Abstract
The present study aimed to develop an efficient superabsorbent hydrogel for water conservation and slow release of Ethephon in sandy soil. Herein, carboxymethyl cellulose (CMC) was grafted via a free radical polymerization technique with acrylamide and 2-Acrylamido-2-methylpropanesulfonic acid (AMPS) as hydrophilic monomers. The developed CMC-g-(PAM-co-PAMPS) graft copolymer superabsorbent hydrogel was characterized by FTIR, TGA, and SEM analysis to prove the occurrence of the grafting process. Several factors affecting the grafting process were investigated, while maximum grafting (%) value reached 91%. Moreover, increasing AMPS ratio obviously improved the swelling degree of the developed hydrogel with a maximal value of 17,770%. Moreover, addition of 2% of hydrogel to sandy soil potentially enhanced the water retention by 47% compared to pure sandy soil which retained about 7% of water. Besides, increasing Ethephon ratio up to 20% significantly increased its loading (%) value up to 88%, while about 87% of Ethephon was released within 28 days in a slow and sustained manner. The results suggested that the fabricated grafted hydrogel can serve as nutrient carrier and amendment for sandy soil for advanced agricultural applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, the lack of irrigation resources is considered one of the most important problems facing the desert land reclamation sector, since about 70% of water sources are consumed in agricultural fields [1, 2]. The productivity of sandy soils is mostly limited by their low water holding capacity and excessive deep percolation losses [3]. Thus, the water–soil management must aim at increasing water retention in these soils and reducing losses due to deep percolation [4]. Recently, the use of water-holding amendments like polymeric hydrogels for enhancing water and nutrient use efficiency in sandy soils has received much attention [5]. Among these hydrogels, superabsorbent hydrogels (SAHs) are remarkably distinct from hygroscopic materials owing to their multi-dimensional network structure [6]. This kind of polymeric hydrogels demonstrates highly swelling characteristics, which able to absorb and retain water up to thousands of times from their dry weight [7, 8].
SAHs have extensive diverse applications such as medical, pharmaceutical, cosmetics, industrial and agriculture [9]. Therefore, SAHs have been developed to improve the physical properties of sandy soil in view of increasing their water-holding capacity to be comparable to silty clay or loam, increasing water use efficiency, enhancing soil permeability, decreasing deep percolation and reducing evaporation and infiltration rates [10]. In addition, this kind of hydrogel helps sandy soil to remain wet for long periods without irrigation again, provide plants with ultimate moisture and fertilizers/nutrients, boost plant viability and ventilation and consequently, increases the production yield of crops [7, 11, 12].
Cellulose and its derivatives has been considered one of the best choices for developing superabsorbent hydrogels owing to its appealing properties such as abundant in nature, low-cost production, biodegradability, facile modification, non-toxicity and hydrophilicity [13, 14]. Among these derivatives, carboxymethyl cellulose (CMC) is a water-soluble cellulose derivative, consists of β-(1 → 4)-D-glucopyranose of cellulose biopolymer [15]. CMC (sodium salt) has number of carboxymethyl groups (CH2COONa) which generated onto the cellulose backbone and support its solubility. CMC has been effectively applied in various applications including biomedical and water treatment fields as well as industries of detergents, paper, textile and food [16, 17]. Owing to its hydrophilic hydroxyl and carboxylic groups, CMC has been potentially used to fabricate various types of superabsorbent hydrogels, since it can absorb water and moisture [18, 19]. The resultant hydrogel has many outstanding properties, such as high water content, better mechanical properties and acceptable degradation rate, which considered as essential parameters in the agricultural and forestry fields. Grafting with hydrophilic acrylate monomers is the most common effective technique for providing CMC-based superabsorbent hydrogels [20]. Among these acrylate derivatives, acrylamide (AM) and 2-Acrylamido-2-methylpropanesulfonic acid (AMPS) monomers have received much attention for developing SAHs as water retention agents [21, 22]. This is due to their supreme hydrophilic nature resultant from the existence of several hydrophilic groups (–NH2, –SO3H and –OH− groups) along their structures [23, 24]. Many efforts have been made to improve the water absorbency and water retention capacity of PAM and PAMPS hydrogels in addition to simplify their accessibility [25, 26]. Therefore, PAM hydrogel has been applied for reduced water infiltration in irrigation, in addition to its potential use in sandy soil, slit loam and clay loam soils [27]. More recently, hydrogel composed of acrylamide/potassium acrylate/mineral has been employed as soil conditioner under saline conditions [28]. Besides, several SAHs have been constructed based on grafting of AM and AMPS onto numerous natural polymers such as cellulose, chitosan, gelatin and alginate, while their swelling characteristics have been investigated [23, 29, 30]. For instance, highly swellable acrylamide grafted carboxymethyl cellulose hydrogel has been fabricated as soil conditioner for controlled release of agrochemicals (KNO3) [31]. However, few studies reported the evaluation of the swelling profiles of grafted CMC-based superabsorbent hydrogel in sandy soil.
