Abstract
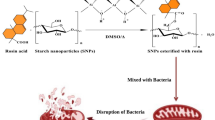
This research aims to prepare potato starch nanoparticles (SNPs) by hydrochloric acid hydrolysis. The esterification reaction of starch nanoparticles with rosin acid is catalyzed by Maghnite-H+ as green catalyst, a non-toxic proton exchanged montmorillonite (sheet silicate clay). The influence of synthesis conditions, including reaction temperature, reaction time, and the amount of catalyst, are assessed using a response surface method relying on a central composite design with three factors and three levels. FTIR spectroscopy analysis elucidates the esterification of SNPs with rosin acid, with the appearance of a new intense peak C=O ester at 1726 cm−1. Scanning electron microscopy observations reveal some atomic/molecules disorder after esterification. X-ray diffraction analysis indicates the presence of the amorphous structure, with a significant decline of the crystallinity upon esterification of SNPs. Thermogravimetric analysis manifests that the thermal stability of esterified SNPs is lower compared to native starch and SNPs. The antioxidant characteristics of SNPs before and after esterification investigated through 1,1-diphenyl- 2-picryl hydrazyl radical scavenging tests demonstrate a dose-dependent increase in the antioxidant activity that is comparable to standard vitamin C (ascorbic acid).










Similar content being viewed by others
References
Hulwani, A.; Zain, M.: Biodegradation behaviour of thermoplastic starch: the roles of carboxylic biodegradation behaviour of thermoplastic starch: the roles of carboxylic acids on cassava starch. J. Polym. Environ. (2018). https://doi.org/10.1007/s10924-017-0978-5
Odian, G.: Principles of Polymerization. Wiley, Hoboken (2004)
Zhu, F.: Encapsulation and delivery of food ingredients using starch based systems. Food Chem. (2017). https://doi.org/10.1016/j.foodchem.2017.02.101
Bule, A.: Starch granules: structure and biosynthesis. Int. J. Biol. Macromol 23, 85–112 (1998)
Eerlingen, R.C.; Delcour, J.A.: Formation, analysis, structure and properties of type III enzyme resistant starch. J. Cereal Sci. 22, 129–138 (1995)
Masina, N., et al.: A review of the chemical modification techniques of starch. Carbohydr. Polym. (2016). https://doi.org/10.1016/j.carbpol.2016.09.094
Topping, D.L.; Clifton, P.M.: Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 81(3), 1031–1064 (2018)
Pe, S.: The molecular structures of starch components and their contribution to the architecture of starch granules: a comprehensive review. Starch-Stärke 62, 389–420 (2010). https://doi.org/10.1002/star.201000013
Hoover, R.: Composition, molecular structure, and physicochemical properties of tuber and root starches: a review. Carbohydr. Polym. 45, 253–267 (2001)
Bras, J.; Dufresne, A.: Starch nanoparticles: a review. Biomacromol 11, 1139–1153 (2010)
Xie, F.; Pollet, E.; Halley, P.J.; Avérous, L.: Progress in polymer science. Prog. Polym. Sci. 38(10–11), 1590–1628 (2013). https://doi.org/10.1016/j.progpolymsci.2013.05.002
Bel Haaj, S.; Magnin, A.; Pétrier, C.; Boufi, S.: Starch nanoparticles formation via high power ultrasonication. Carbohydr. Polym. 92(2), 1625–1632 (2013). https://doi.org/10.1016/j.carbpol.2012.11.022
Kim, H.; Park, S.S.; Lim, S.: Preparation, characterization and utilization of starch nanoparticles. Colloids Surf. B Biointerfaces (2014). https://doi.org/10.1016/j.colsurfb.2014.11.011
Campelo, P.H.; Sant’Ana, A.S.; Pedrosa Silva Clerici, M.T.: Starch nanoparticles: production methods, structure, and properties for food applications. Curr. Opin. Food Sci. 33(1), 136–140 (2020). https://doi.org/10.1016/j.cofs.2020.04.007
