Abstract
Zinc oxide nanoparticles (ZnO NPs) are attractive sunscreens for UV protection, but they still face some challenges due to their white color and skin whitening, and the extent of UV blocking is still limited to UV-B. Therefore, the present work aimed to develop novel sunscreen agents based on ZnO/Fe3O4 hybrid nanocomposites. ZnO/Fe3O4 hybrid nanocomposites were prepared using a facile one-step combustion method and characterized by XRD, FTIR, FESEM, and UV–Vis diffuse reflectance techniques. The in vitro sun protection factor (SPF) and antioxidant activity of the prepared samples were carried out by spectrophotometric methods. The biocompatibility potential was tested on the normal (Human lung fibroblast: WI38) and cancerous (Hepatocellular carcinoma: HEPG-2) cell lines and the antibacterial activity was tested against Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus. Bare ZnO NPs and ZnO/Fe3O4 hybrid nanocomposites had pure structures with average particle sizes on a scale below 100 nm. The samples had antibacterial and antioxidant activity as well as the potential for biocompatibility and anticancer activity (cell viability was 80% for all nanocomposites up to 6.25 μg/mL indicating non-toxicity). The nanocomposite presented good UV absorption and SPF values (SPF = 12.2–14) not too far from those of bare ZnO (SPF = 15.4) and achieved extended protection over the UV and visible range. ZnO/Fe3O4 hybrid nanocomposites are colored compounds that can match a desired tanned skin tone and have biophysical properties that make them a promising future as a cosmetic UV protectant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Excessive and unprotected exposure to the sun's ultraviolet and visible rays is a source of health problems that can affect human skin and health [1, 2]. Natural ultraviolet rays are subdivided into three categories depending on the wavelength range: UV-C (200–290 nm), UV-B (290–320 nm), and UV-A (320–400 nm) [3]. The ozone layer blocks most UV-C, more than 90% of the solar radiation that reaches the Earth's surface is UVA, and less than 10% is UVB [4]. Visible light is recognized by human eyes as various colors that have wavelengths in a range of 400–800 nm [5]. Ultraviolet UV-B, UV-A, and visible light can interact with human skin, but both UV-B and UV-A are harmful to humans except for their role in vitamin D synthesis [6, 7]. UV-B rays can penetrate the epidermis and upper dermis and lead to melanin pigmentation, skin suntan, and sunburn [8, 9]. Also, they can cause skin aging and RNA/DNA damage [10]. Although UV-A is lower in energy than UV-B, it can penetrate deeply into the dermis and make the skin darker [11]. It can reduce skin elasticity by affecting skin collagen and elastin, promote skin photoaging, cause photosensitivity, and may cause skin cancer [12, 13]. As a result of these health damages, the need to develop UV sunscreens with better efficacy and higher safety has increased [14]. Sunscreen filters are classified into two types: organic filters, chemical blocking agents, and inorganic filters, physical blocking agents [15]. Although organic compounds may provide good sunscreen, they have some drawbacks, including chemical instability, an inability to absorb a wide range of UV rays, and the generation of free radicals that damage collagen and skin DNA when the UV is absorbed [16,17,18]. Therefore, physical sunscreens including zinc oxide nanoparticles (ZnO NPs) are the most desirable inorganic materials due to their excellent UV protection [19]. In addition, ZnO NPs as physical sunscreens offer some advantages over organic sunscreens; they have chemical stability, antibacterial activity, low toxicity, and non-irritation of the skin, making them a better choice in sunscreen products for children and those with medical conditions [20–23]. Also, for cosmetic skin protection, easy synthesis has become economically attractive [24]. ZnO can be synthesized using many methods to obtain different nanostructures, including the traditional ceramic approach to modern wet chemical techniques [25, 26]. Among the synthesis methods, green sol–gel combustion method is a promising approach because it is rapid, one-step, environmentally friendly, and cost-effective [27, 28]. This synthesis route uses zinc nitrate as a precursor and organic compounds as a fuel or igniting agent [29]. Organic molecules can be simple molecules such as citric acid, urea, or glycine; or plant extracts containing active molecules [30,31,32,33]. The application of ZnO NPs for UV protection still faces some challenges due to their white color and skin whitening, and the UV blocking range is still limited to UV-A and has not been extended to the UV-B band [34, 35]. Accordingly, research has focused on mixing zinc oxide with safe organic or inorganic agents to improve its effectiveness as a sunscreen [36]. Bio-metals such as iron and its oxides (magnetite: Fe3O4) are promising for modifying biomaterials agents. They are non-toxic, biodegradable, and biocompatible as well as iron having essential roles in cell and tissue functions [37, 38]. These properties have created a scientific interest in developing them as multifunctional agents for various biomedical applications [39]. Magnetite nanoparticles are used for biomedical applications such as MRI contrast agents, antimicrobials, tissue engineering and regeneration, targeted drug delivery, cancer therapy, and biosensors [40, 41]. Therefore, the objective and novelty of this work are solving the challenges inherent in ZnO NPs as an inorganic sunscreen by hybridizing it with a bio-metal oxide (Fe3O4) as a colored oxide that can protect skin from visible light and non-toxic, biodegradable upon entry into the human body. Hybrid physical sunscreens are expected to attract increased interest due to their non-toxicity, biocompatibility, and extended radiation-blocking potential for both the ultraviolet (UVA) and visible rays. Here, a series of ZnO/Fe3O4 hybrid nanocomposites were synthesized by using a facile one-step combustion method. The biophysical characterizations of the synthesized hybrid nanocomposite were characterized using XRD, FTIR, SEM, and UV–vis diffuse reflectance techniques. The in-vitro determination of sun protection factor (SPF) for synthesized products was performed by spectrophotometric means. The antioxidant activity of the samples was tested by DPPH radical scavenging activity. The biocompatibility of synthesized products was tested on the normal (Human lung fibroblast: WI38) and cancerous (Hepatocellular carcinoma: HEPG-2) cell lines. Their antibacterial activity was tested against Gram-negative Escherichia coli (E. coli) and Gram-positive Staphylococcus aureus (S. aureus).
