Abstract
The production of the amino polysaccharide (chitosan) from crustacean sources has faced many hindrances due to environmental, seasonal and noneconomic issues. On the other hand, mycogenic chitosan has many advantages that make it suitable for many medical and nutritional applications over the non-mycological counterparts. A number of fungal isolates have been screened for chitosan production, where the most potent fungal isolate has been genetically identified using 18S rDNA and selected to be the focus of the current study. The factors affecting chitosan production by the selected fungal isolate have been studied and numerically optimized and validated using Box–Behnken design. The produced chitosan has been collected, purified and characterized for the degree of deacetylation (DDA), molecular weight (MW), water-binding (WBC) and fat-binding capacities (FBC). Results showed that Aspergillus terreus (F3) was the most potent chitosan-producing fungal isolate with maximum validated productivity (2.92 g/l) at the following conditions: glucose, 35.6 g/l; (NH4)2SO4, 4.6 g/l; CaCl2, 0.29 g/l; and pH 7.9 at 23.2 °C for 10 days. The purified chitosan has the following characteristics: 71.9%, DDA; 54.1165 KD, MW; 58.6%, WBC; and 47.6%, FBC. The features and applications of fungal chitosan are not fully uncovered which necessitates further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Worldwide, huge annual quantities of synthetic polymers (about 140 million tons) are produced [1]. These synthetic polymers have a limited degradation rate and adverse effect on the environment and the eco-systems. This fact necessitates replacement of synthetic with biogenic polymers. Chitosan—a cationic biopolymer obtained from the hard outer skeleton of shellfish and other crustacean shells—is composed of the amino sugars: β-(1–4)-linked d-glucosamine and N-acetyl-d-glucosamine (NAG) [2, 3]. A linear backbone linked with glycosidic bonds comprises the molecular structure of chitosan. The protonated amine groups of chitosan are responsible for its positive charge in most physiological fluids [4].
Nontoxicity (the intravenous large dose (20 g) of GlcNAc does not reveal toxicity or alter the blood glucose concentration) [5], biocompatibility, biodegradability, cationic properties, permeability-enhancing properties and bioadhesion are all useful features characterizing biologically produced chitosan from others [6]. It can be processed in different forms including tablets, films, threads, membranes and microparticles/nanoparticles which allow its use in a variety of food, agricultural, medical and pharmaceutical purposes [6]. These biological properties foster its application in many fields [7].
Chitosan has been used in agriculture as biopesticide and in wine-making industry as a fining agent [8]. In medicine, it has been reported that the encapsulation of active compounds in chitosan allows its protection against various environmental factors and increases the drug control releasing system [9] and, aggressively, binds to both microbial and mammalian cells. Chitosan-based particles have been extensively studied for delivery of many active compounds including anti-cancer agents, therapeutic proteins, genes, antigens [10]. Chitosan is hypoallergenic and has antimicrobial and blood clotting properties. It is use in bandages and other hemostatic agents have been approved in the USA and Europe to assist in dermal drug delivery and bleeding reduction [11]. Its role as a soluble dietary fiber has been investigated [12] and as a nano-carrier it has been found to increase the intestinal transport [13]. Chitosan has been reported to have anti-obesity properties [14]; therefore, it has been used as a dietary supplement for weight reduction in obese individuals. This feature is back to the positively charged amino groups in chitosan that binds to the negatively charged lipids reducing their gastrointestinal uptake and lowering serum cholesterol [14]. Subsequently, chitosan has the potential of atherosclerotic risk reduction. Furthermore, it has been reported to accelerate osteoblast, bone and connective tissue formation [15]. Chitosan has been used in water purification and found many applications in the cosmetic and fabric industry [16]. Many reviews and research papers have discussed properties and various applications of chitosan [17,18,19].
Some fungi belong to the class of Zygomycetes have been reported to contain chitosan in their cell walls including Absidia spp., Gongronella spp., Rhizopus spp. and Aspergillus spp. [20, 21]. However, the production of chitosan from fungi is a species-specific and highly related species may have different productivity. The production of chitosan from microbial (i.e., fungal) source has gained increasing interest [22] because it has many advantages over crustacean sources [23]. Fungal chitosan has a constant composition, available throughout the year; wastes and industrial by-products can be used for its production and free from heavy metal; therefore, demineralization step is not required for its purification [1]. The bulk production, however, of fungal chitosan is facing a challenge of high cost of production media and low production of chitosan [19]. The cost-effective production of chitosan requires simple strategies, cheap technologies and culture media [24].
The current work aims to use the biotechnological methods for screening and production of fungal chitosan. In addition, statistical techniques, modeling and maximizing chitosan yield at the optimum conditions is to be used for an economic production process. Furthermore, we aim to conduct a scaling-up trial for mycogenic chitosan. The produced chitosan is to be monitored for some of its physicochemical characteristics.