Avella, M.; De Vlieger, J.J.; Emanuela, M.; Fischer, S.; Vacca, P.; Grazia, M.: Food chemistry biodegradable starch/clay nanocomposite films for food packaging applications. Food Chem. 93, 467–474 (2005). https://doi.org/10.1016/j.foodchem.2004.10.024
Jiang, S.; Liu, C.; Wang, X.; Xiong, L.; Sun, Q.: Physicochemical properties of starch nanocomposite films enhanced by self-assembled potato starch nanoparticles. LWT Food Sci. Technol. 69, 251–257 (2016). https://doi.org/10.1016/j.lwt.2016.01.053
Rodrigues, A.; Emeje, M.: Recent applications of starch derivatives in nanodrug delivery. Carbohydr. Polym. 87(2), 987–994 (2012). https://doi.org/10.1016/j.carbpol.2011.09.044
Fan, Y.; Picchioni, F.: Modification of starch: a review on the application of ‘green’ solvents and controlled functionalization. Carbohydr. Polym. 241, 116350 (2020). https://doi.org/10.1016/j.carbpol.2020.116350
Chen, Q., et al.: Recent progress in chemical modification of starch and its applications. RSC Adv. 5(83), 67459–67474 (2015). https://doi.org/10.1039/c5ra10849g
Ashogbon, A.O.; Akintayo, E.T.: Recent trend in the physical and chemical modification of starches from different botanical sources: a review. Starch-Stärke 66, 41–57 (2014). https://doi.org/10.1002/star.201300106
Alcázar-alay, S.C.; Angela, M.; Meireles, A.: Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci. Technol. 35(2), 215–236 (2015)
Din, Z.; Xiong, H.; Fei, P.: Physical and chemical modification of starches: a review. Crit. Rev. Food Sci. Nutr. 57(12), 2691–2705 (2015). https://doi.org/10.1080/10408398.2015.1087379
Byrne, F.P., et al.: Tools and techniques for solvent selection: green solvent selection guides. Sustain. Chem. Process. 4, 1–24 (2016). https://doi.org/10.1186/s40508-016-0051-z
Syahariza, Z.A.; Li, E.; Hasjim, J.: Extraction and dissolution of starch from rice and sorghum grains for accurate structural analysis. Carbohydr. Polym. 82(1), 14–20 (2010). https://doi.org/10.1016/j.carbpol.2010.04.014
Liu, X.; Zhang, R.; Zhu, J.; Jiang, Y.: Synthesis and properties of full bio-based thermosetting resins from rosin acid and soybean oil: the role of rosin acid derivatives. Green Chem. (2013). https://doi.org/10.1039/c3gc00095h
Wilbon, P.A.; Chu, F.; Tang, C.: Progress in renewable polymers from natural terpenes, terpenoids, and rosin. Macromol. Rapid Commun. 34(1), 8–37 (2013)
Division, P.M.; Centre, M.S.: Rosin: a renewable resource for polymers and polymer chemicals. Prog. Polym. Sci. 14, 297–338 (1989)
Zaoui, A.; Mahendra, V.; Mitchell, G.; Cherifi, Z.; Harrane, A.: Design, synthesis and thermo-chemical properties of rosin vinyl imidazolium based compounds as potential advanced biocompatible materials. Waste Biomass Valorization (2019). https://doi.org/10.1007/s12649-019-00691-0
Kherroub, D.E.; Belbachir, M.; Lamouri, S.; Chikh, K.: Cationic ring opening polymerization of octamethylcyclotetrasiloxane using a cost-effective solid acid catalyst (maghnite-H+). Iran. J. Sci. Technol. Trans. A Sci. 43(1), 75–83 (2019). https://doi.org/10.1007/s40995-017-0269-y
Yahiaoui, A.; Belbachir, M.; Hachemaoui, A.; De Chimie, L.; De Chimie, D.: An acid exchanged montmorillonite clay-catalyzed synthesis of polyepichlorhydrin. Int. J. Mol. Sci. 4, 548–561 (2003)
Sinha Ray, S.; Okamoto, M.: Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog. Polym. Sci. 28(11), 1539–1641 (2003). https://doi.org/10.1016/j.progpolymsci.2003.08.002
Harrane, A.; Belaouedj, M.A.; Meghabar, R.; Belbachir, M.: Bulk polycondensation of lactic acid by Maghnite-H+ a non-toxic catalyst. J. Polym. Res. 19(2), 1–5 (2012). https://doi.org/10.1007/s10965-011-9785-1
