Abstract
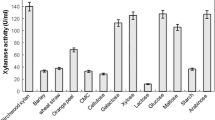
Paper de-inking is one of the critical processes in pulp and paper industry as it is ecofriendly and energy saving. This process requires microbial enzymes such as xylanases with ability to withstand harsh bioprocess conditions. Microbulbifer is a halophilic genus with ability to produce hydrolytic enzymes that could be applied in the biotechnological industry. So far, none of the xylanases from this genus have been studied, particularly in paper de-inking process. Therefore, in this study, the xylanase of a new halophilic bacterium, Microbulbifer sp. strain CL37, was characterized. Strain CL37 produced maximum amount of xylanase at 14th hour of incubation at 30 °C. The xylanase demonstrated optimal activity at 70 °C and pH 7. The xylanase was stable at wide range of NaCl (0–14%, w/v), in the presence of Al3+, Ca2+, Co2+, Cu+, Cu2+, Fe2+, Fe3+, Mn2+, Zn2+, acetone, chloroform, ethanol, sodium deoxycholate, Triton X-100, Tween 20, 40, 60, and 80, indicating that it is a halotolerant enzyme with high stability in various additives. The xylanase also demonstrated its ability to de-ink paper with considerably high efficiency (159%) as compared to other strains. The valuable characteristics possessed by xylanase of strain CL37 could potentially benefit to de-inking process in paper industry.






Similar content being viewed by others
Availability of Data and Material
The 16S rRNA gene (1475 bp) of Microbulbifer sp. strain CL37 is available at GenBank under accession of MK256319.
Code Availability
Not applicable.
References
Enache, M.; Teodosiu, G.; Itoh, T.; Kamekura, M.; Stan-Lotter, H.: Halophilic microorganisms from man-made and natural hypersaline environments: physiology, ecology, and biotechnological potential. In: Stan-Lotter, H.; Fendrihan, S. (Eds.) Adaption of Microbial Life to Environmental Extremes: Novel Research Results and Application, pp. 201–226. Springer International Publishing, Cham (2017)
Yin, J.; Chen, J.-C.; Wu, Q.; Chen, G.-Q.: Halophiles, coming stars for industrial biotechnology. Biotechnol. Adv. 33(7), 1433–1442 (2015)
Zakaria, M.R.; Lam, M.Q.; Chen, S.J.; Karim, M.H.A.; Tokiman, L.; Yahya, A.; Shamsir, M.S.; Chong, C.S.: Genome sequence data of Mangrovimonas sp. strain CR14 isolated from mangrove forest at Tanjung Piai National Park, Malaysia. Data Brief 30, 105658 (2020)
Thevarajoo, S.; Selvaratnam, C.; Goh, K.M.; Hong, K.W.; Chan, X.Y.; Chan, K.-G.; Chong, C.S.: Vitellibacter aquimaris sp. Nov., a marine bacterium isolated from seawater. Int. J. Syst. Evol. Microbiol. 66(9), 3662–3668 (2016)
Singh, S.; Gupta, M.; Gupta, Y.: Microbial life at extreme of salt concentration: adaptation strategies. In: Singh, R.P.; Manchanda, G.; Maurya, I.K.; Wei, Y. (Eds.) Microbial Versatility in Varied Environments: Microbes in Sensitive Environments, pp. 35–49. Springer, Singapore (2020)
Liu, C.; Baffoe, D.K.; Zhan, Y.; Zhang, M.; Li, Y.; Zhang, G.: Halophile, an essential platform for bioproduction. J. Microbiol. Methods 166, 105704 (2019)
Chen, G.-Q.; Jiang, X.-R.: Next generation industrial biotechnology based on extremophilic bacteria. Curr. Opin. Biotechnol. 50, 94–100 (2018)
