Abstract
The habitat accommodation model (HAM) is a theoretical framework that predicts wildlife community recovery based on their habitat requirements. While post-fire habitat-related research is well documented in the Mediterranean basin, studies specifically focusing on HAM are scarce. Here, we described the small mammal assemblage in a Mediterranean area ~3 years after a fire, specifically examining three functional small mammalian categories: ground-foraging insectivorous, ground-foraging herbivorous/granivorous, and arboreal-foraging species. The study was conducted in Monte Pisano (Italy), where fire burnt ~12 km2 in September 2018. A stratified random sampling was adopted, basing on burnt status and forest type. In each of the 50 sites, during late spring-summer 2021, 12 hair-tubes were deployed, and collected hairs were taxa-attributed based on morphology. A presence/absence dataset was built, and db-RDA was used to explore assemblage composition, and single-species occupancy models to test specific hypotheses. The relative abundance of ground-foraging herbivorous/granivorous was higher in the burnt area, characterised by a dense undergrowth, which could be related to anti-predatory strategies and food opportunities. Insectivorous could be in a recolonisation phase, masking their earlier absence, which could explain why their abundance was not associated with any factor tested. Arboreal-foraging species were associated with forest type, indicating a primary role for tree cover and other factors such as rocky cover and likely in situ survival. The HAM was overall confirmed also in Mediterranean basin ecosystems. This may facilitate predictions about post-fire animal successions, which in turn may provide valuable insights into post-fire management practices and biodiversity conservation strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
From time immemorial, fires have profoundly shaped ecosystems globally (Prodon 1987; Bowman et al. 2009; Pausas and Keeley 2009; Kelly et al. 2020). In recent decades, as a consequence of climate change, accumulation of biomass, and agricultural land abandonment (Moreira and Russo 2007; Fernandes et al. 2013; Bowman et al. 2020; Jones et al. 2020), fires have become even more frequent and intense, and this pattern is expected to become increasingly pronounced (Flannigan et al. 2009; Bowman et al. 2020; Jones et al. 2020). It is therefore increasingly necessary to understand the multiple and complex impacts on ecosystems and wildlife (Pausas and Parr 2018; González et al. 2022). There are both direct and indirect effects of fires on wildlife (Engstrom 2010).
Direct effects can cause injury or death of individuals by flames, smoke inhalation, or heat stress (Whelan et al. 2002; Nimmo et al. 2021). Fire-induced mortality rates are considered to be low for wildlife (∼3%, Jolly et al. 2022), although it is assumed to vary widely among taxa, due to features such as species vagility, adaptive traits, and shelter location (Pausas and Parr 2018; Nimmo et al. 2019). Small mammals, for instance, are typically thought to be highly vulnerable to fires. However, some burrowing species are known to experience little direct mortality from fires (Vernes 2000; Letnic et al. 2005). Conversely, mortality is typically higher in species that take refuge above ground (Simons 1991; Koprowski et al. 2006). Understanding these dynamics is essential as they play a key role in shaping the overall post-fire recovery process (Puig-Gironès et al. 2018; Hale et al. 2022). Indirect effects include changes in vegetation structure and composition. For instance, early post-fire conditions may involve increased prey mortality due to higher predation rates resulted to increased predation risk, and a limited availability of food resources (Banks et al. 2011; Morris et al. 2011; Leahy et al. 2016).
After a fire, communities go through secondary ecological succession that re-colonises the disturbed ecosystem (Fox 1982; Monamy and Fox 2000). In fire-prone areas such as those of Mediterranean ecosystems, many plant species have evolved adaptive traits that enable them to survive and take advantage of fires (Pausas et al. 2004; Pausas and Keeley 2009; Keeley et al. 2011). Due to these adaptations (e.g., seed germination triggered by fire, Keeley and Pausas 2018) and post-fire environmental conditions (e.g., increases in light and soil nutrients, Keeley and Babr-Keeley 1999), post-fire vegetation recovery in Mediterranean ecosystems is usually quick, and within 2 or 3 years, the undergrowth may become denser than surrounding unburnt areas (Trabaud 1994; Torre and Díaz 2004; Kayes et al. 2010; Tessler et al. 2016). By contrast, reestablishment of the tree canopy may take decades (Keeley et al. 2011; Senf and Seidl 2022).