2 Materials and Methods
2.1 Media and Growth Conditions
Three culture media were used for chitosan production; Potato-Dextrose-Broth (PDB) medium; containing (g/l): 4, Potato Starch (from infusion) and 20, Dextrose, Czapek Dox broth medium [25]; containing (g/l): 30, Sucrose; 2, NaNO3; 1, K2HPO4; 0.5, MgSO4. 7H2O; 0.5, KCl and 0.01, FeSO4. 7H2O, and C1 medium [26]; containing (g/l): 20, Glucose; 15, Yeast extract; 1, NaCl; 5, (NH4)2SO4; 1, K2HPO4; 5, MgSO4. 7H2O and 0.1, CaCl2. If not otherwise mentioned, all tests were conducted in 250 ml Erlenmeyer flasks with 50 ml medium at pH 7, 30 °C and 120 rpm horizontal shaking for 7 days.
2.2 Selection of the Fungal Isolate and Production Medium
Six laboratory fungal isolates, coded as: “F1,” “F3,” “L2,” “F5k,” “F18” and “FM,” have been evaluated for biomass dry weight, chitosan productivity (both represented in (g/l) ± standard deviation) and specific chitosan productivity (Eq. 1) using potato dextrose broth (PDB) medium and the most potent chitosan-producing isolate has been selected for further investigations according to the current study. These isolates have been, previously, isolated from cultivated soils except for F5k from sewage water, Kafr El Shaikh Governorate, Egypt, and were preserved in refrigerator on potato dextrose agar (PDA) medium and re-cultured monthly. All tests have been performed in triplicates.
The most potent fungal isolate has been cultured in the different broth nutrient media: PDB, Czapek Dox and C1, where the best medium for chitosan production has been selected.
2.3 Chitosan Isolation and Purification
At the end of cultivation period, fungal mycelia were harvested using Whatman filter paper (No. 1). The fungal cells have been washed twice with distilled water and dried at 65 °C till constant weight is obtained. The dried cells were, finely, ground, suspended in NaOH solution (1 N) and autoclaved at 121 °C for 15 min. The alkali-insoluble fractions were collected and washed with distilled water to neutral pH with the aid of centrifugation at 12,000g for 15 min. The neutral residues were then extracted using acetic acid (2%) at 95 °C for 8 h, centrifuged and the acid insoluble materials were discarded. The pH of the supernatant was adjusted to 10 using NaOH (2 N). The solution was allowed to precipitate, centrifuged at 12,000g for 15 min, washed with distilled water, ethanol (95%), and acetone and finally dried at 60 °C to constant chitosan weight per grams [27, 28].
2.4 Molecular Identification of the Most Promising Fungal Isolate
DNA sequence analysis of 18S rDNA was used for the characterization of the selected fungal isolate. This test was performed by Sigma Scientific Services Co., Giza, Egypt (http://sigmaeg-co.com/), where the DNA was extracted by Quick-DNA™ Fungal/Bacterial Microprep Kit (Zymo Research #D6007), amplified using Maxima Hot Start PCR Master Mix (Thermo K1051), purified using GeneJET™ PCR Purification Kit (Thermo K0701) and sequenced by ABI 3730xl DNA sequencer (SeqGen, Inc., Torrance, USA) using forward (ITS1) and reverse primers (ITS4). Data were recorded in National Center for Biotechnology Information (https://blast.ncbi.nlm.nih.gov) and accession number was provided.
2.5 Statistical Modeling Chitosan Production
For statistical modeling and optimization of chitosan production, six; nutritional and physical, factors have been selected to conduct the current research, namely; glucose, (NH4)2SO4, CaCl2, pH, incubation temperature and incubation period at three levels using Box–Behnken (response surface method) as shown in Table 1. Modeling and optimization process have been taken place using Design-Expert (version 7.0.0) software (Stat-Ease Inc., Minneapolis, MN, USA).
After carrying out all the runs, the response (chitosan dry weight (g/l)) has been recorded and the model was analyzed using partial sum of squares (type III) analysis of variance (ANOVA).
2.6 Validation of the Model Results
At the end of the model data analysis, the optimum production conditions have been numerically optimized and practically applied where the results compared to confirm and validate the model results for future simulation and prediction of test results.
2.7 Pilot-Scale Production of Chitosan
BioFlo 310; 5-L working volume bioreactor, (Eppendorf New Brunswick™, Germany) has been used for pilot-scale production of chitosan, by the fungal isolate under investigation, in a batch process at the optimum conditions, 1vvm, aeration flow rate and 200 rpm, agitation (using Rushton turbine impellers).
3 Characterization of Chitosan
3.1 Degree of Deacetylation (DDA)
For determining the degree of deacetylation (DDA), the powdered chitosan sample was prepared by blending and triturated with KBr in mortar. The produced mixture was compacted to form a disk. The disk was oven-dried at 80 °C for 16 h and then cooled in a desiccator before analysis [28]. The spectrum of chitosan was obtained using FTIR instrument (JASCO, FT/IR-6100typeA, Japan) within a frequency range of 4000–400 cm−1. The degree of deacetylation (DDA) was evaluated by recording absorbance at 1655 cm−1 and 3450 cm−1 which represent the absorbance of amide-I and OH groups in chitosan. The spectrum of chitosan was used to calculate the degree of deacetylation (DDA) according to the following equation [29]:
where A1655 is the absorbance at 1655 cm−1 and A3450 is the absorbance at 3450 cm−1; the factor 1.33 represents the ratio of A1655/A3450 for fully N-acetylated chitosan.