Peng, X.; Yang, G.; Shi, Y.; Zhou, Y.; Zhang, M.; Li, S.: Box-Behnken design based statistical modeling for the extraction and physicochemical properties of pectin from sunflower heads and the comparison with commercial low-methoxyl pectin. Sci. Rep. 10(1), 1–10 (2020). https://doi.org/10.1038/s41598-020-60339-1
Abd, G.; Mahmoud, E.; Ryhan, S.: Nitrogen, amino acids, and carbon as control factors of riboflavin production by Novosphingobium panipatense-SR3 (MT002778). Curr. Microbiol. 78(4), 1577–1589 (2021). https://doi.org/10.1007/s00284-021-02376-1
Arun, V.V.; Saharan, N.; Ramasubramanian, V.; Rani, A.M.B.; Salin, K.R.: Multi-response optimization of Artemia hatching process using split-split-plot design based response surface methodology. Sci. Rep. (2017). https://doi.org/10.1038/srep40394
Solomon, D.; Kiflie, Z.; Van Hulle, S.: Using Box-Behnken experimental design to optimize the degradation of Basic Blue 41 dye by Fenton reaction. Int. J. Ind. Chem. (2020). https://doi.org/10.1007/s40090-020-00201-5
Gülcin, I.: Antioxidant activity of food constituents: an overview. Arch. Toxicol. 86(3), 345–391 (2012). https://doi.org/10.1007/s00204-011-0774-2
Dai, J.; Mumper, R.J.: Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15, 7313–7352 (2010). https://doi.org/10.3390/molecules15107313
Draoua, Z.; Harrane, A.; Belbachir, M.: Amphiphilic biodegradable poly(ε-caprolactone)-poly (ethylene glycol)–poly(ε-caprolactone) triblock copolymer synthesis by maghnite-H+ as a green catalyst. J. Macromol. Sci. A Pure Appl. Chem. 52(2), 130–137 (2015). https://doi.org/10.1080/10601325.2015.980763
Putaux, J.; Molina-boisseau, S.; Momaur, T.; Dufresne, A.: Platelet nanocrystals resulting from the disruption of waxy maize starch granules by acid hydrolysis. Biomacromol 4, 1198–1202 (2003)
Lin, R.; Li, H.; Long, H.; Su, J.; Huang, W.: Synthesis of rosin acid starch catalyzed by lipase. Biomed. Res. Int. (2014). https://doi.org/10.1155/2014/647068
Almeida, M.; Erthal, R.; Padua, E.; Silveira, L.; Am, L.: response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76, 965–977 (2008). https://doi.org/10.1016/j.talanta.2008.05.019
Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R.: A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 89(3), 217–233 (2011). https://doi.org/10.1016/j.fbp.2010.04.008
Nasir, N.M.; Abdulmalek, E.; Zainuddin, N.: Preparation and optimization of water-soluble cationic sago starch with a high degree of substitution using response surface methodology. Polymers (Basel) 12(11), 1–13 (2020). https://doi.org/10.3390/polym12112614
Ahmad, A., et al.: Box-Behnken response surface design of polysaccharide extraction from rhododendron arboreum and the evaluation of its antioxidant potential. Molecules 25(17), 3835 (2020). https://doi.org/10.3390/molecules25173835
Qin, Y.; Liu, C.; Jiang, S.; Xiong, L.; Sun, Q.: Characterization of starch nanoparticles prepared by nanoprecipitation: influence of amylose content and starch type. Ind. Crop. Prod. 87, 182–190 (2016). https://doi.org/10.1016/j.indcrop.2016.04.038
Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S.: Starch retrogradation: a comprehensive review. Rev. Food Sci. Food Saf. 14, 568–585 (2015). https://doi.org/10.1111/1541-4337.12143
Xin, J.; Wang, Y.; Liu, T.; Lin, K.; Chang, L.; Xia, C.: Biosysthesis of corn starch palmitate by lipase novozym. Int. J. Mol. Sci. 13(6), 7226–7236 (2012). https://doi.org/10.3390/ijms13067226