2 Experimental
2.1 Preparation of ZnO/Fe3O4 Hybrid Nanocomposite
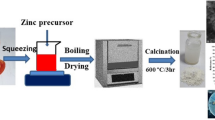
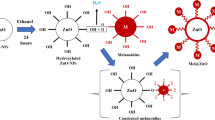
ZnO-magnetite hybrid nanocomposites were prepared using analytical grade Zn(NO3)2⋅6H2O (zinc nitrate hexahydrate, Oxford Lab, India), Fe(NO3)3⋅9H2O (ferric nitrate nonahydrate (RANKEM, India), and citric acid (ACS, 99.5 + %, Elnaser Co., Egypt). A series of ZnO/Fe3O4 hybrid nanocomposites, with different Fe3O4/ZnO weight ratios of 0, 0.1, 0.2, and 0.3, were synthesized using the one-step combustion method. Typically, 30 ml aqueous solutions of zinc precursor were mixed and stirred with 30 ml of different concentrations of iron precursor, as shown in Table 1. Then, 30 ml of aqueous citric acid solution was added and stirred into aqueous solutions. Citric acid was added as a fuel for the combustion reaction and the solutions were preheated on a hot plate to evaporate the water to obtain the viscous solutions and form a dry foam. The initially mixed solution gives a white color with pure zinc precursor while it gives a brown color to solutions of zinc precursor and iron precursor. When the water was completely evaporated, with the pure zinc precursor, the solution changed to a yellowish-yellow gel while with the zinc precursor and ferric iron solutions it changed to dark brown. By increasing the heating time, foam swelling occurred, and the dried foam subsequently underwent auto-ignition and self-propagating combustion, and powdery samples were obtained. Powder samples were collected and roasted at 600 °C for 2 h in an electric oven.
2.2 Characterization of Zinc Oxide/Fe3O4 Hybrid Nanocomposite
Powder X-ray diffraction (XRD) was used to characterize the crystal structure and phase composition of the samples. The XRD patterns were collected over the range of 2θ from 20° to 80° on an X-ray diffractometer (XRD: Bruker-D8 Advance, Germany) with Cu/Kα radiation (λ = 1.5418 Å) operating at 40 kV and 30 mA. The crystal phases were identified for the samples by comparing their peak positions with those of the Joint Committee for Powder Diffraction Standards (JCPD Card No. 36-1451) for ZnO and (JCPD Card No. 19-0629) for Fe3O4. A Field emission scanning electron microscope (FESEM: JEOL JSM 6510 IV) was used to analyze the topography, shape, and size of the formed nanoparticles. Before observation, the powdery samples were put on a carbon-coated mounting grid and the used scanning scales were set at 100,000×, and 200,000×. The mean particle size of the samples was calculated from the FE-SEM images by counting 75 calibrated particles using ImageR software. Fourier transformation infrared spectroscopy (FITR: Jasco FT/IR-400) was used to identify the chemical functional groups for the synthesized samples. The powdery samples were ground separately and mixed with KBr and then the FTIR spectra of the samples were recorded over a frequency range from 400 to 4000 cm−1 with a resolution of 4 cm−1.
2.3 Antibacterial Activity
The antibacterial activity of synthetic samples was evaluated by the agar-well diffusion method against Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria. Stock solutions of ZnO NPs and ZnO/Fe3O4 hybrid nanocomposites were prepared by dispersing 2 mg/ml in sterile water. Pure cultures of bacteria were grown on nutrient broth at 37 °C for 24 h and then sterile cotton swabs were used for a homogenous swap of each strain on each plate. Wells were prepared using inverted micro tips (6 mm). Dispersed samples were individually poured into each well while distilled water was used as a negative control. The plates were incubated at 37 °C for 24 h and the antibacterial activities were assessed by measuring the diameter of the zone of inhibition.