3.2 Intrinsic Viscosity and Molecular Weight [30]
For this purpose, chitosan has been dried at 60 °C and allowed to cool in desiccator. Chitosan solution was prepared in acetate buffer (0.5 M AcOH–0.2 M NaOAc) at different concentrations. The reduced viscosity of these solutions was determined from the mean flow times of five replica using borosilicate glass Ostwald U-shaped viscometer at ≈ 30 °C. The kinematic viscosity of polymer solutions is assumed to be equal to their dynamic viscosity.
The kinematic viscosity (v) of chitosan was determined from its mean flow times compared with the acetate buffer. The viscosities of the buffer and chitosan solutions were used to calculate the relative viscosity, specific viscosity and reduced viscosity using the following relationships:
where η is dynamic viscosity and ρ is the fluid density.
where t is the mean flow time of chitosan solution and ts is the mean flow time of acetate buffer.
where c is the chitosan concentration in (g/dl).
Huggins’ plot, a plot of reduced viscosity against chitosan concentration, was applied to determine the intrinsic viscosity according to Huggins equation [31]:
The intrinsic viscosity (η) was used to determine chitosan molecular weight based on the viscosity average molecular weight (in Dalton) according to Mark–Houwink equation [32].
where (M) is the viscosity average molecular weight; (K) and (a) are constant values depend on the polymer and solvent types. For chitosan and acetate buffer used, these constants are 3.5 × 10–4 and 0.76, respectively [33]. Three concentrations of chitosan solutions were used in this experiment.
3.3 Water-Binding Capacity (WBC)
The modified method of Knorr [34] was used to measure chitosan WBC. In brief, a centrifuge tube containing 0.5 g of a well dried chitosan was weighed, filled with 10 ml of distilled water and vigorously mixed for 1 min. The tube was left for 30 min in room temperature with 5 s. intermittent shaking every 10 min. The tube was centrifuged for 25 min at RCF (1409g). The supernatant was decanted and the tube was reweighed. Finally, WBC was calculated using the following equation:
3.4 Fat-Binding Capacity (FBC)
The modified method of Knorr [34] was used to measure chitosan FBC, exactly, as mentioned in WBC with replacement of distilled water with oil (a mixture of soybean and sunflower oils). Finally, FBC was calculated using the following equation:
4 Results
4.1 Selection of the Most Potent Fungal Isolate(s)
The six tested fungal isolates showed different biomass and chitosan productivity on PDB medium (Table 2). The isolate (F18) showed the maximum biomass dry weight at 9.908 ± 1.836 g/l, whereas the isolate (F3) revealed the maximum chitosan productivity at 0.067 ± 0.003 (g/l) and (F5k) represented the maximum specific chitosan dry weight at 2.201%.
4.2 Selection of Production Medium
According to the results of fungal isolate selection, F3 has been selected to conduct the current research. Testing of F3 on different production media (Table 3) showed that C1 was the most suitable production medium with chitosan absolute dry weight and specific productivity of 1.069 g/l and 8.13% (81.3 g/kg biomass dry weight), respectively.
4.3 Molecular Identification of the Isolate (F3)
DNA sequence analyses of 18S rDNA for the selected fungal isolate (F3) revealed that it is highly related to Aspergillus terreus with 100% query coverage and 100% percent identity as shown in molecular phylogenetic tree (Fig. 1). The data were recorded in National Center for Biotechnology Information (NCBI) and taken the Accession number of “MK633874.”
4.4 Statistical Modeling of Chitosan Production by Aspergillus terreus
The actual and predicted results, along with residuals, the difference between predicted and actual values, of Box–Behnken model for production of chitosan by Aspergillus terreus are shown in Table 4. The results showed that the minimum and maximum chitosan productivity were 0.052 and 3.138 g/l, respectively. Figure 2 shows the relationship between actual and predicted chitosan results with (R2 = 0.9752) which reveals good fitting to the curve and highly related scheme.
4.5 Analysis of Variance (ANOVA) of Box–Behnken Model
Analysis of variance (Table 5) of Box–Behnken model for production of chitosan by Aspergillus terreus revealed that the model is significant as the F value was 12.08. The model terms C, D, F, AD, AE, BD, CD, CF, DE, DF, EF, C2, D2, E2, ABD, ACF, CDF, A2D, A2F, AB2, B2C, B2D, B2F and C2E were significant because the values of “Prob > F” were less than 0.05. The model “lack of fit” F value of 0.26 implies that the lack of fit is not significant and the results fit to the model data.
The model R2 was 0.975 and the adjusted-R2 was 0.894. The model “adequate precision,” which measures the signal-to-noise ratio, was 19.813, which indicates an adequate signal.
Chitosan production by A. terreus can be represented in terms of actual factor as follows:
A: glucose; B: (NH4)2SO4; C: CaCl2; D: pH; E: incubation temperature; F: incubation period.
4.6 Numerical Optimization and Validation of the Process Conditions
According to Eq. (12), chitosan production by A. terreus has been numerically optimized for the different factors to obtain maximum production of chitosan. The data showed that the maximum chitosan yield was 3.138 g/l under the following conditions: 35.6 g/l, glucose; 4.6 g/l, (NH4)2SO4; 0.29 g/l, CaCl2 and pH 7.9 at 23.2 °C for 10 days. The practical application of these conditions resulted in chitosan (2.92 g/l). These results reveal that the model is valid for 93.05%. The dried chitosan product is shown in Fig. 3.