One of the theoretical frameworks used to understand how faunal communities adapt and respond to changing environmental conditions after fire is the Habitat Accommodation Model (HAM; Fox 1982; Monamy and Fox 2000, 2010). The HAM makes it possible to predict the successional sequence of faunal communities after fire, taking into account crucial habitat requirements for species. According to the HAM, species have a range of tolerance to certain environmental factors, including food availability, temperature, humidity, and other critical parameters that directly affect their survival and reproductive success. These factors are closely linked to different features of vegetation structure, such as height, species composition and density, which in turn affect cover and food availability. As a result, species would enter the post-fire successions when the local vegetation structure meets their environmental requirements, and leave or undergo a numerical reduction when the conditions are outside of their optimal range (Fox 1982, 2022; Monamy and Fox 2010). Among mammals, the model has been confirmed for small mammal assemblage of temperate environments (e.g., Monamy and Fox 2000, 2010; Fox et al. 2003). The HAM is also indirectly supported by additional studies (e.g., Torre and Díaz 2004; Swan et al. 2015; Torre et al. 2022) which, although not directly discussing the HAM, highlight the role of vegetation structure in small mammals post-fire succession.
Other frameworks have been proposed for different environmental conditions, such as the state-and-transition model proposed by Letnic et al. (2004) for arid areas.
However, it is essential to recognise that small mammals’ succession is a complex process influenced by a number of factors. These include the recovery type (i.e., by in situ survival or recolonisation; Puig-Gironès et al. 2018; Hale et al. 2022), the dispersal capacity of the species (Banks et al. 2011, 2017; Swan et al. 2016), and the age and sex of the individuals (Banks et al. 2017; Puig-Gironès and Pons 2023).
Within the small mammal assemblages, ground-foraging granivorous and herbivorous are usually the earliest species joining post-fire secondary succession (Fox 1982; Haim and Izhaki 1994, 2000; Puig-Gironès et al. 2018; Torre et al. 2023). Early successional Mediterranean habitats typically have abundant quantities of seeds and seedlings (Ne’eman et al. 1993), plus dense undergrowth mainly composed of pioneer species (Trabaud 1994; Torre and Díaz 2004; Keeley et al. 2011). Therefore, these mammals may thrive in these early suitable habitats, from both improved feeding conditions and more favourable microclimatic ones, characterised by higher humidity and lower temperatures (Haim and Izhaki 1994; Keeley et al. 2011). However, while overall understorey cover may provide reduced predation risk (Torre and Díaz 2004; Puig-Gironès et al. 2018), this may not be the case with ambush predators (Eby et al. 2013; Gigliotti et al. 2022). In addition, predators may actively react to fires, for example, by hunting where prey are most abundant (i.e., burnt habitats; McGregor et al. 2014; Leahy et al. 2016; Geary et al. 2020), triggering complex predator-prey relationships (Torre and Díaz 2004; Geary et al. 2020; Doherty et al. 2022; Puig-Gironès and Pons 2023).
Insectivores have high requirements in terms of both microclimate and food (Torre and Díaz 2004; Greenberg et al. 2007; Torre et al. 2023), so they could probably be found in mid-successional stages (Fox 1990), although data are scarce (Greenberg et al. 2007; Zwolak and Foresman 2007; Torre et al. 2023). Like herbivorous and granivorous species, they also may face lower predation risk in areas with a thicker herbaceous cover (Torre and Díaz 2004; Puig-Gironès et al. 2018). By contrast, arboreal species have distinct habitat requirements. Indeed, they are usually found in late-successional forests for feeding and nesting needs (Fox 1990; Zwolak and Foresman 2007; Chia et al. 2015; Linnell et al. 2018).