2.4 Cell Culture and Cell Viability Assay
This study used two cell lines: human lung fibroblast (WI-38) and human hepatocellular carcinoma (HepG2) cell lines which were obtained from VACSERA (Cairo, Egypt). Cell lines were cultured in RPMI medium (RPMI-1640: Lonza, USA), 10% fetal bovine serum (GIBCO, UK), and antibiotics (100 units/ml penicillin and 100 µg/ml streptomycin) at 37 °C in a 5% CO2 incubator. The cytotoxic activity of the samples with cell lines was evaluated using the MTT assay (Sigma Aldrich). Cell lines (100 μl) were seeded in a 96-well plate at a density of 1*104 at 37 °C for 48 h in a humid atmosphere (95% air and 5% CO2). After incubation, cells were mixed with different concentrations of nanocomposites suspensions (0, 1.56, 3.125, 6.25, 12.5, 25, 50, and 100 μg/ml) and incubated for 24 h in a humidified atmosphere (95% air and 5% CO2). Then 20 µl of MTT solution (0.5 mg/ml in PBS) was added and incubated for 4 h, and 100 µl of dimethyl sulfoxide (DMSO: Sigma Aldrich) was added into each well to dissolve the formed MTT formazan. A plate reader (EXL 800, USA) was used to measure and record the colorimetric assay at an absorbance of 570 nm. The percentage of viable cells (viability (%)) was calculated by the formula:
2.5 Antioxidant Activity: DPPH Radical Scavenging Activity Assay
The 1,1-diphenylpicrylhydrazyl (DPPH: Sigma Aldrich) test was used to determine the antioxidant capacity of the samples in a spectrophotometric manner at OD517 nm while ascorbic acid was used as a standard. The samples were dispersed in methanol at different concentrations from 10 to 100 μg/ml and the control was ascorbic acid. The ability of samples to scavenge DPPH free radicals was evaluated by the following formula:
2.6 Optical Properties of the Samples
Ultraviolet–visible diffuse reflectance spectroscopy (DRS: Shimadzu UV-2050) was used to characterize the optical absorption properties of the solid nanocomposite samples. Barium sulfate was used as a reference and spectra were recorded in the wavelength range from 200 to 800 nm at room Temperature. One gram of each sample was dispersed in 100 ml of absolute ethanol (Elnaser Co., Egypt).
2.7 In Vitro Investigation of the Sun Protection Factor (SPF)
An in vitro assessment of the SPF of the samples was obtained from absorbance measurements recorded every 5 nm in the wavelength range of 290–320 nm using UV–vis spectroscopy (Labomed UVS-2700). The SPF of the samples was calculated using the following Mansur equation [42]:
where CF = 10 is the correction factor, λ is the wavelength, A is the sample absorbance, I is the solar intensity spectrum, and EE is the erythemal effect.
3 Results and Discussion
3.1 Characterization of Zinc Oxide/Fe3O4 Hybrid Nanocomposites
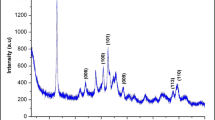
Figure 1 shows the XRD analysis of a bare ZnO sample (a) and different ZnO/Fe3O4 hybrid nanocomposites with different Fe/Zn weight ratios of 0.1 (b), 0.2 (c), and 0.3 (d).
The XRD patterns show the formation of zinc oxide (ZnO) and magnetite (Fe3O4) which were a result of the combustion method. All XRD reflections for the samples can only be indexed into two phases: zinc oxide (ZnO: JCPDS 36-1451) and magnetite phase (Fe3O4: JCPDS 19-0629). No other secondary phases belonging to zinc or iron oxides were detected. For the bare ZnO sample (Fig. 1a), all the diffraction peaks of ZnO can be assignable to the hexagonal wurtzite structure of ZnO without any zinc phase, indicating that the samples have a pure phase structure. For the ZnO/Fe3O4 hybrids (Fig. 1b–d), the diffraction peaks can be attributed to ZnO and those in the spinel phase of Fe3O4. FT-IR analysis examined the main chemical bonds that characterize the ZnO and Fe3O4 structures as Zn–O and Fe–O, respectively. Figure 2 shows FTIR analysis of the functional groups of ZnO NPs (Fig. 2a) and ZnO/Fe3O4 hybrid nanocomposites (Fig. 2b, c, d). Table 2 shows that all FT-IR spectra have bending and stretching vibration peaks between 1633 to 1643 cm−1 and 3413 to 3432 cm−1, respectively that characterize water molecules on the surface of the synthetic nanoparticles. The characteristic peaks at 434 cm−1 and 453 cm−1 correspond to the Zn–O vibrations. The overlapping bands ranging from (570–700 cm−1) characterize the vibrational mode of the Fe–O bonds belonging to the crystalline lattice of Fe3O4.
The morphology, size, and microstructure of the samples were investigated by FESEM as shown in Fig. 3. The FESEM images reveal the formation of sphere-like nanoparticles with an almost homogeneous size distribution. The particle size distribution of the samples has average particle sizes on a scale below 100 nm with a narrow particle size distribution. The bare ZnO NPs have an average size of 48 ± 27 nm (Fig. 3a, h) while the introduction of Fe3O4 significantly changes the average particle size of the nanocomposites: 0.1Fe/ZnO NPs (62 ± 18 nm (Fig. 3b, g)), 0.2Fe/ZnO NPs (64 ± 25 nm (Fig. 3c, f)), and 0.3Fe/ZnO NPs (71 ± 19 nm (Fig. 3d, e)).
3.2 Antibacterial activity
Figure 4 shows the zones of inhibition of bare ZnO NPs and ZnO/Fe3O4 hybrid nanocomposites against the two bacterial strains (E. coli. and S. aureus) by measuring the diameters of the inhibition zone after 24 h. The bare ZnO NPs and ZnO/Fe3O4 hybrid nanocomposites showed good antibacterial activity against pathogenic bacteria as shown in Table 3. All samples are more effective against S. aureus than E. coli. Inhabitation zones of ZnO/Fe3O4 hybrid nanocomposites are an indicator of the bacteriostatic potential of synthesized nanocomposites. For S. aureus, the nanocomposites had diameter inhabitation zone values (=12–13 mm) not too far from those of bare ZnO NPs (= 11 mm) but For S. aureus, their values from 8 to 10 mm are not too far away for the ZnO NPs (= 7 mm).