The 3D plotting (Fig. 4) shows the interaction relations between the different factors and their effect on chitosan production.
4.7 Pilot-Scale Production of Chitosan by Aspergillus terreus
The results (Table 6, Fig. 5) showed a progressive increase in biomass production up to the 4th day of incubation and decreased afterward. However, these results are not representative of the fact because it has been noted the formation of mycelial coagulation and accumulation of fungal cells that hindered the agitation and aeration process. Furthermore, the data obtained from this batch were underestimated due to the inability to collect representative samples as shown in the fluctuation of results and the mycelial accumulation.
5 Characteristics of Chitosan Produced by Aspergillus terreus
5.1 FTIR Determination of Degree of Deacetylation
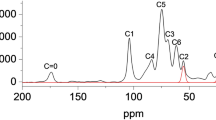
According to Eq. (2) suggested by Domszy and Roberts [29] and the FTIR spectrum (Fig. 6) of the chitosan produced by A. terreus, the degree of deacetylation was calculated to be 71.9%.
The results of viscosity determination and calculations of VAMW indicated that the molecular weight of the produced chitosan is about 54.1165 KD. On the other hand, according to Knorr [24] and Eqs. (10) and (11), the WBC and FBC were 58.6% and 47.6%, respectively.
6 Discussion
Chitosan is an eco-friendly biopolymer and is considered the second major abundant polymer on earth after cellulose and it does not appear in cellulose-producing organisms [15]. Although its main sources are crustaceans’ shells and insects’ exoskeletons, it has been reported in many fungal strains in a considerable amount [35]. However, fungal chitosan production has many advantages over its production from marine animals’ skeletons [36], most importantly, the absence of geographical and seasonal limitations [37]. Contrary to fungi, chitosan does not present, naturally, in crustacean or insects’ structures and is obtained by aggressive deacetylation of their chitin which may result in low-grade mixed chitosan structures. Chitosan is highly versatile and found many applications in various fields due to the presence of (–NH2) group [15]. A lot of trials have been made to synthesize new functional derivatives to improve its solubility and applications [38, 39]. The merit of chitosan depends on its ability to create the optimal response in the desired environment along with minimum side effects. Therefore, it is considered a potential candidate for drug development [7].
Through the current study, six fungal isolates have been evaluated for their production of chitosan in the PDB medium. PDB is one of the most widely used media for the growth and propagation of fungi [40]. Although the isolate (F18) showed the maximum biomass production (9.908 ± 1.836 g/l), its chitosan productivity was very low compared with FM, F1 and F3. On the other hand, the isolate (F3) showed the maximum absolute chitosan productivity (0.067 ± 0.003 g/l). Chitosan-specific production was the highest (2.201%) in the case of F5k; however, it showed very low biomass and chitosan productivity. On these bases, the isolate F3 has been selected for further investigation because it showed the maximum absolute (0.067 ± 0.003 g/l) and specific (1.76%) chitosan productivity along with reasonable biomass production. The final chitosan yield obtained by Aspergillus niger (0.24 mg/ml) [41] and Rhizopus oryzae (5.63 g/kg) [42] was reasonably compared with the yield obtained by the isolate F3 before optimization processes. Chitosan yield obtained by F3 was expected to exceed the previously mentioned values, after optimization, because the chitosan content of F3 was 1.76% which is very low compared with that listed by [43] for Cunninghamella blakesleeana, Rhizopus delemar, Absidia coerulea and Mortierella isabelina, which was 9.4, 7.1, 10.4 and 6.7 (%), respectively. Therefore, the data reveal that optimization of the production conditions of F3 may result in an improvement in biomass and chitosan productivity.
Three nutritional media, PDB, Czapek Dox and C1, have been screened on the isolate (F3) to select the most suitable medium for chitosan production. The data showed that C1 was the most suitable medium based on biomass (13.137 ± 0.041 g/l) and chitosan dry weight (1.069 ± 0.01 g/l). Also, the data showed that the fungal chitosan content is not in a rational relation with fungal biomass and varies with difference in nutritional medium. Pochanavanich and Suntornsuk [27] reported that the fungal chitosan content is species-specific and depends on the cultivation conditions, production medium and growth phase. This step resulted in 3.46 X increase in the biomass dry weight and 15.96 X increase in the chitosan dry weight.
Molecular identification of the most promising fungal isolate (F3) revealed 100% query coverage and 100% percent identity to Aspergillus terreus. The data have been registered in the National Center for Biotechnology Information (NCBI) and given the Accession number of “MK633874.”