Herein, we aimed in this study to fabricate efficient superabsorbent hydrogel for water conservation and controlled release of nutrients. CMCwas grafted using AM and AMPS acrylate monomers, N,N-methylenebisacrylamide (MBA) as a crosslinker and potassium persulfate (KPS) as an initiator. The developed CMC-g-poly(AM-co-AMPS) superabsorbent hydrogel was verified its chemical structure, thermal stability and morphological properties using FTIR, TGA and SEManalysis tools, respectively. Factors affecting the grafting process were optimized. Furthermore, the swelling profiles of the grafted hydrogel were studied under several affecting conditions. Ethephon was loaded into the developed hydrogel, while its loading efficiency and release profile were clarified. Effect of CMC-g-poly (AM-co-AMPS) superabsorbent hydrogel on the water retention in sandy soil was also explored. Besides, studies of sandy soil amended hydrogel were performed to investigate the water retention and Ethephon release profiles.
2 Experimental
2.1 Materials
Carboxymethyl cellulose sodium salt, (CMC; DS = 0.7; M.wt; 90,000), acrylamide (AM; assay > 98%; Mw 71.08), 2-Acrylamido-2-methylpropanesulfonic acid (AMPS; assay > 98%; M.wt: 207.25), N, N´-methylenebisacrylamide (MBA; Mw. 154.17) and potassium persulfate (KPS; purity 99%, Mw 270.322) were acquired from Sigma-Aldrich chemicals Ltd (Germany). Sodium hydroxide (Mw 40 g/mol; purity 99%) and acetone (Mw58.08; purity 99%) were purchased from EL-Nasr pharmaceutical Co. (Egypt). Sandy soil was collected from New Borg El Arab desert, Alexandria, Egypt.
2.2 Fabrication of CMC- Graft Copolymer Hydrogel
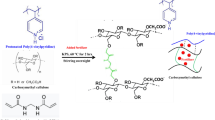
Superabsorbent CMC-g-poly(AM-co-AMPS) hydrogel was prepared according to the previously reported free radical polymerization technique [20]. In brief, a known amount of CMC (2–8%; w/v) was dissolved under continuous stirring in hot distilled water. KPS (0.025–0.1% w/v) added to CMC solution to initiate the generation of active sites. AM monomers were dissolved in distilled water and subsequently added to CMC solution. After 5 min, AMPS solution was added to the grafting medium, while the final concentration of used AM and AMPS was fixed to be 6% (w/w) comprising 4:2, 3:3 and 2:4 of AMPS: AM ratio. Finally, MBA was added with final concentrations of 0.025–1% (w/v). The grafting process was performed in shaking water bath at different grafting temperatures (50–80 °C), while the grafting time was studied in the range of 1–4 h. The resultant hydrogel was set aside overnight (post grafting), washed by excess of distilled water to remove the unreacted monomers, while acetone was used for squeezing water and extraction of homopolymers. The obtained grafted hydrogel was dried overnight at 60 °C. The dried xerogels were crushed and fractionated into nearly equal particle size (500 µm–1 mm). The proposed preparation mechanism and a laboratory image of freshly prepared CMC-g-poly (AM-co-AMPS) hydrogel are shown in Fig. 1. The grafting percentage (GP %) is determined according to equations [23]:
where Wa is weight of pristine CMC and Wb is weight of grafted hydrogel.
2.3 Characterization
The chemical composition of the fabricated hydrogel was confirmed utilizing Fourier transform infrared (Shimadzu FTIR-8400 S, Japan). The thermal stability was examined in the range of 25–800 °C using thermogravimetric analyzer (Shimadzu TGA-50, Japan) under a heating rate (20 °C/ min). Moreover, the surface morphology was investigated by a scanning electron microscope (Joel-Jsm 6360LA, Japan).
2.4 In vitro Swelling Studies
The swelling experiments were conducted according to the author’s previous work [20]. An accurate amount of dried samples was immersed in distilled water (pH 7.2) and allowed to swell for a definite time period (1–6 h) at room temperature. The swollen hydrogel samples were taken out and pressed in between two filter papers to eliminate the excess of water and followed by weighing using an electronic balance. In addition, same experiment was performed using tap water to examine the impacts of dissolved salts on the swelling profile. The variation of the swelling degree in each cycle was estimated.
The swelling degree (S.D %) of hydrogel was determined as a function of time according to the following equation:
where Wt is the weight of swollen hydrogel sample at time t, and W0 represent the weight of the xerogel sample.
2.5 Ethephon Loading and In Vitro Release Studies
In this study, Ethephon was loaded into hydrogel via the reported immersing method [32]. The loading process was achieved by soaking the dried samples into aqueous solution of Ethephon (10, 20 and 30% and allowed to swell for 24 h. The swollen hydrogels was dried, while the loading (%) was calculated according to the following equation:
where W1 and W0 are the weights of Ethephon in the loaded and dry hydrogels, respectively.
Besides, the Ethephon release (%) was estimated after time interval 1–24 h, while the quantity of released Ethephon was measured as a function of phosphonic groups using ion exchange capacity assay.
2.6 Sandy Soil Experiments
2.6.1 Water Retention in Sandy Soil
A plastic measuring cylinder (1000 mL, height 40 cm, area 39.57 cm2) was perforated from the bottom in the form of small holes of approximately equal size, then a filter paper was placed on the bottom. Next, accurate amount (0.5–2%; w/w) of dried CMC-g-poly (AM- co- AMPS) xerogel was mixed with sandy soil (particle size 500 µm -1 mm) and placed in the bottom of the cylinder followed by covering with 10 cm of sandy soil. Tap water was poured slowly into the soil from the top of the tube for continuous 24 h as a watering time to ensure the complete saturation of hydrogel inside sandy soil and followed by weighing. Water retention (%) of sandy soil amended with CMC-g- poly (AM-co-AMPS) superabsorbent hydrogel was evaluated for consecutive 28 days according to the following equation:
where W2 and W1 are the weights of sandy soil mixed with hydrogel and pure sandy soil, respectively, after watering for 24 h.