3.3 Cell viability
Figure 5 shows the effect of the bare Zn NPs (a) and the ZnO/Fe3O4 hybrid nanocomposites (b, c, d) on both WI-38 and HepG2 cell lines. Samples at concentrations above 6.25 μg/ml had apparent cytotoxicity on WI-38 and HepG2 cells at all the investigation times at 24 h test. Compared to ZnO NPs (a), 0.1Fe/ZnO NPs (b), 0.2Fe/ZnO NPs (c), and 0.3Fe/ZnO NPs (d) had a higher effect on cell viability of WI-38 but showed a lower effect on HepG2 cells at all concentrations. The results showed that bare ZnO NPs and ZnO/Fe3O4 hybrid nanocomposites have biocompatibility potential with anticancer activity at low concentrations.
3.4 Antioxidant Activity
The antioxidant activity of bare ZnO NPs and the ZnO/Fe3O4 hybrid nanocomposites was determined using DPPH free radicals. A higher IC50 value indicates lower scavenging activity; a low ability of the samples to act as a DPPH scavenger. Table 4 shows the effect of different concentrations of ZnO NPs and the ZnO/Fe3O4 hybrid nanocomposites on DPPH radical antioxidant activity. The results indicated that the synthesized nanoparticles are moderate free radical scavengers. However, the ZnO/Fe3O4 hybrid nanocomposites showed lower scavenging activity of DPPH than bare ZnO NPs for all concentrations from 10 to 100 μg/ml. The DPPH activity of the samples increased with increasing concentration of synthetic samples indicating a dose-dependent behavior. At concentrations 10–100 µg/ml, ZnO NPs showed a scavenging activity ranging from 23 to 95% with an average IC50 value, 30.14 ± 0.18. The antioxidant activity was close to that of standard ascorbic acid at 100 µg/ml (94%).
3.5 Optical Properties of the Samples
Figure 6A shows the color change of powders and solutions for (a) bare ZnO NPs and ZnO/Fe3O4 hybrid nanocomposites (b, c, d). Figure 6B shows the reflectance spectra of the samples: ZnO/Fe3O4 hybrid nanocomposites (b, c, d) synthesized using the combustion method, as well as their comparison with the spectrum of bare ZnO NPs (a) used in sunscreen applications. ZnO/Fe3O4 hybrid nanocomposites are colored compounds that can match a desired tanned skin tone and have biophysical properties that make them a promising future as a cosmetic UV protectant. In the UV–visible diffused reflectance spectra, low reflectance values of the samples indicate high absorption power of the corresponding radiative wavelength region [47].
ZnO NPs with hexagonal wurtzite crystal structure showed very low diffuse reflectance behavior in the entire UV-B region (280–320 nm) and the UV region (320–372 nm) that is part of the UV-A region (320–400 nm) [48, 49]. They can efficiently absorb ultraviolet rays; they showed a broad and intense absorption peak at 200–400 nm (absorption maximum at 372 nm), which corresponds to a very low diffuse reflectance (~ 4%) in the UV range below 370 nm [50]. ZnO NPs did not show absorption capacity in the visible light region, and the diffuse reflectance values of ZnO NPs start to increase suddenly at 372 nm, which means losing their ability to absorb radiation, especially in the visible light region [51]. All the ZnO/Fe3O4 hybrid nanocomposites showed very low diffuse reflection behavior in the entire UV-B region (280–320 nm) and the UV-A region (320–400 nm). It was also found that 0.1Fe/ZnO sample (Fig. 6b) still has diffuse reflectance in the UV region (absorption maximum at 370–372 nm) which is identical to that of the ZnO NPs sample. However, the 0.2Fe/ZnO sample (Fig. 6c) and the 0.3Fe/ZnO sample (Fig. 6d) underwent very slight increases in UV reflectance at (~ 8%) and (~ 12%), respectively. Therefore, the presence of the Fe3O4 phase did not strongly change the UV absorption capacity of ZnO NPs. However, the optical responses of the Fe3O4/ZnO hybrids showed an obvious progressive absorption and a red shift in the visible light range. While the reflectance values of the hybrid samples increased with a slight gradient in the region from 300 to 500 nm and then with a high gradient up to 700 nm. Therefore, these hybrid samples are considered promising for sunscreen formulations and personal care products. Increasing the ratio of Fe3O4/ZnO led to further improvement of the red shift in the range from 400 to 660 nm. For sunscreen applications, several studies have doped synthetic ZnO NPs with a variety of metals including K, Na, Al, Fe, and Cu to modify their optical properties [52,53,54]. The results show that the efficiency of bare ZnO NPs reaches about 70–90% on UV absorption in the range of 200–400 nm, with the maximum absorption at 372 nm [20, 21, 21]. The results also show that ZnO NPs are optically transparent, as they do not absorb light in the visible range (400–700 nm). The doped ZnO showed a high ability to absorb UV light with an efficiency of 80–90% [50]. Moreover, the metallic activation of transition elements such as Fe and Cu affected the absorption in the visible region compared to that of Al and Zn [50, 53, 54]. Therefore, the presence of the hybrid Fe3O4 phase can enhance the visible light absorption capacity of ZnO NPs. Moreover, the ZnO/Fe3O4 hybrid nanocomposite showed a brown color close to the tanning effect, which may be suitable for use in sunscreens. The ability of ZnO nanoparticles to absorb ultraviolet radiation mostly depends on their chemical nature, phase composition, and particle size [55]. Here in this work, all zinc oxide/Fe3O4 hybrid nanocomposites exhibited high adsorption capacity in the UV region almost similar to that of bare ZnO nanoparticles. This behavior can be explained by the fact that despite the formation of the Fe3O4 phase in the hybrid samples, no change or modification occurred in the ZnO phase, and the hexagonal phase remains in all the hybrid samples as confirmed by X-ray diffraction results. The hybrid samples showed a decrease in particle size compared to the ZnO sample.