Thatoi, H.; Behera, B.C.; Mishra, R.R.; Dutta, S.K.: Biodiversity and biotechnological potential of microorganisms from mangrove ecosystems: a review. Ann. Microbiol. 63(1), 1–19 (2013)
Alves, K.J.; da Silva, M.C.P.; Cotta, S.R.; Ottoni, J.R.; van Elsas, J.D.; de Oliveira, V.M.; Andreote, F.D.: Mangrove soil as a source for novel xylanase and amylase as determined by cultivation-dependent and cultivation-independent methods. Braz. J. Microbiol. 51(1), 217–228 (2020)
Lam, M.Q.; Oates, N.C.; Thevarajoo, S.; Tokiman, L.; Goh, K.M.; McQueen-Mason, S.J.; Bruce, N.C.; Chong, C.S.: Genomic analysis of a lignocellulose degrading strain from the underexplored genus Meridianimaribacter. Genomics 112(1), 952–960 (2020)
Liew, K.J.; Teo, S.C.; Shamsir, M.S.; Sani, R.K.; Chong, C.S.; Chan, K.-G.; Goh, K.M.: Complete genome sequence of Rhodothermaceae bacterium RA with cellulolytic and xylanolytic activities. 3 Biotech 8(8), 1–8 (2018)
Chen, S.J.; Lam, M.Q.; Thevarajoo, S.; Abd Manan, F.; Yahya, A.; Chong, C.S.: Genome analysis of cellulose and hemicellulose degrading Micromonospora sp. CP22. 3 Biotech 10(4), 160 (2020)
Teo, S.C.; Liew, K.J.; Shamsir, M.S.; Chong, C.S.; Bruce, N.C.; Chan, K.-G.; Goh, K.M.: Characterizing a halo-tolerant GH10 xylanase from Roseithermus sacchariphilus strain RA and its CBM-truncated variant. Int. J. Mol. Sci. 20(9), 2284 (2019)
Liew, K.J.; Ngooi, C.Y.; Shamsir, M.S.; Sani, R.K.; Chong, C.S.; Goh, K.M.: Heterologous expression, purification and biochemical characterization of a new endo-1, 4-β-xylanase from Rhodothermaceae bacterium RA. Protein Expr. Purif. 164, 105464 (2019)
Singh, B.: Production, characteristics, and biotechnological applications of microbial xylanases. Appl. Microbiol. Biotechnol. 103(21–22), 8763–8784 (2019)
Chakdar, H.; Kumar, M.; Pandiyan, K.; Singh, A.; Nanjappan, K.; Kashyap, P.L.; Srivastava, A.K.: Bacterial xylanases: biology to biotechnology. 3 Biotech 6(2), 150 (2016)
Walia, A.; Guleria, S.; Mehta, P.; Chauhan, A.; Parkash, J.: Microbial xylanases and their industrial application in pulp and paper biobleaching: a review. 3 Biotech 7(1), 11 (2017)
Singh, A.; Varghese, L.M.; Yadav, R.D.; Mahajan, R.: A pollution reducing enzymatic deinking approach for recycling of mixed office waste paper. Environ. Sci. Pollut. Res. 27(36), 45814–45823 (2020)
Saxena, A.; Singh Chauhan, P.: Role of various enzymes for deinking paper: a review. Crit. Rev. Biotechnol. 37(5), 598–612 (2017)
Lee, C.K.; Ibrahim, D.; Omar, I.C.: Enzymatic deinking of various types of waste paper: efficiency and characteristics. Process. Biochem. 48(2), 299–305 (2013)
Maity, C.; Ghosh, K.; Halder, S.K.; Jana, A.; Adak, A.; Mohapatra, P.K.D.; Pati, B.R.; Mondal, K.C.: Xylanase isozymes from the newly isolated Bacillus sp. CKBx1D and optimization of its deinking potentiality. Appl. Biochem. Biotechnol. 167(5), 1208–1219 (2012)
Parte, A.C.: LPSN—–List of Prokaryotic names with Standing in Nomenclature (bacterio.net), 20 years on. Int. J. Syst. Evol. Microbiol. 68(6), 1825–1829 (2018)
Jonnadula, R.; Ghadi, S.C.: Purification and characterization of β-agarase from seaweed decomposing bacterium Microbulbifer sp. strain CMC-5. Biotechnol. Bioprocess. Eng. 16(3), 513–519 (2011)