2.6.2 Ethephon Release in Sandy Soil
The release of Ethephon from hydrogel mixed with sandy soil was evaluated under different heights of sandy layers (10 cm). The cumulative release percentage of Ethephon was investigated for defiant time period (1–28 days), while the amount of used hydrogel was fixed at 2% w/w.
3 Results and Discussion
3.1 FTIR Analysis
FTIR spectra of neat CMC and its grafted copolymer are shown in Fig. 2A. The observed characteristic absorption band at 3287 cm−1 is assigned to the stretching vibration of –OH groups of CMC [33, 34]. In addition, the detected absorption bands at 1410 and 1318 cm−1 are associated with CH2 and –OH bending vibration [35]. The presence of a strong absorption band at 1582 cm−1confirms the antisymmetrical stretching of COOH group in carboxylic acid salts, while the band at 1018 cm−1 is related to the symmetrical stretching of C–O in secondary alcohols (–CHROH) of anhydroglucose units, respectively [36]. On the other hand, the IR spectrum of the grafted superabsorbent hydrogel displayed that the characteristic bands of CMC, PAM and PAMPS were observed. The characteristic band of OH groups of CMC was significantly shifted after the grafting process from 3287 cm−1 to the higher wavelength at 3340 cm−1 in addition to changing its peak intensity as a result of their overlapping with the NH2 groups of the copolymer. The stretching vibration of 1651, 1436 and1032 cm−1 are associated with NH bending (Amide II band), C–N stretching of secondary amide (–CONHR) and CH–O–CH2 group [37, 38]. Moreover, the observed new bands at 1106 and 1564 cm−1 are assigned to S=O group of AMPS and C=O stretching mode of amide groups of AM proving the evidence of the graft copolymerization process on CMC backbone. The noticeable changes in the chemical structure of CMC prove the occurrence of the graft copolymerization process.
3.2 Thermal Analysis
The thermal properties of native CMC and the fabricated CMC-g-poly (AM- co- AMPS) hydrogel were examined as deliberated in Fig. 2B and Table 1. At the initial degradation stage, all samples displayed initial weight loss in the range of 11.4–18.2% (up to 120 °C), which could be ascribed to evaporation of moisture content from tested samples [39]. With increasing temperature, the developed grafted hydrogel samples showed better thermal stability compared to pure CMC which could be explained by the generated crosslinked network structure between CMC and acrylate monomers (i.e., AM and AMPS). The noticed weight losses could be associated with the decarboxylation of CMC, decomposition of the cyclic product and loss of CO2 from the polysaccharide [20]. In addition, the thermal stability was slightly improved with increasing AMPS ratio in the hydrogel matrix, where about 41.53% was lost by CMC-g-poly (AM-co-AMPS [2:4]) sample compared to 47.18% which was recorded by sample with a lower MPS ratio (i.e., CMC-g-poly (AM-co-AMPS [2:4])). These observations were confirmed through investigation of the required temperature for samples to loss their half weight (T50% oC) which was recorded 423–452 °C in case of grafted samples compared to 297 °C for original CMC proving the decent thermal stability of the fabricated CMC-g-poly (AM- co- AMPS) hydrogel.
3.3 Morphological Characterization
The SEM images of the developed dried pure CMC and CMC-g-poly (AM-co-AMPS) hydrogel were performed to visualize the hydrogel morphological changes. Figure 3A clarified that the surface of CMC displayed granular structure with large particles, while it completely changed after the grafting process (Fig. 3B) to be wrinkled surface with spacers between its grains [18]. The changes in the morphological structures could be attributed to the chemical grafting of CMC by AM and AMPS monomers in addition to their polarity difference which might affect the morphological properties of the developed hydrogel.
3.4 Optimization of the Grafting Process
Figure 4 displays the consequence of varies affecting grafting conditions on the grafting (%) values. Figure 4A shows that increasing the CMC concentration from 2 to 6%, significantly improved the grafting percent from 43 to 91%, and then, a slight decrease was observed (84%) with the further increase up to 8% These results could be attributed to increasing the number of initiated active sites on CMC backbone by increasing its concentration up to 6%, consequently, a large number of AM and AMPS monomeric units will be grafted on CMC followed with an increase in the grafting (%) [18]. However, further increasing CMC concentration up to 8% could increase the viscosity of the grafting medium, which hinder the monomers to be attached to the initiated active sites on CMC backbone, and the grafting percent decreases accordingly [40]. On the other hand, impact of AM and AMPS monomers ratio on the grafting process is studied as shown in Fig. 4B. The gained results signified that samples with higher AMPS ratio (AM: AMPS (2:4)) recorded the highest grafting (%) value. Factually, increasing the monomers concentration promotes their diffusibility toward the initiated sites on the CMC backbone, which subsequently increases the grafting yield. Also, increasing AMPS ratio could improve the copolymerization process which affects directly the grafting of both AM and AMPS monomers on to CMC backbone.