3.6 Sun Protection Factor Performance
Table 5 shows the required parameters (EE(λ) × I(λ)) required to determine SPF values and the SPF values calculated for all samples according to the Mansur method. EE(λ) was calculated by Sayre et al. [56]. An in vitro sun protection factor (SPF) test was used to determine the capacity of the synthesized nanocomposites in absorbing UV radiation. An SPF value is defined as the amount of ultraviolet energy needed to achieve a minimum dose of erythema on skin covered with sunscreen compared to the amount of UV energy required to reach a minimum dose of erythema on sun-protected skin. The results of calculations of the SPF values of bare ZnO NPs and ZnO/Fe3O4 hybrid nanocomposites showed that increasing the percentage of Fe3O4 can change the SPF values. The higher the SPF values of sunscreens, the more effective it is in protecting the skin from the dangerous effects of UV rays. The nanocomposites provided good UV absorption and SPF values (SPF = 12.2–14) not too far from those of bare ZnO (SPF = 15.4) and achieved protection extended over the UV and visible range. Bare ZnO NPs had the highest SPF value while 0.3Fe/ZnO sample had the lowest SPF value compared to other hybrid nanocomposites, being 20% less attractive than its value for ZnO NPs. Although the addition of Fe3O4 reduced the response of ZnO to UV absorption, it increased protection for a fraction of visible light.
4 Conclusions
In this study, ZnO NPs and Fe3O4/ZnO hybrid nanocomposites were successfully prepared by a facile one-step combustion and characterized by different analyses. The samples are pure structures and showed antibacterial and antioxidant activity. They had biocompatibility potential for normal cells up to concentrations 6.25 μg/ml indicating no susceptible to toxicity but show higher toxicity for cancerous cells. Although, all Fe3O4/ZnO hybrid nanocomposites can effectively absorb UV light as ZnO NPs, they also show a red shift in the range of 400–660 nm and progressively absorb the visible light by increasing the wavelengths in this range. ZnO/Fe3O4 hybrid nanocomposites are colored compounds that can match a desired tanned skin tone and have biophysical properties that make them a promising future as a cosmetic UV protectant. The prepared sunscreen nanocomposites have SPF values (SPF = 12.2–14) almost close to that of ZnO NPs (SPF = 15.4) that confirm their effective absorption of the UV radiation. They showed the ability to extend the radiation-blocking capabilities of both ultraviolet (UVA) and visible radiation.
5 Statement of Significance
The authors used a facile one-step combustion method for synthesizing novel zinc oxide/Fe3O4 hybrid nanocomposites. Zinc oxide/Fe3O4 hybrid nanocomposites were synthesized using aqueous solutions of zinc nitrate and ferric nitrate as precursors. The nanocomposites properties were investigated using a variety of techniques: XRD, FTIR, FESEM, and UV–vis diffuse reflectance techniques. The in vitro sun protection factor (SPF), antioxidant activity, antibacterial activity, and biocompatibility potential was tested on the normal and cancerous cell lines. The optical results showed the ability of the nanocomposites to extend the radiation blocking capabilities of both UV and visible radiation. ZnO/Fe3O4 hybrid nanocomposites are colored compounds that can match a desired tanned skin tone and have biophysical properties that make them a promising future as a cosmetic UV protectant.