It is known that the post-fire response of small mammal assemblages depends on the type of ecosystem affected by fire and the fire regime (e.g., Diffendorfer et al. 2012; Griffiths and Brook 2014; Chia et al. 2015; Culhane et al. 2022; Puig-Gironès and Pons 2023). Our current knowledge of post-fire small mammal assemblage dynamics is mostly based on studies in Australian and American ecosystems (e.g., Kelly et al. 2011; van Mantgem et al. 2015; Culhane et al. 2022; Hale et al. 2022). By contrast, in other ecosystems, such as the Mediterranean ones in Southern Europe, these dynamics have been poorly studied (Sainz-Elipe et al. 2012; Torre et al. 2022; Puig-Gironès and Pons 2023), such as for other European animal communities (e.g., invertebrates, Santos et al. 2009; Radea and Arianoutsou 2012; reptiles, Santos and Poquet 2010; Santos and Cheylan 2013; birds, Rost et al. 2012; Puig-Gironès et al. 2017, and large mammals, Soyumert et al. 2010, 2020). Although HAM performs well for small mammalian assemblages, we lack knowledge about how fires shape these communities in Mediterranean basin (Puig-Gironès et al. 2018; Torre et al. 2023), and we cannot predict their future impact, when fires are predicted to become more frequent and severe (Jolly et al. 2015; Soyumert et al. 2020). Recognising and addressing this knowledge gap is essential for the comprehensive study of fire ecology, as well as the development of effective biodiversity conservation strategies in fire-prone Mediterranean regions (Syphard et al. 2009; Van Wagtendonk 2009).
Our main aim was to describe the assemblage of small mammals in a Mediterranean area ~3 years after a fire. In addition, we tested specific hypotheses for the three ecological functional groups of small mammals (ground-foraging herbivorous/granivorous, insectivorous and arboreal), as their habitat requirements differ in terms of resources.
Hyp. 1: As food resources and cover provided by restoration of the vegetation of the burnt area could make this environment particularly suitable for ground-foraging herbivorous and granivorous (Torre and Díaz 2004; Puig-Gironès and Pons 2020), we expect ground-foraging herbivorous and granivorous to be more abundant in the burnt areas than in unburnt ones.
Hyp. 2: Taxa such as ground-foraging insectivorous requiring late successional microhabitats characterised by the occurrence of woody debris, moisture, and leaf litter (Greenberg et al. 2007), other than particular food resources (Canova and Fasola 1993; Greenberg et al. 2007), are unlikely to find burnt areas particularly suitable. Therefore, we predict a lower abundance of ground-foraging insectivorous species in burnt compared to unburnt areas.
Hyp. 3: Arboreal species require mature forest for hiding, feeding, and nesting (Wauters et al. 2000; Linnell et al. 2018). In the absence of a canopy, we predict the arboreal species’ abundance to be lower in the highly severe burnt area compared to other areas.
Materials and methods
Study area
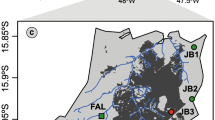
Our study area of ~18 km2 was located in the Monte Pisano mountain system (northern Tuscany, Italy; Fig. 1). The highest peak of the mountain chain is Mt. Serra (917 m). The climate is typical Mediterranean, humid in winter, and arid in summer (Rapetti and Vittorini 1994). The natural vegetal associations of Monte Pisano are dominated mainly by holm oak Quercus ilex L., European hop-hornbeam Ostrya carpinifolia Scop., and common alder Alnus glutinosa L. However, these associations are very reduced, as over time they have been supplanted by olive groves and vineyards, and by introduced chestnut Castanea sativa Mill. and maritime pine Pinus pinaster Aiton forests. At the shrub layer, Mediterranean scrub is common, with species such as tree heather Erica arborea L., green heather E. scoparia L., strawberry tree Arbutus unedo L., sage-leaved rock-rose Cistus salviifolius L., Montpellier cistus C. monspeliensis L., and common gorse Ulex europaeus L. (Bertacchi et al. 2004).
Satellite map of the study area and its location on the Italian peninsula (red star). The 50 squares correspond to cells; the red and green squares were located in the pine and in the chestnut forests, respectively. Triangles correspond to the sampling sites, roughly triangular areas in whose vertices small mammals were sampled. In the figure, the sites were coloured according to the sampling period
The small mammal assemblage of Monte Pisano included 14 shrews and rodent species (Etruscan shrew Suncus etruscus, bicolored shrew Crocidura leucodon, lesser white-toothed shrew C. suaveolens, Sorex sp., house mouse Mus domesticus, wood mouse Apodemus sylvaticus, yellow-necked wood mouse A. flavicollis, Savi’s pine vole Microtus savii, Norway rat Rattus norvegicus, black rat R. rattus, garden dormouse Eliomys quercinus, hazel dormouse Muscardinus avellanarius, edible dormouse Glis glis, and red squirrel Sciurus vulgaris (Perfetti 2009; Santini et al. 2012), in addition to two Talpa spp., the crested porcupine Hystrix cristata, and the hedgehog Erinaceus europaeus (Perfetti 2009).