Parameters affecting the grafting process; A CMC (%), B AM: AMPS ratio, C MBA (%), D KPS, E Grafting time and F Grafting temperature for the preparation of CMC-g-poly (AM-co-AMPS) hydrogel. All measurements were accomplished in triplicate (n = 3), and data obtained were expressed as mean standard deviation (± SD)
The consequence of MBA concentration on the grafting process was studied in the range of 0.025–0.1% as shown in Fig. 4C. A significant increase in the grafting (%) from 71 to 91% was noticed with increasing crosslinker concentration from 0.025 to 0.075% as a result of increasing the affinity of AM and AMPS monomers to be copolymerized through the grafting process, which reflects positively on the formation of three-dimensional network structure [20]. On contrast, further increasing MBA up to 0.1% leads to phase separation in addition to formation of denser network which limit the diffusion of AM and AMPS monomers and acting in favor of homopolymers formation. Besides, increasing initiator (KPS) concentration up to 0.075% (Fig. 4D) significantly increased the grafting (%) value due to increasing number of free radicals that provide more active sites on the CMC backbone. Consequently, a great number of AM and AMPS units will participate in the grafting process resulting an increase in the grafting (%) value. However, further increasing the KPS concentration up to 0.1% leads to fast termination of the process through the bimolecular collisions, producing short macromolecular chains and consequently, the grafting (%) values decreases.
Variation of the grafting time from 1 to 2 h has a positive impact on the grafting process, since maximal grafting (%) was accomplished after 2 h (91%) compared to only 58% after 1 h as a result of increasing the number of initiated active sites by increasing the grafting time (Fig. 4E). Nevertheless, further increasing time up to 4 h has no observable effect on the grafting (%) value, since all monomers have been grafted on the available active sites on CMC backbone. Finally, Fig. 4F revealed that increasing temperature of the grafting medium from 50 to 60 °C promoted the grafting process. These results could be ascribed by increasing rate of the thermal dissociation of KPS with an increase in temperature in addition to improving the diffusion rate of AM and AMPS monomers from the solution phase to the swellable CMC phase, and thus, the grafting (%) value increases. On contrary, the grafting (%) value was decreased with the further increase in temperature up to 80 °C which recorded the lowest value of 73% due to the rapid termination step that directly decreases the number of grafted monomers chains on CMC surface.
3.5 Evaluation of Swelling Profiles
3.5.1 Impacts of Swelling Time and Total Dissolved Salts
Figure 5 clarified that increasing the swelling time has a positive consequence on the swelling profile of the developed superabsorbent hydrogel. Therefore, the swelling degree was increased extensively from 6700 to 17,770% with an increase in the swelling time from 1 to 5 h using distilled water. Later, the swelling degree value was nearly constant with further increasing time up to 6 h, since the hydrogel network reached the equilibrium swelling within 5 h. These results could be elucidated by increasing the number of water molecules that penetrate into the hydrogel network, causing an increase in the swelling degree value. Thus, the equilibrium swelling time was chosen as 5 h for the subsequent experiments. On the other hand, the impact of total dissolved salts (TDS) on the swelling degree was examined using distilled and tap water, which were used as swelling mediums with TDS values of 1.6 and 488 ppm, respectively. The depicted results in Fig. 5 referred also that the swelling degree was much reduced in tap water compared to that in distilled water. This is due to the existence of ions in tap water which has a profound outcome on the swelling profile of the developed hydrogel [41]. Increasing the TDS concentration in the swelling medium potentially lessens the mobile ion concentration difference between the hydrogel and the external swelling medium. Consequently, a reduction in the volume of the hydrogel network takes place, resulting in a decrease in the swelling degree.
3.5.2 Impacts of Hydrogel Compositions
Figure 6a shows the impact of variation of CMC concentration on the swelling degree of the developed CMC-g-poly (AM-co-AMPS) superabsorbent hydrogel using distilled water (pH 7.2) at constant the other affecting conditions (AM: AMPS (2:4), MBA (0.075%), KPS (0.075%), and equilibrium swelling time (5 h)). The results signified that that increasing CMC concentration from 2 to 6% in the hydrogel matrix clearly improved the swelling degree from 7430 to 17,770% owing to the nature of CMC, since it contains various OH and COOH groups which impart the hydrophilicity of the hydrogel network. Consequently, the affinity of water molecules to diffuse into the gel matrix increases resulting in a greater swelling degree of hydrogel network. However, the swelling degree decreased to 15,440% with further increase in CMC concentration up to 8% due to increasing density of the hydrogel network, and consequently, the diffusion rate of water molecules decreases resulting a decrease in the swelling degree value [19]. The gained swelling degree values were higher than those obtained by other reported SAHs. Similar study referred that increasing CMC concentration in the CMC-g-PAM hydrogel significantly increased the swelling degree value to a maximal value of 15,800% [18]. Other reported studies concluded that the swelling ratios of crosslinked CMC superabsorbent hydrogel and biohybrid composite hydrogel containing acrylamide/potassium 3-sulfopropyl methacrylate/sodium alginate/bentonite were 1162 and 2055% compared to 17,770% in this study [42, 43].