Chitosan production by Aspergillus terreus has been statistically modeled using Box–Behnken (response surface model (RSM)), and the data showed a high correlation coefficient (R2 = 0.9752) between actual and predicted results indicating a significant model [44] which has been confirmed with ANOVA. The lack-of-fit parameter showed insignificant results (F value of 0.26) and adequate precision of (19.813) indicating an adequate signal of the model. Finally, the model is compatible with the obtained data and can be used to navigate the design space. The ANOVA showed that glucose and incubation temperature did not have a significant direct effect on chitosan production; however, their interaction with the other factors showed significant effects. These results assert the importance of statistical modeling of valuable products in biotechnological and industrial processes. The model has been numerically optimized where the optimum conditions have been predicted to obtain the maximum probable yield of chitosan. Accordingly, the optimum conditions were glucose (35.6 g/l), (NH4)2SO4 (4.6 g/l), CaCl2 (0.29 g/l) and pH 7.9 at 23.2 °C for 10 days to obtain (3.138 g/l) chitosan. In accordance with our results, glucose, (NH4)2SO4 and CaCl2 were recorded as significant nutritional factors for the production of chitosan by Absidia coerulea [26]. In addition, glucose has been proven a significant factor in chitosan production from Mucor racemosus and Cunninghamella elegans [45] and from Aspergillus niger [46].
Experimentally, under these conditions, chitosan yield was 2.92 g/l indicating a model validity of 93.05%. Kim et al. [26] obtained a 2.3 g/l chitosan yield from the mycelia of Absidia coerulea under optimized conditions. In a study by [46], the maximum chitosan productivity (1.93 g/l) was obtained by Aspergillus niger. The overall medium components and conditions were (g/l): 35.6, glucose; 15, yeast extract; 4.6, (NH4)2SO4; 0.29, CaCl2; 1, K2HPO4; 1, NaCl and 5, MgSO4.7H2O. Medium pH is adjusted at 7.9 and incubation is at 23.2 °C for 10 days.
Production of chitosan has been discussed in both solid and submerged fermentation levels [47]. Production using either method has its own pros and cons. With the focus on biomass productivity, solid-state fermentation (SSF) is favored according to Favela-Torres et al. [48] due to the absence of catabolite repressors [49]. The most difficulty facing SSF of chitosan production from fungi is the recovery of fungal biomass from the fermentation system [47] and heterogeneous substrate [50]. This difficulty sometimes pushes scientists to favor submerged-state fermentation (SmF) for chitosan production over SSF. On the other hand, other cases reported that biomass levels of SmF either exceed that SSF or are comparable with it [51, 52]. The submerged fermentation systems have dominated most of chitosan production studies [26, 45]. However, studies on SSF should be continued and evaluated.
In a trial for pilot-scale production of chitosan by Aspergillus terreus, BioFlo 310; 5 l bioreactor has been used under optimum nutritional and physical conditions. The process could not be completed by the end of the recommended incubation period due to the formation of mycelial coagulation and fungal cells’ accumulation that resulted in failure of agitation and aeration processes and, subsequently, un-controlled production system. To date, without a clear scientific reason, the production of fungal chitin and chitosan has not been scaled up for industrial production. However, our trial to perform scaling-up based on problem-solving process should pave the way for a clear economic industry.
Further studies have been conducted for assigning some characters of the produced chitosan including FTIR for determining the degree of deacetylation (DDA), viscosity for calculation of viscosity average molecular weight (VAMW), water-binding capacity (WBC), and fat-binding capacity (FBC). Measurements and calculations revealed that the produced chitosan has a degree of deacetylation (DDA), viscosity average molecular weight (VAMW), water-binding capacity and fat-binding capacity were 71.9%, 54.12 KD, 58.6% and 47.6%, respectively. These characteristics are critical parameters in determining the possible fields of application [53,54,55,56]. According to [9], most of the biological activities of glycans are due to their physicochemical properties.
The degree of deacetylation (DDA) of chitosan represents the balance between N-d-glucosamine and N-acetyl- d-glucosamine [57] and reflects the cationic chitosan charge after its dissolution in a weak acid [58]. DDA is highly related to the solubility of chitosan, where they are positively correlated, and subsequently the possible field of application. Chitosan is considered chitin in its deacetylated form. Chitosan with degree of deacetylation (DD) more than 60% [more than 60% of D-glucosamine and less than 40% of N-acetyl-d-glucosamine] is, usually, considered as soluble in dilute acidic solutions [55]. According to Sorlier et al. [58], chitosan DDA varies based on the method of manufacture in the range (70–100%).
Viscosity is one of the important characteristics of biopolymers as it reflects its ability to enhance the solution viscosity. In addition, the intrinsic viscosity of chitosan solution may correlate with its molecular mass as has been reported by Mark–Houwink equation [32]. The data obtained in the current study (VAMW = 54.12 KD) are matched with that reported by Nwe and co-workers [59, 60] who concluded that the viscosity-average molecular weight of fungal chitosan ranges from 20 to 70 kDa. The low molecular weight chitosan has many biomedical applications and as hypocholesterolemic agents [61, 62], whereas high molecular weight yields high viscous solutions that limit its exploitation in agricultural, health and food sectors [63].
Fungal chitosan production has approved many usages and advantages over crustacean chitosan. Aspergillus terreus was revealed as a promising chitosan producer. Statistical modeling became an important tool in biotechnological and bio-production processes. The produced chitosan differs from one fungal strain to another; therefore, it should be, routinely, evaluated and quality-controlled before its application. Aspergillus terreus can be used for biotechnological production of chitosan under submerged-state fermentation. Further studies are to be conducted for troubleshooting and control of the current production system (SmF). The medium C1 used by Kim et al. [26] with modifications is highly recommended for fungal growth and chitosan production. More studies are to be conducted to transfer chitosan production by Aspergillus terreus from laboratory to industrial scale.