Liu, H.; Zeng, L.; Jin, Y.; Nie, K.; Deng, L.; Wang, F.: Effect of different carbon sources on cellulase production by marine strain Microbulbifer hydrolyticus IRE-31-192. Appl. Biochem. Biotechnol. 188(3), 741–749 (2019)
Lee, H.-J.; Lee, Y.-S.; Choi, Y.-L.: Cloning, purification, and characterization of an organic solvent-tolerant chitinase, MtCh509, from Microbulbifer thermotolerans DAU221. Biotechnol. Biofuels. 11(1), 1–14 (2018)
Ohta, Y.; Hatada, Y.; Nogi, Y.; Miyazaki, M.; Li, Z.; Akita, M.; Hidaka, Y.; Goda, S.; Ito, S.; Horikoshi, K.: Enzymatic properties and nucleotide and amino acid sequences of a thermostable β-agarase from a novel species of deep-sea Microbulbifer. Appl. Microbiol. Biotechnol. 64(4), 505–514 (2004)
Hatada, Y.; Mizuno, M.; Li, Z.; Ohta, Y.: Hyper-production and characterization of the ι-carrageenase useful for ι-carrageenan oligosaccharide production from a deep-sea bacterium, Microbulbifer thermotolerans JAMB-A94T, and insight into the unusual catalytic mechanism. Mar. Biotechnol. 13(3), 411–422 (2011)
Lam, M.Q.; Vodovnik, M.; Zorec, M.; Chen, S.J.; Goh, K.M.; Yahya, A.; Md Salleh, M.; Ibrahim, Z.; Tokiman, L.; McQueen-Mason, S.J.; Bruce, N.C.; Chong, C.S.: Robertkochia solimangrovi sp. nov., isolated from mangrove soil, and emended description of the genus Robertkochia. Int. J. Syst. Evol. Microbiol. 70(3), 1769–1776 (2020)
Lane, D.J.: 16S/23S rRNA sequencing. In: Stackebrandt, E.M.G. (Ed.) Nucleic Acid Techniques in Bacterial Systematics, pp. 125–175. John Wiley and Sons, Chichester (1991)
Lam, M.Q.; Nik Mut, N.N.; Thevarajoo, S.; Chen, S.J.; Selvaratnam, C.; Hussin, H.; Jamaluddin, H.; Chong, C.S.: Characterization of detergent compatible protease from halophilic Virgibacillus sp. CD6. 3 Biotech 8(2), 104 (2018)
Wright, E.S.; Yilmaz, L.S.; Noguera, D.R.: DECIPHER, a search-based approach to chimera identification for 16S rRNA sequences. Appl. Environ. Microbiol. 78(3), 717 (2012)
Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J.: Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 67(5), 1613–1617 (2017)
Kumar, S.; Stecher, G.; Tamura, K.: MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33(7), 1870–1874 (2016)
Saitou, N.; Nei, M.: The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4(4), 406–425 (1987)
Smibert, R.; Krieg, N.: Phenotypic characterization. In: Gerhardt, P.; Murray, R.; Wood, W.; Krieg, N. (Eds.) Methods for General and Molecular Bacteriology, pp. 607–654. American Society for Microbiology, Washington (1994)
Bailey, M.J.; Biely, P.; Poutanen, K.: Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. 23(3), 257–270 (1992)
Thomas, L.; Ushasree, M.V.; Pandey, A.: An alkali-thermostable xylanase from Bacillus pumilus functionally expressed in Kluyveromyces lactis and evaluation of its deinking efficiency. Bioresour. Technol. 165, 309–313 (2014)
González, J.; Mayer, F.; Moran, M.; Hodson, R.; Whitman, W.: Microbulbifer hydrolyticus gen. nov., sp. nov., and Marinobacterium georgiense gen. nov., sp. nov., two marine bacteria from a lignin-rich pulp mill waste enrichment community. Int. J. Syst. Evol. Microbiol. 47(2), 369–376 (1997)
Moh, T.H.; Furusawa, G.; Amirul, A.A.-A.: Microbulbifer aggregans sp. Nov., isolated from estuarine sediment from a mangrove forest. Int J Syst Evol Microbiol 67(10), 4089–4094 (2017)