Similar to CMC, increasing AMPS monomer concentration in the copolymer ratio has a positive impact on the swelling performance compared to AM monomer as shown in Fig. 6b. Therefore, maximal value of 17,770% was recorded by the highest AMPS ratio (AM: AMPS [2:4]) compared to 12,360% which was recorded by the lowest AMPS ratio (AM: AMPS [4:2]). In fact, both AMPS and AM are hydrophilic monomers which are expected to enrich the hydrophilicity of the hydrogel network. In addition, the strong hydrophilic -SO3H and -CONH2 groups of AMPS monomers have much affinity to bind with water molecules compared to the NH2 groups of AM monomers [44]. At the same time, the synergistic consequence of hydrogen bonding between hydrophilic groups of hydrogel network and water molecules and between various groups was improved [45].
On the other hand, the effect of the extent of MBA crosslinking on the swelling degree was studied as shown in Fig. 6c. The results refereed that increasing MBA concentration beyond 0.075% has an adverse effect on the swelling degree values, which obviously decreased from 17,770 to 14,660% with increasing MBA concentration up to 0.1%. These observations could be attributed to increase in the crosslinking density of the fabricated hydrogel network which becomes inflexible, resulting in a decrease in the diffusion rate of water molecules through the hydrogel followed with a decrease in the swelling degree values with the further increase in MBA concentration [46]. At concentrations lower than 0.075%, the hydrogel shape is diminished noticeably due to the formation of very loosely crosslinked networks with very low gel strength. The effect of the KPS initiator concentration was studied also by varying the concentration from 0.0025 to 0.1% as shown in Fig. 6d. It was noticed that the swelling degree was increased initially with an increase in KPS concentration up to 0.075% as a result of increase in the free radicals that generated by decomposition of KPS initiator, and consequently, the number of grafted hydrophilic monomeric chains increased followed by increasing the swelling degree. However, increasing KPS concentration up to 1% causes a fast termination of the graft-copolymerization process, which leads directly to formation of hydrogel with short macromolecular chains and high network density. Therefore, the diffusion rate of water molecules into the hydrogel network decreases, resulting in a decrease in the swelling degree values [20, 47].
3.6 Evaluation of Ethephon Loading and In Vitro Release
Ethephon (10, 20 and 30%) was loaded into the developed hydrogel matrix. Figure 7a showed that a significant improvement in the loading (%) from 72 to 88% was observed with increasing Ethephon ratio from 10 to 20%, while further increase up to 30% has no observable impact on the loading (%) value. The higher loading values confirm the successful loading process into the fabricated CMC-g-poly (AM- co- AMPS [2:4]) hydrogel. The in vitro Ethephon release performance was studied at pH 7.2 for sequential 24 h as shown in Fig. 7b. The results demonstrated that a regular release profile of Ethephon was accomplished with an increase in the release time up to 5 h with a maximal release value reached 71%. Thereafter, a slow release performance was attained with increasing time up to 24 h. In fact, the potential release of Ethephon from the hydrogel matrix was occurred after the penetration of water, which causes swelling of the hydrogel network, followed by diffusion alongside the aqueous pathways to the surface [41].
3.7 Water Retention and Ethephon Release in Sandy Soil
Research evidence suggests that when the soil is treated with water hydrogel composite, the water volumetric content of the soil increases significantly, and when the surrounding soil dries, the stored water is released back slowly into the soil. Water retention of sandy soil amended with CMC-g-poly (AM-co-AMPS) superabsorbent hydrogel was evaluated as deliberated in Fig. 8a. The results clarified that the water retention properties of sandy soil were much improved after amendment by the developed hydrogel [23, 48]. The water loss for all tested samples was increased gradually at the first 7 days, and then, a slow rate was noticed with increasing time up to 28 days. The developed superabsorbent hydrogels helped sandy soil to remain wet for long periods without irrigation again. Therefore, increasing hydrogel ratio from 0.5 to 2% significantly boosted the water holding capacity of sandy soil, since the sandy soil amended with 2% hydrogel obviously retained about 43% of water compared to only 7% which was retained by pure sandy soil. In fact, extra water runs across the surface of sandy soil and its loose texture makes them easily exposed to the erosion [4, 49]. In addition, the low water holding capacity of sandy soils combined with their low fertility potentially reduces the possibilities choose of crop production. Increasing the water retention in case of sandy soil amended by hydrogel could be ascribed to the existence of existence of hydrophilic OH, NH2, COOH and SO3H groups which potentially impart the hydrophilicity of hydrogel matrix. Consequently, water uptake by the hydrogel network increases, resulting in an improvement in the water retention in sandy soil. It can be concluded that the addition of hydrogel to sandy soil meaningfully altered the water holding capacity to be analogous to silty clay and loam. A similar phenomenon was also observed by others [50].
On the other hand, the results shown in Fig. 8b clarified that a sustained release of Ethephon took place with a maximum cumulative release value reached 17, 66 and 87% after 1, 7 and 28 days, respectively, using 2% hydrogel mixed with sandy soil. These observations could be associated with the long time period for swelling of the hydrogel network due to the applied pressure that caused pressure by sandy layers which delays the swelling, and consequently, a slow Ethephon release occurs [39]. It can be concluded from the swelling and release studies that the fabricated superabsorbent hydrogel significantly improved the water holding capacity of sandy soil, decreased the water loss and regulated the released amount of Ethephon for a long time period. Similar observations have been reported by other authors [51,52,53].