References
Lucas, R.M.: An epidemiological perspective of ultraviolet exposure-public health concerns. Eye Contact Lens. 37, 168–175 (2011). https://doi.org/10.1097/ICL.0b013e31821cb0cf
Gilchrest, B.A.: Sun exposure and vitamin D sufficiency. Am. J. Clin. Nutr. 88, 570S-577S (2008). https://doi.org/10.1093/ajcn/88.2.570S
Parrish, J.A.; Anderson, R.R.; Urbach, F.; Pitts, D.; Parrish, J.A.; Anderson, R. R.: The spectrum of electromagnetic radiation: UV-A in perspective. UV-A Biol. In: UV-A: Biological Effects of Ultraviolet Radiation with Emphasis on Human Responses to Longwave Ultraviolet. pp. 1–6 (1978). https://doi.org/10.1007/978-1-4684-2475-1_1
Leiter, U.; Keim, U.; Garbe, C.: Epidemiology of skin cancer: update. Sunlight 2019. Vitamin D Skin Cancer pp. 123–139 (2020). https://doi.org/10.1007/978-3-030-46227-7
Giannos, S.A.; Kraft, E.R.; Lyons, L.J.; Gupta, P.K.: Spectral evaluation of eyeglass blocking efficiency of ultraviolet/high-energy visible blue light for ocular protection. Optom. Vis. Sci. 96, 513 (2019). https://doi.org/10.1097/OPX.0000000000001393
Bissett, D.L.; Hannon, P.D.; Orr, T.V.: Wavelength dependence of histological, physical, and visible changes in chronically UV-irradiated hairless mouse skin. Photochem. Photobiol. 50, 763–769 (1989). https://doi.org/10.1111/j.17511097.1989.tb029
Antwis, R.E.; Browne, R.K.: Ultraviolet radiation and Vitamin D3 in amphibian health, behaviour, diet and conservation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 154, 184–190 (2009). https://doi.org/10.1016/j.cbpa.2009.06.008
Costin, G.-E.; Hearing, V.J.: Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 21, 976–994 (2007). https://doi.org/10.1096/fj.06-6649rev
Baron, E.D.; Suggs, A.K.: Introduction to photobiology. Dermatol. Clin. 32, 255–266 (2014). https://doi.org/10.1016/j.det.2014.03.002
Tiwari, V.; Wilson, D.M.: DNA damage and associated DNA repair defects in disease and premature aging. Am. J. Hum. Genet. 105, 237–257 (2019). https://doi.org/10.1016/j.ajhg.2019.06.005
Wang, F.; Smith, N.R.; Tran, B.A.P.; Kang, S.; Voorhees, J.J.; Fisher, G.J.: Dermal damage promoted by repeated low-level UV-A1 exposure despite tanning response in human skin. JAMA Dermatol 150, 401–406 (2014). https://doi.org/10.1001/jamadermatol.2013.8417
Yagi, M.; Yonei, Y.: Glycative stress and anti-aging: 7. Glycative stress and skin aging. Glycative Stress Res 5, 50–54 (2018). https://doi.org/10.3793/jaam.8.23
DeBuys, H.V.; Levy, S.B.; Murray, J.C.; Madey, D.L.; Pinnell, S.R.: Modern approaches to photoprotection. Dermatol. Clin. 18, 577–590 (2000). https://doi.org/10.1016/S0733-8635(05)70208-4
Paul, S.P.: Ensuring the safety of sunscreens, and their efficacy in preventing skin cancers: challenges and controversies for clinicians, formulators, and regulators. Front. Med. (2019). https://doi.org/10.3389/fmed.2019.00195
Schneider, S.L.; Lim, H.W.: Review of environmental effects of oxybenzone and other sunscreen active ingredients. J. Am. Acad. Dermatol. 80, 266–271 (2019). https://doi.org/10.1016/j.jaad.2018.06.033
Damiani, E.; Rosati, L.; Castagna, R.; Carloni, P.; Greci, L.: Changes in ultraviolet absorbance and hence in protective efficacy against lipid peroxidation of organic sunscreens after UVA irradiation. J. Photochem. Photobiol. B Biol. 82, 204–213 (2006). https://doi.org/10.1016/j.jphotobiol.2005.03.011
Morabito, K.; Shapley, N.C.; Steeley, K.G.; Tripathi, A.: Review of sunscreen and the emergence of non-conventional absorbers and their applications in ultraviolet protection. Int. J. Cosmet. Sci. 33, 385–390 (2011). https://doi.org/10.1111/j.1468-2494.2011.00654
Shanbhag, S.; Nayak, A.; Narayan, R.; Nayak, U.Y.: Anti-aging and sunscreens: paradigm shift in cosmetics. Adv. Pharm. Bull. 9, 348 (2019). https://doi.org/10.15171/apb.2019.042
Sasani Ghamsari, M.; Alamdari, S.; Han, W.; Park, H.-H.: Impact of nanostructured thin ZnO film in ultraviolet protection. Int. J. Nanomedicine (2017). https://doi.org/10.2147/IJN.S118637
Mueen, R.; Lerch, M.; Cheng, Z.; Konstantinov, K.: Na-doped ZnO UV filters with reduced photocatalytic activity for sunscreen applications. J. Mater. Sci. 55, 2772–2786 (2020). https://doi.org/10.1007/s10853-019-04122-2
Vieira, C.O.; Grice, J.E.; Roberts, M.S.; Haridass, I.N.; Duque, M.D.; Lopes, P.S.: ZnO: SBA-15 nanocomposites for potential use in sunscreen: preparation, properties, human skin penetration and toxicity. Skin Pharmacol. Physiol. 32, 32–42 (2019). https://doi.org/10.1159/000491758
Vinardell, M.P.; Llanas, H.; Marics, L.; Mitjans, M.: In vitro comparative skin irritation induced by nano and non-nano zinc oxide. Nanomaterials 7, 56 (2017). https://doi.org/10.3390/nano7030056