Yoon, J.-H.; Kim, I.-G.; Oh, T.-K.; Park, Y.-H.: Microbulbifer maritimus sp. nov., isolated from an intertidal sediment from the Yellow Sea, Korea. Int. J. Syst. Evol. Microbiol. 54(4), 1111–1116 (2004)
Camacho, M.; del Carmen, M.-C.M.; Redondo-Gómez, S.; Rodríguez-Llorente, I.; Schumann, P.; Klenk, H.-P.: Microbulbifer rhizosphaerae sp. nov., isolated from the rhizosphere of the halophyte Arthrocnemum macrostachyum. I. Int. J. Syst. Evol. Microbiol. 66(4), 1844–1850 (2016)
Prakash, S.; Veeranagouda, Y.; Kyoung, L.; Sreeramulu, K.: Xylanase production using inexpensive agricultural wastes and its partial characterization from a halophilic Chromohalobacter sp. TPSV 101. World J. Microbiol. Biotechnol. 25(2), 197–204 (2009)
Giridhar, P.V.; Chandra, T.: Production of novel halo-alkali-thermo-stable xylanase by a newly isolated moderately halophilic and alkali-tolerant Gracilibacillus sp. TSCPVG. Process. Biochem. 45(10), 1730–1737 (2010)
Baumgartner, R.A.; Deramo, V.A.; Beaven, M.A.: Constitutive and inducible mechanisms for synthesis and release of cytokines in immune cell lines. J. Immunol. 157(9), 4087–4093 (1996)
Liu, Z.; Zhao, X.; Bai, F.: Production of xylanase by an alkaline-tolerant marine-derived Streptomyces viridochromogenes strain and improvement by ribosome engineering. Appl. Microbiol. Biotechnol. 97(10), 4361–4368 (2013)
Wang, G.; Wu, J.; Yan, R.; Lin, J.; Ye, X.: A novel multi-domain high molecular, salt-stable alkaline xylanase from Alkalibacterium sp. SL3. Front. Microbiol. 7, 2120 (2017)
Lin, X.; Hetharua, B.; Lin, L.; Xu, H.; Zheng, T.; He, Z.; Tian, Y.: Mangrove sediment microbiome: adaptive microbial assemblages and their routed biogeochemical processes in Yunxiao mangrove national nature reserve, China. Microb. Ecol. 78(1), 57–69 (2019)
Kathiresan, K.: Salt-tolerant microbes in mangroves: ecological role and bioprospecting potential. In: Dagar, J.C.; Yadav, R.K.; Sharma, P.C. (Eds.) Research Developments in Saline Agriculture, pp. 237–255. Springer, Singapore (2019)
Qeshmi, F.I.; Homaei, A.; Fernandes, P.; Hemmati, R.; Dijkstra, B.W.; Khajeh, K.: Xylanases from marine microorganisms: a brief overview on scope, sources, features and potential applications. Biochim. Biophys. Acta Proteins Proteom. 140312 (2019)
Lee, H.J.; Kim, I.J.; Kim, J.F.; Choi, I.-G.; Kim, K.H.: An expansin from the marine bacterium Hahella chejuensis acts synergistically with xylanase and enhances xylan hydrolysis. Bioresour. Technol. 149, 516–519 (2013)
Guo, B.; Chen, X.-L.; Sun, C.-Y.; Zhou, B.-C.; Zhang, Y.-Z.: Gene cloning, expression and characterization of a new cold-active and salt-tolerant endo-β-1, 4-xylanase from marine Glaciecola mesophila KMM 241. Appl. Microbiol. Biotechnol. 84(6), 1107 (2009)
Cai, Z.-W.; Ge, H.-H.; Yi, Z.-W.; Zeng, R.-Y.; Zhang, G.-Y.: Characterization of a novel psychrophilic and halophilic β-1, 3-xylanase from deep-sea bacterium, Flammeovirga pacifica strain WPAGA1. Int. J. Biol. Macromol. 118, 2176–2184 (2018)
Han, Z.; Shang-guan, F.; Yang, J.: Characterization of a novel cold-active xylanase from Luteimonas species. World J. Microbiol. Biotechnol. 34(8), 123 (2018)