4 Conclusion
This investigation deals with the continuous development of superabsorbent hydrogels as amendments for sandy soil. An efficient superabsorbent CMC-g- poly (AM-co-AMPS) hydrogel was fabricated for (i) improving the water holding capacity of sandy soil and (ii) efficient loading and sustained release of Ethephon. The structure, morphological changes and thermal stability of the developed grafted hydrogel were examined. The influences of the grafting variables on the grafting (%) and swelling profiles were thoroughly investigated. The results refereed that the grafted hydrogel displayed a high degree of swelling, while about 88% of Ethephon was successfully loaded into the hydrogel matrix. Moreover, water retention of sandy soil was greatly improved after the addition of hydrogel, while the retention time was extended. Therefore, sandy soil amended with 2% of hydrogel could retain about 43% of water, while a sustained release profile took place with a maximum cumulative release value of Ethephon reached 88% after 28 days. Overall, the gained nominates the potential use of the fabricated grafted hydrogel in the agricultural applications as soil conditioner with a dual function, to reduce the water consumption in sandy soil in addition to carrying nutrients and fertilizers.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kazeminejadfard, F.; Hojjati, M.R.: Preparation of superabsorbent composite based on acrylic acid-hydroxypropyl distarch phosphate and clinoptilolite for agricultural applications. J. Appl. Polym. Sci. 136(16), 47365 (2019)
Elbeih, S.F.: Evaluation of agricultural expansion areas in the Egyptian deserts: A review using remote sensing and GIS. Egypt. J. Remote Sens. Space Sci. Part 2 24(3), 889–906 (2021)
Ndede, E.O.; Kurebito, S.; Idowu, O.; Tokunari, T.; Jindo, K.: The potential of biochar to enhance the water retention properties of sandy agricultural soils. Agronomy 12, 311 (2022)
Shahid, S.A.; Qidwai, A.A.; Anwar, F.; Ullah, I.; Rashid, U.: Improvement in the water retention characteristics of sandy loam soil using a newly synthesized Poly(acrylamide-co-acrylic Acid)/AlZnFe2O4 superabsorbent hydrogel nanocomposite material. Molecules 17, 9397–9412 (2012)
Abobatta, W.: Impact of hydrogel polymer in agricultural sector. Adv. Agric. Environ. Sci. 1(2), 59–64 (2018)
Yu, J.; Shi, J.G.; Ma, X.; Dang, P.F.; Yan, Y.L.; Mamedov, A.I.; Shainberg, I.; Levy, G.J.: Superabsorbent polymer properties and concentration effects on water retention under drying conditions. Soil Sci. Soc. Am. J. 81, 889–901 (2017)
Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C.: Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: a review. Eur. Polymer J. 72, 365–385 (2015)
Ahmed, E.M.: Hydrogel: preparation, characterization, and applications : a review. J. Adv. Res. 6(2), 105–121 (2015)
Zohuriaan-Mehr, M.J.; Omidian, H.; Doroudiani, S.; Kabiri, K.: Advances in non-hygienic applications of superabsorbent hydrogel materials. J. Mater. Sci. 45, 5711–5735 (2010)
Dar, S.B.; Mishra, D.; Zahida, R.; Afshana, B.B.: Hydrogel: to enhance crop productivity per unit available water under moisture stress agriculture. Bull. Environ., Pharm. Life Sci. 6(10), 129–135 (2017)
Miller, V.S.; Naeth, M.A.: Hydrogel and organic amendments to increase water retention in anthroposols for land reclamation. Appl. Environ. Soil Sci., 11 (2019)
Montesano, F.F.; Parente, A.; Santamaria, P.; Sannino, A.; Serio, F.: Biodegradable superabsorbent hydrogel increases water retention properties of growing media and plant growth. Agric. Agric. Sci. Proced. 4, 451–458 (2015)
Chang, C.; Zhang, L.: Cellulose-based hydrogels: present status and application prospects. Carbohyd. Polym. 84, 40–53 (2011)
Ma, J.; Li, X.; Bao, Y.: Advances in cellulose-based superabsorbent hydrogels. RSC Adv. 5, 59745–59757 (2015)
Liu, J.; Zhang, C.; Zhang, L.; Miao, D.; Sui, S.; Deng, F.; Zhu, P.: Preparation and properties of carboxymethyl cellulose hydrogels. Ferroelectrics 547(1), 37–43 (2019)