Data availability
All data generated and/or analyzed during this study are included in this published article.
References
Akila, R.M.: Fermentative production of fungal Chitosan, a versatile biopolymer (perspectives and its applications). Adv. Appl. Sci. Res. 5(4), 157–170 (2014)
Świątek, M.A.; Tenconi, E.; Rigali, S.; van Wezel, G.P.: Functional analysis of the N-Acetylglucosamine metabolic genes of Streptomyces coelicolor and role in control of development and antibiotic production. J. Bacteriol. 194(5), 1136–1144 (2012)
Ali-Komi, D.E.; Hamblin, M.R.: Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. (Indore) 4(3), 411–427 (2016)
Fee, M.; Errington, N.; Jumel, K.; Illum, L.; Smith, A.; Harding, S.E.: Correlation of SEC/MALLS with ultracentrifuge and viscometric data for chitosans. Eur. Biophys. J. 32, 457–464 (2003)
Liu, Y.; Dentin, R.; Chen, D.; Hedrick, S.; Ravnskjaer, K.; Schenk, S.; Milne, J.; Meyers, D.J.; Cole, P.; Yates, J.: 3rd A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature 456, 269–273 (2008)
Muzzarelli, R.A.A.: Review: Chitins and chitosans as immunoadjuvants and non-allergenic drug carriers. Mar. Drugs 8, 292–312 (2010)
Chen, J.K.; Shen, C.R.; Liu, C.L.: Review: N-Acetylglucosamine: production and applications. Mar. Drugs 8, 2493–2516 (2010). https://doi.org/10.3390/md8092493
Xu, P.; Su, Y.: Study on fresh keeping effect of chitosan antistaling agent for several kinds of fruits. Adv. J. Food Sci. Technol. 5(12), 1577–1579 (2013)
Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, Á.: Functional characterization of chitin and chitosan. Curr. Chem. Biol. 3, 203–230 (2009)
Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y.: Review: Anticancer and anti-inflammatory properties of chitin and chitosan oligosaccharides. J. Funct. Biomater. 6, 33–49 (2015)
Gómez-Rivas, L.; Escudero-Abarca, B.I.; Aguilar-Uscanga, M.G.; Hayward-Jones, P.M.; Mendoza, P.; Ramírez, M.: Selective antimicrobial action of chitosan against spoilage yeasts in mixed culture fermentations. J. Ind. Microbiol. Biotechnol. 31(1), 16–22 (2004)
Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B.: Chitosan-based nanomaterials: a state-of-the-art review. Int. J. Biol. Macromol. 59, 46–58 (2013)
Furda, I.: Interaction of dietary fiber with lipids—mechanistic theories and their limitations. New Dev. Diet. Fiber Adv. Exp. Med. Biol. 270, 67–82 (1990)
Jun, S.C.; Jung, E.Y.; Hong, Y.H.; Park, Y.; Kang, D.H.; Chang, U.J.; Suh, H.J.: Anti-obesity effects of chitosan and psyllium husk with L-ascorbic acid in guinea pigs. Int. J. Vitam. Nutr. Res. 82(2), 113–120 (2012)
Dutta, P.K.; Dutta, J.; Tripathi, V.S.: Chitin and chitosan: chemistry, properties and applications. J. Sci. Ind. Res. 63, 20–31 (2004)
Yong, S.K.; Shrivastava, M.; Srivastava, P.; Kunhikrishnan, A.; Bolan, N.: Environmental applications of chitosan and its derivatives. Rev. Environ. Contam. Toxicol. 233, 1–43 (2015)
Habibi, A.; Karami, S.; Varmira, K.; Hadadi, M.: Key parameters optimization of chitosan production from Aspergillus terreus using apple waste extract as sole carbon source. Bioprocess Biosyst. Eng. 44, 283–295 (2021)
Joseph, S.M.; Krishnamoorthy, S.; Paranthaman, R.; Moses, J.A.; Anandharamakrishnan, C.: A review on source-specific chemistry, functionality, and applications of chitin and chitosan. Carbohydr. Polym. Technol. Appl. 2, 100036 (2021)
Crognale, S.; Russo, C.; Petruccioli, M.; D’Annibale, A.: Chitosan production by fungi: current state of knowledge, future opportunities and constraints. Fermentation 8, 76 (2022)
Ruiz-Herrera, J.; Sentandreu, R.R.; Martinez, J.P.: Chitin biosynthesis in fungi. In: Dilip, K.A.; Richard, P.E.; Mukerji, K.G. (Eds.) Handbook of Applied Mycology. Fungal Biotechnology, Vol. 4, pp. 281–312. Marcel Dekker, New York (1992)
Hon, D.N.S.: Chitin and chitosan: medical applications. In: Dumitri, S. (Ed.) Polysaccharides in Medicinal Application, pp. 631–649. Marcel Dekker, New York (1996)
Niederhofer, A.; Müller, B.W.: A method for direct preparation of chitosan with low molecular weight from fungi. Eur. J. Pharm. Biopharm. 57, 101–105 (2004)
Sebastian, J.; Rouissi, T.; Brar, S.K.: Chapter 14: Fungal chitosan: prospects and challenges. In: Gopi, S.; Thomas, S.; Pius, A. (Eds.) Handbook of Chitin and Chitosan, pp. 419–452. Elsevier, Amsterdam (2020)