Osmond, M.J.; Mccall, M.J.: Zinc oxide nanoparticles in modern sunscreens: an analysis of potential exposure and hazard. Nanotoxicology 4, 15–41 (2010). https://doi.org/10.3109/17435390903502028
Taghizadeh, S.-M.; Lal, N.; Ebrahiminezhad, A.; Moeini, F.; Seifan, M.; Ghasemi, Y.: Green and economic fabrication of zinc oxide (ZnO) nanorods as a broadband UV blocker and antimicrobial agent. Nanomaterials 10, 530 (2020). https://doi.org/10.3390/nano10030530
Bakhori, S.K.M.; Mahmud, S.; Azaldin, N.Q.; Nadzri, N.F.N.M.; Zakaria, S.; Chuan, H.T.: Characterisation and larvicidal effects of different zinc oxide nanoparticles against Aedes aegypti larvae. Mater. Today Proc. 1, 23–45 (2023). https://doi.org/10.1016/j.matpr.2023.02.282
Srivastava, S.; Manjhi, J.: Surface characterization of zinc oxide nanoparticles synthesized via chemical route. Curr. Nanomater. 8, 175–181 (2023). https://doi.org/10.2174/2405461507666220603115619
Mustafa, S.M.; Barzinjy, A.A.; Hamad, A.H.: An environmentally friendly green synthesis of Co2+ and Mn2+ Ion Doped ZnO nanoparticles to improve solar cell efficiency. J. Environ. Chem. Eng. (2023). https://doi.org/10.1016/j.jece.2023.109514
Li, W.; You, Q.; Zhang, J.; Li, W.; Xu, H.: Green synthesis of antibacterial LFL-ZnO using L plantarum fermentation liquid assisted by ultrasound-microwave. J. Alloys Compd. 947, 169697 (2023). https://doi.org/10.1016/j.jallcom.2023.169697
Khoshbin, R.; Haghighi, M.; Margan, P.: Combustion dispersion of CuO–ZnO–Al2O3 nanocatalyst over HZSM-5 used in DME production as a green fuel: effect of citric acid to nitrate ratio on catalyst properties and performance. Energy Convers. Manag. 120, 1–12 (2016). https://doi.org/10.1016/j.enconman.2016.04.076
Kalia, S.; Dhiman, V.; Basandrai, D.; Prasad, N.: Antibacterial activities of Bi-Ag co-doped cobalt ferrite and their ZnO/Ag nanocomposite/s. Inorg. Chem. Commun. 150, 110382 (2023). https://doi.org/10.1016/j.inoche.2022.110382
Sharma, N.; Sahay, P.P.: Solution combustion synthesis of Dy-doped ZnO nanoparticles: an investigation of their structural, optical and photoluminescence characteristics. J. Lumin. 257, 119655 (2023). https://doi.org/10.1016/j.jlumin.2022.119655
Hezam, A.; Abutaha, N.; Almekhlafi, F.A.; Saeed, A.M.N.; Abishad, P.: Wadaan MA Smart plasmonic Ag/Ag2O/ZnO nanocomposite with promising photothermal and photodynamic antibacterial activity under 600 nm visible light illumination. J. Photochem. Photobiol. A Chem. 435, 114322 (2023). https://doi.org/10.1016/j.jphotochem.2022.114322
Surendra, B.S.; Swamy, M.M.; Shamala, T.; Rao, S.; Pramila, S.: Development of enhanced electrochemical sensor and antimicrobial studies of ZnO NPs synthesized using green plant extract. Sensors Int. 3, 100176 (2022). https://doi.org/10.1016/j.sintl.2022.100176
Rabani, I.; Lee, S.-H.; Kim, H.-S.; Yoo, J.; Hussain, S.; Maqbool, T.: Engineering-safer-by design ZnO nanoparticles incorporated cellulose nanofiber hybrid for high UV protection and low photocatalytic activity with mechanism. J. Environ. Chem. Eng. 9, 105845 (2021). https://doi.org/10.1016/j.jece.2021.105845
Vigneshwaran, N.; Kumar, S.; Kathe, A.A.; Varadarajan, P.V.; Prasad, V.: Functional finishing of cotton fabrics using zinc oxide–soluble starch nanocomposites. Nanotechnology 17, 5087 (2006). https://doi.org/10.1088/0957-4484/17/20/008
Kaur, R.; Bhardwaj, S.K.; Chandna, S.; Kim, K.-H.; Bhaumik, J.: Lignin-based metal oxide nanocomposites for UV protection applications: A review. J. Clean. Prod. 317, 128300 (2021). https://doi.org/10.1016/j.jclepro.2021.128300
Yew, Y.P.; Shameli, K.; Miyake, M.; Khairudin, N.B.B.A.; Mohamad, S.E.B.; Naiki, T.: Green biosynthesis of superparamagnetic magnetite Fe3O4 nanoparticles and biomedical applications in targeted anticancer drug delivery system: a review. Arab. J. Chem. 13, 2287–2308 (2020). https://doi.org/10.1016/j.arabjc.2018.04.013
Gao, G.; Li, J.; Zhang, Y.; Chang, Y.-Z.: Cellular iron metabolism and regulation. Brain Iron Metabolism CNS Diseases, pp. 21–32 (2019). https://doi.org/10.1007/978-981-13-9589-5
Morsy, R.; Hosny, M.; Reicha, F.; Elnimr, T.: Development and characterization of multifunctional electrospun ferric oxide-gelatin-glycerol nanofibrous mat for wound dressing applications. Fibers Polym. 17, 2014–2019 (2016). https://doi.org/10.1007/s12221-016-6915-8
Abuzeid, A.M.; Saafan, S.A.; Salem, M.L.; Elnouby, M.S.; Morsy, R.: Magnesium and gadolinium doping of superparamagnetic magnetite nanoparticles as T2 contrast nanoagents for magnetic resonance imaging. MRS Commun. 12, 944–951 (2022). https://doi.org/10.1557/s43579-022-00280-8