Jin, H.; Xu, H.; Wang, N.; Yang, L.; Wang, Y.; Yu, D.; Ouyang, X.K.: Fabrication of Carboxymethylcellulose/metal-organic framework beads for removal of Pb(II) from Aqueous solution. Materials 12(6), 942 (2019)
Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B.: Recent Developments of Carboxymethyl Cellulose. Polymers 13, 1345 (2021)
Mohy Eldin, M.S.; Omer, A.M.; Soliman, E.A.; Hassan, E.A.: Superabsorbent polyacrylamide grafted carboxymethyl cellulose pH sensitive hydrogel: I. Preparation and characterization. Desalin. Water Treat. 51(16–18), 3196–3206 (2013)
Nan, C.; Fitrah, N.; Norhazlin, Z.; Mansor, A.A.: Preparation and swelling study of CMC hydrogel as potential superabsorbent. Pertan. J. Sci. Technol. 27(1), 489–498 (2019)
Mohy Eldin, M.S.; El-Sherif, H.M.; Soliman, E.A.; Elzatahry, A.A.; Omer, A.M.: Polyacrylamide-grafted carboxymethyl cellulose: Smart pH-sensitive hydrogel for protein concentration. J. Appl. Polym. Sci. 122(1), 469–479 (2011)
Yudi, W.; Simeng, L.; Gang, C.: Impacts of mechanical and chemical factors on the water-holding capacity of polyacrylamide in sandy: models and mechanisms. Soil Research 59, 501–510 (2021)
Kabiri, K.; Zohuriaan-Mehr, M.J.; Kheirabadi, M.H.; M,: Solvent-, ion- and pH-specific swelling of poly(2-acrylamido-2-methylpropane sulfonic acid) superabsorbing gels. J. Polym. Res. 17, 203–212 (2010)
Mohy Eldin, M.S.; Omer, A.M.; Soliman, EA..; Hassan, E.A.: Polyacrylamide-grafted gelatin: Swellable hydrogel delivery system for agricultural applications. Food Composition and Analysis: Methods and Strategies, 187–212 (2014)
Wang, Y.; Shi, X.; Wang, W.; Wang, A.: Synthesis, characterization, and swelling behaviors of a pH-responsive CMC-g-poly(AA-co-AMPS) superabsorbent hydrogel}. Turk. J. Chem. 37, 149–159 (2013)
Czarnecka, E.; Nowaczyk, J.: Synthesis and characterization superabsorbent polymers made of starch, acrylic acid, acrylamide, poly(Vinyl Alcohol), 2-hydroxyethyl methacrylate, 2-acrylamido-2-methylpropane sulfonic acid. Int. J. Mol. Sci. 22, 4325 (2021)
Guo, Y.; Guo, R.; Shi, X.; Lian, S.; Zhou, Q.; Chen, Y.; Liu, W.; Li, W.: Synthesis of cellulose-based superabsorbent hydrogel with high salt tolerance for soil conditioning. Int. J. Biol. Macromol. Part A 209, 1169–1178 (2022)
Ning, S., Jumai, H., Wang, Q., Zhou, B., Su, L., Shan, Y., Zhang, J.: Comparison of the effects of polyacrylamide and sodium carboxymethylcellulose application on soil water infiltration in sandy loam soils. Adv. Polym. Technol., 1-7 (2019)
Costa, M.; Freire, A.; Lourenço, D.; Rodrigues, R.; de Sousa, J.; de Andrade, P.; Feitosa, J.; Mota, A.: Hydrogel composed of potassium acrylate, acrylamide, and mineral as soil conditioner under saline conditions. Sci. Agric. (2022). https://doi.org/10.1590/1678-992x-2020-0235
Ibrahim, A.; Sayed, A.; Abd El-Wahab, H.; Sayah, M.: Synthesis of Poly(Acrylamide-Graft-Chitosan) hydrogel: optimization of the grafting parameters and swelling studies. Am. J. Polym. Sci. Technol. 5, 55–62 (2019)
Mirdarikvande, S.; Sadeghi, H.; Godarzi, A.; Alahyari, M.: Effect of pH, and salinity onto swelling properties of hydrogels based on H-alginate-g-poly(AMPS). Biosci., Biotechnol. Res. Asia 11(1), 205–209 (2014)
Bajpai, A.; Giri, A.: Water sorption behaviour of highly swelling (carboxy methylcellulose-g-polyacrylamide) hydrogels and release of potassium nitrate as agrochemical. Carbohyd. Polym. 53(3), 271–279 (2003)
Kenawy, E.; Azaam, M.M.; El-nshar, E.M.: Sodium alginate-g-poly(acrylic acid-co-2-hydroxyethyl methacrylate)/montmorillonite superabsorbent composite: preparation, swelling investigation and its application as a slow-release fertilizer. Arab. J. Chem. 12(6), 847–856 (2019)
Yang, F.; Li, G.; He, Y.G.; Ren, F.X.; Wang, G.X.: Synthesis, characterization, and applied properties of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbohyd. Polym. 78, 95–99 (2009)
Abd El-Monaem, E., Omer, A., El-Subruiti, G., Mohy-Eldin, MS., Eltaweil, A.: (2022) Zero-valent iron supported-lemon derived biochar for ultra-fast adsorption of methylene blue. Biomass Conversion and Biorefinery, 1–13.