Rafael, O.H.D.; Fernándo, Z.G.L.; Abraham, P.T.; Alberto, V.L.P.; Guadalupe, G.S.; Pablo, P.J.: Production of chitosan-oligosaccharides by the chitin-hydrolytic system of Trichoderma harzianum and their antimicrobial and anticancer effects. Carbohydr. Res. 486, 107836 (2019)
Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D.: Standard Methods for the Examination of Water and Wastewater, 20th edn. APHA, Washington, DC (1998)
Kim, W.J.; Lee, W.G.; Theodore, K.; Chang, H.N.: Optimization of culture conditions and continuous production of chitosan by the fungi, Absidia coerulea. Biotechnol. Bioprocess Eng. 6, 6–10 (2001)
Pochanavanich, P.; Suntornsuk, W.: Fungal chitosan production and its characterization. Lett. Appl. Microbiol. 35, 17–21 (2002)
Hussain, M.R.; Iman, M.; Maji, T.K.: Determination of degree of deacetylation of chitosan and their effect on the release behavior of essential oil from chitosan and chitosan gelatin complex microcapsules. Int. J. Adv. Eng. Appl. 2(4), 4–12 (2013)
Domszy, J.G.; Roberts, G.A.F.: Evaluation of infrared spectroscopic techniques for analysing chitosan. Die Makromol. Chem. 186(8), 1671–1677 (1985)
Hossain, M.S.; Iqbal, A.: Production and characterization of chitosan from shrimp waste. J. Bangladesh Agril. Univ. 12(1), 153–160 (2014)
Wang, T.; Turhan, M.; Gunasekaran, S.: Selected properties of pH-sensitive, biodegradable chitosan- poly(vinyl alcohol) hydrogel. Polym. Int. 53, 911–918 (2004)
Wang, W.; Bo, S.Q.; Li, S.Q.; Qin, W.: Determination of the Mark-Houwink equation for chitosans with different degrees of deacetylation. Int. J. Biol. Macromol. 13, 281–285 (1991)
Terbojevidh, M.; Cosani, A.: Molecular weight determination of chitin and chitosan. In: Muzzarelli, R.A.A.; Peter, M.G. (eds.) Chitin Handbook, pp 87–101. European Chitin Society (1997)
Knorr, D.: Functional properties of chitin and chitosan. J. Food Sci. 47, 593–595 (1982)
Abo Elsoud, M.M.; El Kady, E.M.: Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Centre 43(59), 1–12 (2019)
Chien, R.; Yen, M.; Mau, J.: Antimicrobial and antitumor activities of chitosan from Shiitak estipes, compared to commercial chitosan from crab shells. Carbohydr. Polym. 138(1), 259–264 (2016)
White, S.A.; Farina, P.R.; Fulton, I.: Production and isolation of chitosan from Mucor rouxii. Appl. Environ. Microb. 38(2), 323–328 (1979)
Kubota, N.: Permeability properties of chitosan-transition metal complex membrane. J. Appl. Poly. Sci. 64, 819 (1997)
Yao, K.D.; Zhao, F.; Li, F.; Yin, Y.J.: Chitosan-based gels. In: Schwartz, M. (Ed.) Encyclopedia of Smart Materials, pp. 182–190. Wiley, New York (2002)
Atlas, R.M.; Parks, L.C.: Handbook of Microbiological Media. CRC Press, Boca Raton (1993)
Chhabra, R.; Sachdeva, A.; Mathur, G.; Sharma, P.; Goswami, N.; Jain, C.K.; Sharma, S.K.; Mathur, A.: Enhanced production of fungal chitosan from Aspergillus niger using statistical optimization. J. Chitin Chitosan Sci. 2, 70–74 (2014)
Khalaf, S.A.: Production and characterization of fungal chitosan under solid-state fermentation conditions. Int. J. Agric. Biol. 6(6), 1033–1036 (2004)
Miyoshi, H.; Shimura, K.; Watanabe, K.; Onodera, K.: Characterization of some fungal chitosans. Biosci. Biotechnol. Biochem. 56(12), 1901–1905 (2014). https://doi.org/10.1271/bbb.56.1901
Schober, P.; Boer, C.; Schwarte, L.A.: Correlation coefficients: appropriate use and interpretation. Anesth. Analg. 126(5), 1763–1768 (2018)
Amorim, R.; Da Silva, V.; De Souza, W.; Fukushima, K.; Campos-Takaki, G.M.: Faster chitosan production by Mucoralean strains in submerged culture. Braz. J. Microbiol. 32(1), 20–23 (2001)
Logesh, A.R.; Thillaimaharani, K.A.; Sharmila, K.; Kalaiselvam, M.; Raffi, S.M.: Production of chitosan from endolichenic fungi isolated from mangrove environment and its antagonistic activity. Asian Pac. J. Trop. Biomed. 2(2), 140–143 (2012)
Sitanggang, A.B.; Sophia, L.; Wu, H.S.: MiniReview: Aspects of glucosamine production using microorganisms. Int. Food Res. J. 19(2), 393–404 (2012)
Favela-Torres, E.; Cordova-Lṑpez, J.; Garcἱa-Rivero, M.; Gutἱerrez-Rojas, M.: Kinetics of growth of Aspergillus niger during submerged, agar surface and solid state fermentations. Process Biochem. 33, 103–107 (1998)