Yu, H.; Zhao, S.; Fan, Y.; Hu, C.; Lu, W.; Guo, L.: Cloning and heterologous expression of a novel halo/alkali-stable multi-domain xylanase (XylM 18) from a marine bacterium Marinimicrobium sp. strain LS-A18. Appl. Microbiol. Biotechnol. 103(21–22), 8899–8909 (2019)
Uday, U.S.P.; Majumdar, R.; Tiwari, O.N.; Mishra, U.; Mondal, A.; Bandyopadhyay, T.K.; Bhunia, B.: Isolation, screening and characterization of a novel extracellular xylanase from Aspergillus niger (KP874102.1) and its application in orange peel hydrolysis. Int. J. Biol. Macromol. 105, 401–409 (2017)
Beg, Q.; Kapoor, M.; Mahajan, L.; Hoondal, G.: Microbial xylanases and their industrial applications: a review. Appl. Microbiol. Biotechnol. 56(3–4), 326–338 (2001)
Polizeli, M.; Rizzatti, A.; Monti, R.; Terenzi, H.; Jorge, J.A.; Amorim, D.: Xylanases from fungi: properties and industrial applications. Appl. Microbiol. Biotechnol. 67(5), 577–591 (2005)
Ko, J.K.; Ko, H.; Kim, K.H.; Choi, I.-G.: Characterization of the biochemical properties of recombinant Xyn10C from a marine bacterium, Saccharophagus degradans 2–40. Bioprocess. Biosyst. Eng. 39(4), 677–684 (2016)
Sanjivkumar, M.; Silambarasan, T.; Palavesam, A.; Immanuel, G.: Biosynthesis, purification and characterization of β-1, 4-xylanase from a novel mangrove associated actinobacterium Streptomyces olivaceus (MSU3) and its applications. Protein Exp. Purif. 130, 1–12 (2017)
Liu, X.; Huang, Z.; Zhang, X.; Shao, Z.; Liu, Z.: Cloning, expression and characterization of a novel cold-active and halophilic xylanase from Zunongwangia profunda. Extremophiles 18(2), 441–450 (2014)
Viikari, L.; Alapuranen, M.; Puranen, T.; Vehmaanperä, J.; Siika-Aho, M.: Thermostable enzymes in lignocellulose hydrolysis. In: Olsson, L. (Ed.) Biofuels: Advances in Biochemical Engineering/Biotechnology, Vol. 108, pp. 121–145. Springer, Berlin (2007)
Kumar, V.; Marín-Navarro, J.; Shukla, P.: Thermostable microbial xylanases for pulp and paper industries: trends, applications and further perspectives. World J. Microbiol. Biotechnol. 32(2), 34 (2016)
Torres, G.G.; Figueroa-Galvis, I.; Munoz-Garcia, A.; Polania, J.; Vanegas, J.: Potential bacterial bioindicators of urban pollution in mangroves. Environ. Pollut. 255, 113293 (2019)
Bai, W.; Xue, Y.; Zhou, C.; Ma, Y.: Cloning, expression and characterization of a novel salt-tolerant xylanase from Bacillus sp. SN5. Biotechnol. Lett. 34(11), 2093–2099 (2012)
Hung, K.-S.; Liu, S.-M.; Tzou, W.-S.; Lin, F.-P.; Pan, C.-L.; Fang, T.-Y.; Sun, K.-H.; Tang, S.-J.: Characterization of a novel GH10 thermostable, halophilic xylanase from the marine bacterium Thermoanaerobacterium saccharolyticum NTOU1. Process. Biochem. 46(6), 1257–1263 (2011)
Palavesam, A.: Investigation on lignocellulosic saccharification and characterization of haloalkaline solvent tolerant endo-1, 4 β-d-xylanase from Halomonas meridiana APCMST-KS4. Biocatal. Agric. Biotechnol. 4(4), 761–766 (2015)
Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J.: Strategies for stabilization of enzymes in organic solvents. ACS Catal. 3(12), 2823–2836 (2013)
Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K.: Lipase catalysis in organic solvents: advantages and applications. Biol. Proced. Online 18(1), 2 (2016)