Comanescu, C.: Magnetic nanoparticles: current advances in nanomedicine, Drug Delivery and MRI. Chemistry (Easton) 4, 872–930 (2022). https://doi.org/10.3390/chemistry4030063
Fonseca, A.P.; Rafaela, N.: Determination of sun protection factor by UV-vis spectrophotometry. Health Care (Don. Mills) 1, 1000108 (2013). https://doi.org/10.4172/hccr.1000108
Indubala, E.; Dhanasekar, M.; Sudha, V.; Malar, E.J.P.; Divya, P.; Sherine, J.: L-Alanine capping of ZnO nanorods: increased carrier concentration in ZnO/CuI heterojunction diode. RSC Adv. 8, 5350–5361 (2018). https://doi.org/10.1039/C7RA12385J
Yusefi, M.; Shameli, K.; Yee, O.S.; Teow, S.-Y.; Hedayatnasab, Z.; Jahangirian, H.: Green synthesis of Fe3O4 nanoparticles stabilized by a Garcinia mangostana fruit peel extract for hyperthermia and anticancer activities. Int. J. Nanomed. 16, 2515 (2021). https://doi.org/10.2147/IJN.S284134
Janaki, A.C.; Sailatha, E.; Gunasekaran, S.: Synthesis, characteristics and antimicrobial activity of ZnO nanoparticles. Spectrochim Acta Part A Mol. Biomol. Spectrosc. 144, 17–22 (2015). https://doi.org/10.1016/j.ceramint.2013.04.110
Thangeeswari, T.; George, A.T.; Kumar, A.A.: Optical properties and FTIR studies of cobalt doped ZnO nanoparticles by simple solution method. Indian J. Sci. Technol. 9, 4 (2016). https://doi.org/10.17485/ijst/2016/v9i1/85776
De Lima, J.F.; Martins, R.F.; Neri, C.R.; Serra, O.A.: ZnO: CeO2-based nanopowders with low catalytic activity as UV absorbers. Appl. Surf. Sci. 255, 9006–9009 (2009). https://doi.org/10.1016/j.apsusc.2009.06.071
Wang, Y.Y.; Yu, H.-Y.; Yang, L.; Abdalkarim, S.Y.H.; Chen, W.-L.: Enhancing long-term biodegradability and UV-shielding performances of transparent polylactic acid nanocomposite films by adding cellulose nanocrystal-zinc oxide hybrids. Int. J. Biol. Macromol. 141, 893–905 (2019). https://doi.org/10.1016/j.ijbiomac.2019.09.062
Aditya, A.; Chattopadhyay, S.; Gupta, N.; Alam, S.; Veedu, A.P.; Pal, M.: ZnO nanoparticles modified with an amphipathic peptide show improved photoprotection in skin. ACS Appl. Mater. Interfaces 11, 56–72 (2018). https://doi.org/10.1021/acsami.8b08431
Dao, D.V.; van den Bremt, M.; Koeller, Z.; Le, T.K.: Effect of metal ion doping on the optical properties and the deactivation of photocatalytic activity of ZnO nanopowder for application in sunscreens. Powder Technol. 288, 366–370 (2016). https://doi.org/10.1016/j.powtec.2015.11.030
Taghavi Fardood, S.; Ramazani, A.; Moradi, S.; Azimzadeh Asiabi, P.: Green synthesis of zinc oxide nanoparticles using arabic gum and photocatalytic degradation of direct blue 129 dye under visible light. J. Mater. Sci. Mater. Electron. 28, 13596–13601 (2017). https://doi.org/10.1007/s10854-017-7199-5
Au, B.W.C.; Chan, K.Y.: Sodium and potassium doped P-type ZnO films by sol-gel spin-coating technique. Appl. Phys. 123, 1–9 (2017). https://doi.org/10.1007/s00339-017-1099-7
Porrawatkul, P.; Nuengmatcha, P.; Kuyyogsuy, A.; Pimsen, R.; Rattanaburi, P.: Effect of Na and Al doping on ZnO nanoparticles for potential application in sunscreens. J. Photochem. Photobiol. B Biol. 240, 112668 (2023). https://doi.org/10.1016/j.jphotobiol.2023.112668
Le, T.H.; Bui, A.T.; Le, T.K.: The effect of Fe doping on the suppression of photocatalytic activity of ZnO nanopowder for the application in sunscreens. Powder Technol. 268, 173–176 (2014). https://doi.org/10.1016/j.powtec.2014.08.043
Nguyen, N.T.; Nguyen, T.M.N.; Le, N.T.; Le, T.K.: Suppressing the photocatalytic activity of ZnO nanoparticles by Al-doping for the application in sunscreen products. Mater. Technol. 35, 349–355 (2020). https://doi.org/10.1080/10667857.2019.1684733
Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E.: A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 29, 559–566 (1979). https://doi.org/10.1111/j.1751-1097.1979.tb07090.x
Acknowledgements
The authors are greatly thankful to Tanta University for support.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding.
Author information
Authors and Affiliations
Contributions
All authors have contributed to writing and editing the manuscript and methodology. All authors have read and approved the manuscript.
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elbrolesy, A., Elhussiny, F.A., Abdou, Y. et al. Facile Synthesis and Biophysical Characterization of Novel Zinc Oxide/Fe3O4 Hybrid Nanocomposite as a Potentially Active Agent in Sunscreens. Arab J Sci Eng 49, 1083–1093 (2024). https://doi.org/10.1007/s13369-023-08082-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-023-08082-3