Pozo, C.; Rodríguez-llamazares, S.; Bouza, R.; Barral, L.; Castaño, J.; Müller, N.: Study of the structural order of native starch granules using combined FTIR and XRD analysis study of the structural order of native starch granules using combined FTIR and XRD analysis. J. Polym. Res. 25, 1–8 (2018). https://doi.org/10.1007/s10965-018-1651-y
Winarti, C.; Surono, I.S.; Uswah, M.: Effect of acid and hydrolysis duration on the characteristics of arrowroot and taro starch nanoparticles. IOP Conf. Ser. Earth Environ. Sci. 309(1), 012039 (2019). https://doi.org/10.1088/1755-1315/309/1/012039
Miskeen, S.; Hong, J.S.; Choi, H.; Kim, J.: Fabrication of citric acid-modified starch nanoparticles to improve their thermal stability and hydrophobicity. Carbohydr. Polym. 253, 117242 (2020). https://doi.org/10.1016/j.carbpol.2020.117242
Cheetham, N.W.H.; Tao, L.: Variation in crystalline type with amylose content in maize starch granules: an X-ray powder diffraction study. Carbohydr. Polym. 36, 277–284 (1998)
Ahmed, I.; Jabeen, M.; Geelani, H.; Ahmad, F.; Saba, I.; Muzaffar, S.: Effect of gamma irradiation on physicochemical properties of Indian Horse Chestnut (Aesculus indica Colebr.) starch. Food Hydrocoll. 35, 253–263 (2014). https://doi.org/10.1016/j.foodhyd.2013.06.002
Zhang, K.; Zhao, D.; Huang, Q.; Huang, J.; Wen, Q.: Physicochemical, structural properties and in vitro digestibility of A- and B-type granules isolated from green wheat and mature wheat starch. Starch-Stärke (2021). https://doi.org/10.1002/star.202100065
Bertoft, E.: Understanding starch structure: recent progress. Agronomy 7, 56 (2017). https://doi.org/10.3390/agronomy7030056
Spinozzi, F.; Ferrero, C.; Perez, S.: The architecture of starch blocklets follows phyllotaxic rules. Sci. Rep. 10(1), 1–16 (2020). https://doi.org/10.1038/s41598-020-72218-w
Lamanna, M.; Morales, N.J.; Lis, N.; Goyanes, S.: Development and characterization of starch nanoparticles by gamma radiation: potential application as starch matrix filler. Carbohydr. Polym. 97(1), 90–97 (2013). https://doi.org/10.1016/j.carbpol.2013.04.081
S. S. Nanoparticles. An investigation of antioxidant and cytotoxic properties of green. no. August 2017 (2015)
Huang, D.; Ou, B.; Prior, R.L.: The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 53, 1841–1856 (2005)
Ahmad, M.; Gani, A.; Hassan, I.; Huang, Q.; Shabbir, H.: Production and characterization of starch nanoparticles by mild alkali hydrolysis and ultra-sonication process. Sci. Rep. 10(1), 1–11 (2020). https://doi.org/10.1038/s41598-020-60380-0
Roche, F.H.: Natural antioxidants exploited commercially (1990)
Cuzzucoli, V., et al.: Intermolecular interaction and solid state characterization of abietic acid/chitosan solid dispersions possessing antimicrobial and antioxidant properties. Eur. J. Pharm. Biopharm. 125, 114–123 (2018). https://doi.org/10.1016/j.ejpb.2018.01.012
Sridhar, K.; Charles, A.L.: Department of tropical agriculture and international cooperation, national pingtung. Food Chem. (2018). https://doi.org/10.1016/j.foodchem.2018.09.040
Cuvelier, M.E.; Berset, C.: Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 30, 25–30 (1995)
Acknowledgements
The authors are grateful for the substantial financial support provided by the General- Direction of Scientific Research and Technology Development (DGRSDT, MESRS, Algeria).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bezzekhami, M.A., Harrane, A., Belalia, M. et al. Green Synthesis of Starch Nanoparticles (SNPs) by Esterification with Rosin Acid Catalyzed by Maghnite-H+ (Algerian Montmorillonite) with Enhanced Antioxidant Activity. Arab J Sci Eng 48, 311–326 (2023). https://doi.org/10.1007/s13369-022-07033-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-022-07033-8