Alhammad, A.; Adewale, P.; Kuttiraja, M.; Christopher, L.P.: Enhancing enzyme-aided production of fermentable sugars from poplar pulp in the presence of non-ionic surfactants. Bioprocess. Biosyst. Eng. 41(8), 1133–1142 (2018)
Wang, F.; Zhang, X.; Zhang, G.; Chen, J.; Sang, M.; Long, Z.; Wang, B.: Studies on the environmentally friendly deinking process employing biological enzymes and composite surfactant. Cellulose 25(5), 3079–3089 (2018)
Holmberg, K.: Interactions between surfactants and hydrolytic enzymes. Colloids Surf. B Biointerfaces 168, 169–177 (2018)
Kamali, M.; Khodaparast, Z.: Review on recent developments on pulp and paper mill wastewater treatment. Ecotoxicol. Environ. Saf. 114, 326–342 (2015)
Acknowledgements
This research was financially sponsored by Industry-International Incentive Grant and Newton Fund Impact Scheme (NFIS) 2020-2021 from Universiti Teknologi Malaysia and Malaysian Industry-Government Group for High Technology with project number 02M34 and 536423090 (RADIS cost centre number for NFIS: 4B568), respectively. This work was supported by the Ministry of Higher Education Malaysia under Fundamental Research Grant Scheme (FRGS/1/2019/WAB13/UTM/02/1). This study was also supported by Universiti Teknologi Malaysia under the Post-Doctoral Fellowship Scheme for the Project: “Harnessing Sustainable Development Opportunities from Oil Palm Empty Fruit Bunch as Food for Black Soldier Fly Larvae”, which granted to Ming Quan Lam. The authors also would like to acknowledge Johor National Parks Corporation for sampling permit (CJB G No. 887005) at Tanjung Piai National Park, Johor. Ming Quan Lam is thankful to Khazanah Watan Postgraduate (PhD) scholarship (scholar ID: 40852) and Post-Doctoral Fellowship Scheme from Yayasan Khazanah and Universiti Teknologi Malaysia, respectively. Ming Hui Mah acknowledges National Postgraduate Fund (NPF) from Universiti Teknologi Malaysia.
Funding
This research was financially sponsored by Industry-International Incentive Grant and Newton Fund Impact Scheme (NFIS) 2020–2021 from Universiti Teknologi Malaysia and Malaysian Industry-Government Group for High Technology with project number 02M34 and 536423090 (RADIS cost centre number for NFIS: 4B568), respectively. This work was supported by the Ministry of Higher Education Malaysia under Fundamental Research Grant Scheme (FRGS/1/2019/WAB13/UTM/02/1).
Author information
Authors and Affiliations
Contributions
Ming Hui Mah performed the experiment, analysed the data, and drafted the manuscript. Ming Quan Lam designed the experiment, assisted in performing the experiment, drafted the manuscript, and provided the expertise. Lili Tokiman, Mohd Farizal Kamaroddin, Zaharah Ibrahim, and Shafinaz Shahir provided the expertise. Chun Shiong Chong conceived the presented idea, designed the experiment, and provided the expertise. All authors read, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Rights and permissions
About this article
Cite this article
Mah, M.H., Lam, M.Q., Tokiman, L. et al. Revealing the Potential of Xylanase from a New Halophilic Microbulbifer sp. CL37 with Paper De-Inking Ability. Arab J Sci Eng 47, 6795–6805 (2022). https://doi.org/10.1007/s13369-021-06400-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-021-06400-1