de Azeredo, L.A.; Gomes, P.M.; Sant’Anna, G.L.; Castilho, L.R.; Freire, D.M.: Production and regulation of lipase activity from Penicillium restrictum in submerged and solid-state fermentations. Curr. Microbiol. 54, 361–365 (2007)
Sparringa, R.A.; Owens, J.D.: Glucosamine content of tempe mould Rhizopus oligosporous. Int. J. Food Microbiol. 47, 153157 (1999)
Mazumder, S.; Basu, S.K.; Mukherjee, M.: Laccase production in solid-state and submerged fermentation by Pleurotus ostreatus. Eng. Life Sci. 9, 45–52 (2009)
Omogbai, B.A.; Ikenebomeh, M.: Solid-state fermentative production and bioactivity of fungal chitosan. J. Microbiol. Biotechnol. Food Sci. 3, 172–175 (2013)
Huang, L.; Chen, J.; Cao, P.; Pan, H.; Ding, C.; Xiao, T.; Zhang, P.; Guo, J.; Su, Z.: Anti-obese effect of glucosamine and chitosan oligosaccharide in high-fat diet-induced obese rats. Mar. Drugs 13, 2732–2756 (2015). https://doi.org/10.3390/md13052732
Ma, J.; Xin, C.; Tan, C.: Preparation, physicochemical and pharmaceutical characterization of chitosan from Catharsius molossus residue. Int. J. Biol. Macromol. 80, 547–556 (2015). https://doi.org/10.1016/j.ijbiomac.2015.07.027
Pan, H.; Yang, Q.; Huang, G.; Ding, C.; Cao, P.; Huang, L.; Xiao, T.; Guo, J.; Su, Z.: Hypolipidemic effects of chitosan and its derivatives in hyperlipidemic rats induced by a high-fat diet. Food Nutr. Res. 60, 31137 (2016). https://doi.org/10.3402/fnr.v60.31137
Hamer, S.N.; Cord-Landwehr, S.; Biarnes, X.; Planas, A.; Waegeman, H.; Moerschbacher, B.M.; Kolkenbrock, S.: Enzymatic production of defined chitosan oligomers with a specific pattern of acetylation using a combination of chitin oligosaccharide deacetylases. Sci. Rep. 5, 8716 (2015). https://doi.org/10.1038/srep08716
Balázs, N.; Sipos, P.: Limitations of pH-potentiometric titration for the determination of the degree of deacetylation of chitosan. Carbohydr. Res. 342(1), 124–130 (2007)
Sorlier, P.; Denuziere, A.; Viton, C.; Domard, A.: A relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules 2, 765–772 (2001)
Nwe, N.; Stevens, W.F.; Tokura, S.; Tamura, H.: Characterization of chitin and chitosan-glucan complex extracted from cell wall of fungus Gongronella butleri USDB 0201 by enzymatic method. Enzyme Microbial Technol. 42, 242251 (2008)
Nwe, N.; Furuike, T.; Tamura, H.: Production of fungal chitosan by enzymatic method and applications in plant tissue culture and tissue engineering: 11 years of our progress, present situation and future prospects. Biopolymers 7(135), 162 (2010)
Sumiyoshi, M.; Kimura, Y.: Low molecular weight chitosan inhibits obesity induced by feeding a high-fat diet long-term in mice. J. Pharm. Pharmacol. 58, 201–207 (2006)
Sathiyaseelan, A.; Shajahan, A.; Kalaichelvan, P.T.; Kaviyarasan, V.: Fungal chitosan based nanocomposites sponges: an alternative medicine for wound dressing. Int. J. Biol. Macromol. 104, 1905–1915 (2017)
Wibowo, S.; Velazquez, G.; Savant, V.; Torres, J.A.: Effect of chitosan type on protein and water recovery efficiency from surimi wash water treated with chitosan-alginate complexes. Bioresour. Technol. 98, 539–545 (2007)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Not applicable.
Author information
Authors and Affiliations
Contributions
All authors certify that they have participated sufficiently in contributing to the intellectual content, concept, and writing the manuscript, and all authors have read and approved the manuscript and ensure that this is the case. MMAE (corresponding author) put the main idea and collected the organisms and materials, designed and analyzed the statistical model and scaling-up; SSM did the production and purification of chitosan; and MSM contributed to the optimization part and confirmed that all listed authors have approved the manuscript before submission, including the names and order of authors; NMS did the characterization part.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abo Elsoud, M.M., Mohamed, S.S., Selim, M.S. et al. Characterization and Optimization of Chitosan Production by Aspergillus terreus. Arab J Sci Eng 48, 93–106 (2023). https://doi.org/10.1007/s13369-022-07163-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-022-07163-z