Elmetwaly, T.; Darwish, S.; Attia, N.; Hassan, R.; El Ebissy, A.; Eltaweil, A.; Omer, A.; El-Seedi, H.; Elashery, S.: Cellulose nanocrystals and its hybrid composite with inorganic nanotubes as green tool for historical paper conservation. Prog. Org. Coat. 168, 106890 (2022)
Lian, Z.; Li, Y.; Xian, H.; Ouyang, X.K.; Lu, Y.; Peng, X.; Hu, D.: EDTA-functionalized magnetic chitosan oligosaccharide and carboxymethyl cellulose nanocomposite: synthesis, characterization, and Pb(II) adsorption performance. Int. J. Biol. Macromol. 165, 591–600 (2020)
Ma, J.; Xia, B.; Yu, P.; An, Y.: Comparison of an emulsion- and solution-prepared acrylamide/AMPS copolymer for a fluid loss agent in drilling fluid. ACS Omega 5(22), 12892–12904 (2020)
Mohy Eldin, M.S.; Omer, A.M.; Wassel, M.A.; Tamer, T.M.; Abd-Elmonem, M.S.; Ibrahim, S.A.: Novel smart pH sensitive chitosan grafted alginate hydrogel microcapsules for oral protein delivery: II. Evaluation of the swelling behavior. Int. J. Pharm. Pharm. Sci. 7(10), 331–337 (2015)
Tamer, T.M.; Omer, A.M.; Hassan, M.A.; Hassan, M.E.; Sabet, M.M.; Mohy Eldin, M.S.: Development of thermo-sensitive poly N-isopropyl acrylamide grafted chitosan derivatives. J. Appl. Pharma. Sci. 5, 1–6 (2015)
Hemvichian, K.; Chanthawong, A.; Suwanmala, P.: Synthesis and characterization of superabsorbent polymer prepared by radiation-induced graft copolymerization of acrylamide onto carboxymethyl cellulose for controlled release of agrochemicals. Radiat. Phys. Chem. 103, 167–171 (2014)
Essawy, H.A.; Ghazy, M.B.; El-Hai, F.A.; Mohamed, M.F.: Superabsorbent hydrogels via graft polymerization of acrylic acid from chitosan-cellulose hybrid and their potential in controlled release of soil nutrients. Int. J. Biol. Macromol. 89, 144–151 (2016)
Karadağ, E.; Öztürk, Z.; Üzüm, Ö.; Kundakc, S.: Swelling performance studies of acrylamide/potassium 3-sulfopropyl methacrylate/sodium alginate/bentonite biohybrid sorbent hydrogels in binary mixtures of water-solvent. J. Encapsul. Adsorpt. Sci 9, 35–61 (2019)
Abdulhameed, A.; Mbuvi, H.; Changamu, E.: Synthesis of cellulose-based superabsorbent hydrogel from rice husk using a microwave. Am. J. Mater. Sci. 10(1), 1–8 (2020)
Soliman, F.M.; Yang, W.; Guo, H.; Shinger, M.I.; Idris, A.M.; Hassan, E.S.: Preparation of carboxymethyl cellulose-g-poly (Acrylic Acid - 2-acrylamido-2-methylpropane sulfonic Acid)/attapulgite superabsorbent composite. Am. J. Polym. Sci. Technol. 2, 11–19 (2016)
Zhang, Y.; Wang, L.; Li, X.; He, P.: Salt-resistant superabsorbents from inverse-suspension polymerization of PEG methacrylate, acrylamide and partially neutralized acrylic acid. J. Polym. Res. 18(2), 157–161 (2010)
Pourjavadi, A.; Barzegar, S.: Mahdavinia GR (2006) MBA-crosslinked Na-Alg/CMC as a smart full-polysaccharide superabsorbent hydrogels. Carbohyd. Polym. 66(3), 386–395 (2006)
Ghasemzadeh, H.; Ghanaat, F.: Antimicrobial alginate/PVA silver nanocomposite hydrogel, synthesis and characterization. J. Polym. Res. 21, 355 (2014)
Kareem, S.A.; Dere, I.; Gungula, D.T.; Andrew, F.P.; Saddiq, A.M.; Adebayo, E.F.; Tame, V.T.; Kefas, H.M.; Joseph, J.; Patrick, D.O.: Synthesis and characterization of slow-release fertilizer hydrogel based on hydroxy propyl methyl cellulose, polyvinyl alcohol. Glycerol Blended Paper. Gels 7, 262 (2021)
Garduque, R.G.; Gococo, B.J.; Yu, C.A.; Nalzaro, P.J.; Tumolva, T.: Synthesis and characterization of sodium carboxymethyl cellulose/sodium alginate/hydroxypropyl cellulose hydrogel for agricultural water storage and controlled nutrient release. Solid State Phenom. 304, 51–57 (2020)
Ni, B.; Liu, M.; Lü, S.: Multifunctional slow-release urea fertilizer from ethylcellulose and superabsorbent coated formulations. Chem. Eng. J. 155, 892–898 (2009)
Liu, L., Kost, J., Fishman, M.L., Hicks K.B: A review: Controlled release systems for agricultural and food applications. In New Delivery Systems for Controlled Drug Release from Naturally Occurring Materials; ACS Publications: Washington, 14, 265–281 (2008)
Wei, H.; Wang, H.; Chu, H.; Li, J.: Preparation and characterization of slow-release and water-retention fertilizer based on starch and halloysite. Int. J. Biol. Macromol. 133, 1210–1218 (2019)
Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Tabar, A.R.: Slow-release NPK fertilizer encapsulated by carboxymethyl cellulose-based nanocomposite with the function of water retention in soil. Mater. Sci. Eng., C 90, 333–340 (2018)
Acknowledgements
This work was supported by the Science, Technology and Innovation Funding Authority (STDF), project No. 25984, Ministry of Scientific Research, Egypt.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The study conception and design were performed by AMO and MSME. Materials preparation, data collection and analysis were performed by AMO, TMT, EMAEl-M, ASE and MEH, REK. The first draft of manuscript was written by AMOManuscript was revised by AMO and MSME. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Omer, A.M., Tamer, T.M., Hassan, M.E. et al. Fabrication of Grafted Carboxymethyl Cellulose Superabsorbent Hydrogel for Water Retention and Sustained Release of Ethephon in Sandy Soil. Arab J Sci Eng 48, 561–572 (2023). https://doi.org/10.1007/s13369-022-07352-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-022-07352-w