02773813.2013.844167
Zhang, L.; Yu, H.: Conversion of xylan and xylose into furfural in biorenewable deep eutectic solvent with trivalent metal chloride added. BioResources 8, 6014–6025 (2013)
Muryanto, M.; Sudiyani, Y.; Abimanyu, H.: Optimization of NaOH alkali pretreatment of oil palm empty fruit bunch for bioethanol. InaJAC 18, 27–35 (2016)
Popescu, A.M.; Donath, C.; Constantin, V.: Density, viscosity and electrical conductivity of three choline chloride based ionic liquids. Bulg. Chem. Commun. 46, 452–457 (2014)
Li, N.; Meng, F.; Yang, H.; Shi, Z.; Zhao, P.; Yang, J.: Enhancing enzymatic digestibility of bamboo residues using a three-constituent deep eutectic solvent pretreatment. Bioresour. Technol. 346, 126639 (2022). https://doi.org/10.1016/j.biortech.2021.126639
Hassan, E.-S.R.E.; Mutelet, F.: Evaluation of miscanthus pretreatment effect by Choline chloride based Deep Eutectic solvents on bioethanol production. Bioresour. Technol. 345, 126460 (2022)
Xu, H.; Peng, J.; Kong, Y.; Liu, Y.; Su, Z.; Li, B.; Song, X.; Liu, S.; Tian, W.: Key process parameters for deep eutectic solvents pretreatment of lignocellulosic biomass materials: a review. Bioresour. Technol. 310, 123416 (2020). https://doi.org/10.1016/j.biortech.2020.123416
Alvarez-Vasco, C.; Ma, R.; Quintero, M.; Guo, M.; Geleynse, S.; Ramasamy, K.K.; Wolcott, M.; Zhang, X.: Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): a source of lignin for valorization. Green Chem. 18, 5133–5141 (2016)
Luo, Y.; Li, Z.; Li, X.; Liu, X.; Fan, J.; Clark, J.H.; Hu, C.: The production of furfural directly from hemicellulose in lignocellulosic biomass: a review. Catal. Today 319, 14–24 (2019)
Triwahyuni, E.; Miftah, A.K.; Muryanto, M.; Maryana, R.; Sudiyani, Y.: Effect of CO2-added steam explosion on oil palm empty fruit bunch for bioethanol production. Cellul. Chem. Technol. 55, 839–847 (2021)
Cara, P.D.; Pagliaro, M.; Elmekawy, A.; Brown, D.R.; Verschuren, P.; Shiju, N.R.; Rothenberg, G.: Hemicellulose hydrolysis catalysed by solid acids. Catal. Sci. Technol. 3, 2057–2061 (2013)
Lee, C.B.T.L.; Wu, T.Y.; Cheng, C.K.; Siow, L.F.; Chew, I.M.L.: Nonsevere furfural production using ultrasonicated oil palm fronds and aqueous choline chloride-oxalic acid. Ind. Crops Prod. 166, 113397 (2021). https://doi.org/10.1016/j.indcrop.2021.113397
Haldar, D.; Purkait, M.K.: A review on the environment-friendly emerging techniques for pretreatment of lignocellulosic biomass: mechanistic insight and advancements. Chemosphere 264, 128523 (2021). https://doi.org/10.1016/j.chemosphere.2020.128523
Li, A.-L.; Hou, X.-D.; Lin, K.-P.; Zhang, X.; Fu, M.-H.: Rice straw pretreatment using deep eutectic solvents with different constituents molar ratios: Biomass fractionation, polysaccharides enzymatic digestion and solvent reuse. J. Biosci. Bioeng. 126, 346–354 (2018). https://doi.org/10.1016/j.jbiosc.2018.03.011
Shen, X.J.; Wen, J.L.; Mei, Q.Q.; Chen, X.; Sun, D.; Yuan, T.Q.; Sun, R.C.: Facile fractionation of lignocelluloses by biomass-derived deep eutectic solvent (DES) pretreatment for cellulose enzymatic hydrolysis and lignin valorization. Green Chem. 21, 275–283 (2019). https://doi.org/10.1039/c8gc03064b
Zhu, Y.; Li, W.; Lu, Y.; Zhang, T.; Jameel, H.; Chang, H.; Ma, L.: Production of furfural from xylose and corn stover catalyzed by a novel porous carbon solid acid in γ-valerolactone. RSC Adv. 7, 29916–29924 (2017)
Wang, M.; Liu, K.; Dai, L.; Zhang, J.; Fang, X.: The structural and biochemical basis for cellulose biodegradation. J. Chem. Technol. Biotechnol. 88, 491–500 (2013). https://doi.org/10.1002/jctb.3987
Liu, Y.; Deak, N.; Wang, Z.; Yu, H.; Hameleers, L.; Jurak, E.; Deuss, P.J.; Barta, K.: Tunable and functional deep eutectic solvents for lignocellulose valorization. Nat. Commun. 12, 5424 (2021)
New, E.K.; Wu, T.Y.; Tien Loong Lee, C.B.; Poon, Z.Y.; Loow, Y.L.; Wei Foo, L.Y.; Procentese, A.; Siow, L.F.; Teoh, W.H.; Nik Daud, N.N.; Jahim, J.M.; Mohammad, A.W.: Potential use of pure and diluted choline chloride-based deep eutectic solvent in delignification of oil palm fronds. Process Saf. Environ. Prot. 123, 190–198 (2019). https://doi.org/10.1016/j.psep.2018.11.015
Guo, Z.; Ling, Z.; Wang, C.; Zhang, X.; Xu, F.: Integration of facile deep eutectic solvents pretreatment for enhanced enzymatic hydrolysis and lignin valorization from industrial xylose residue. Bioresour. Technol. 265, 334–339 (2018)
Ejaz, U.; Sohail, M.: Lignin: a renewable chemical feedstock. In: Handbook of smart materials, technologies, and devices: applications of industry 4.0. Springer, pp 1–15 (2021)
Figueirêdo, M.B.; Hita, I.; Deuss, P.J.; Venderbosch, R.H.; Heeres, H.J.: Pyrolytic lignin: a promising biorefinery feedstock for the production of fuels and valuable chemicals. Green Chem. 24, 4680–4702 (2022)
Liu, Z.-H.; Le, R.K.; Kosa, M.; Yang, B.; Yuan, J.; Ragauskas, A.J.: Identifying and creating pathways to improve biological lignin valorization. Renew. Sustain. Energy Rev. 105, 349–362 (2019)
da Silva, L.V.; López-Sotelo, J.B.; Correa-Guimarães, A.; Hernández-Navarro, S.; Sánchez-Bascones, M.; Navas-Gracia, L.M.; Martín-Ramos, P.; Pérez-Lebeña, E.; Martín-Gil, J.: A kinetic study on microwave-assisted conversion of cellulose and lignocellulosic waste into hydroxymethylfurfural/furfural. Bioresour. Technol. 180, 88–96 (2015). https://doi.org/10.1016/j.biortech.2014.12.089
Duan, C.-J.; Han, X.; Chang, Y.-H.; Xu, J.; Yue, G.-L.; Zhang, Y.; Fu, Y.-J.: A novel ternary deep eutectic solvent pretreatment for the efficient separation and conversion of high-quality gutta-percha, value-added lignin and monosaccharide from Eucommia ulmoides seed shells. Bioresour. Technol. 370, 128570 (2023). https://doi.org/10.1016/j.biortech.2022.128570
Muryanto, M.; Amelia, F.; Izzah, MN.; Maryana, R.; Triwahyuni, E.; Bardant, TB.; Filailla, E.; Sudiyani Y.; Gozan, M.: Delignification of empty fruit bunch using deep eutectic solvent for biobased-chemical production. In: IOP conference series: earth and environmental science. IOP Publishing, p 12013 (2022)
Kohli, K.; Katuwal, S.; Biswas, A.; Sharma, B.K.: Effective delignification of lignocellulosic biomass by microwave assisted deep eutectic solvents. Bioresour. Technol. 303, 122897 (2020)
Acknowledgements
The authors gratefully acknowledged the financial support for publication by The Ministry of Research and Higher Education of Indonesia's financial support through the research grant “Hibah Penelitian Disertasi Doktor” (PDD) 2022 (NKB-1012/UN2.RST/HKP.05.00/2022). The authors also acknowledge the facilities and scientific and technical support from the National Research and Innovation Agency through e-Layanan Sains, Badan Riset dan Inovasi Nasional. This research was supported by the National Research and Innovation Agency of Indonesia and Indonesia Endowment Fund for Education financial support through the research grant “Riset Inovasi untuk Indonesia Maju” (RIIM) 2022 (contract number: 97/IV/KS/11/2022).
Author information
Authors and Affiliations
Contributions
M, MG, and YS designed the work, and M collected and analyzed the data. Meanwhile, M, EMH, and MAD were preparing manuscript. MG and YS supervised the study and also the review and editing of the paper, and all authors were agreed to the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human or animal participants.
Conflict interests
There are no conflicts to declare.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muryanto, M., Sudiyani, Y., Darmawan, M.A. et al. Simultaneous Delignification and Furfural Production of Palm Oil Empty Fruit Bunch by Novel Ternary Deep Eutectic Solvent. Arab J Sci Eng 48, 16359–16371 (2023). https://doi.org/10.1007/s13369-023-08211-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-023-08211-y