Monte Pisano is a fire-prone area, and fires are recurrent. In September 2018, a fire burnt ~12 km2 of vegetation in the south-eastern part of the mountain chain, belonging to the municipalities of Calci, Vicopisano, and Buti (Pisa province). The fire was classified as a mixed-severity fire and considered the most extensive fire event affecting Tuscany in the last 25 years. The portion that burnt at high severity consisted mostly of pine forest, whereas the low severity fire mostly affected the chestnut forest, which survived (Salbitano et al. 2020).
At the understorey level, the burnt area had an overall high density of pioneer plants belonging to the Mediterranean scrub. Conversely, the unburnt areas had a more open understorey and were dominated by species such as tree heather in the pine forest, and bracken fern Pteridium aquilinum (L.) Kuhn and Rubus hirtus Waldst. et Kit in the chestnut forest (Bertacchi et al. 2004; Salbitano et al. 2020).
Study species assemblage
Small mammals were used as a model due to their sensitivity to ecological disturbances, which is attributed to their relatively small home ranges and limited dispersal capacity (Swan et al. 2016; Puig-Gironès et al. 2018; Nimmo et al. 2019). Moreover, they are usually highly sensitive to habitat alterations (Catling 1991; Haim and Izhaki 1994), and their specific life-history traits can lead to diverse responses to environmental changes (Grant 1972; Amori et al. 2008).
Shrews and rodents were categorised into ecological functional groups, based on their feeding habits and habitat use, to relate them to post-fire ecological succession patterns (Fox 1982; Torre and Díaz 2004; Amori et al. 2008; Monamy and Fox 2010).
The three functional groups were (i) ground-foraging insectivorous, (ii) ground-foraging herbivorous/granivorous, and (iii) arboreal-foraging species (Table 1).
Sampling design
A synchronic design was used, comparing the burnt area to a neighbouring undisturbed area. A stratified random sampling (Krebs 1989; MacKenzie and Royle 2005) was used, with two factors: “affected by the September 2018 fire” (hereafter “fire”: “yes” vs “no”) and “forest type” (“pine forest” vs “chestnut forest”). Combinations of the two factors resulted in four habitat categories (UP, unburnt pine forest; BP, burnt pine forest; UC unburnt chestnut forest; BC, burnt chestnut forest). A 200 × 200-m grid was superimposed to the area, although only the cells composed by the four habitats were considered for sampling (N = 168). Among them, 50 cells were chosen at random in proportion to the surface area occupied by habitat category (n UP = 11, n BP = 21, n UC = 12, n BC = 6) (Supplementary 1).
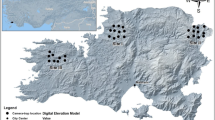
Within each cell, a sampling site (hereafter, site) was selected to be sufficiently distant (>200 m) from all others to be considered independent for target species (Mortelliti et al. 2010) (Fig. 1). The sites consisted of three trees with ∼20 m spacing between them, and approximately at vertices of an equilateral triangle (V1-V2-V3) (Fig. 2A). To survey small mammal assemblage, in each vertex, there was a placement of a set of three hair-tubes (T1-T2-T3), i.e., simple PVC open tubes whose upper internal surface was coated with adhesive tape, containing bait (hazelnut cream, sunflower seeds and mackerel fillets) (Fig. 2D). Hair sampling is an indirect monitoring technique suitable for sampling small mammals (Mortelliti et al. 2010; Chiron et al. 2018; Bertolino et al. 2009). When a small mammal enters the tube, its dorsal hairs adhere to the sticky tape, and are sampled (Suckling 1978; Lindenmayer et al. 1999). These hairs can be examined with an optical microscope to identify species or species-groups (De Marinis and Agnelli 1993; Teerink 2003; Tóth 2017).
Details of the sampling setting used to study the small mammal assemblage ∼3 years after the 2018 fire in Monte Pisano (Tuscany, Italy). A General view of the sampling unit, consisting of three trees spaced ∼20 m apart and approximately at the vertices of an equilateral triangle (V1-V2-V3). Each of the three trees included a pan-pipe (T1 + T2) and a T3 tube. B Focus on a pan-pipe, made by T1 and T2 tubes tied and fixed on the ground. C Focus on a T3 tube, horizontally fixed to a trunk at height ∼1.80 m. D Representation of a hair-tube, a baited open PVC cylinder internally coated with a tape (drawing courtesy of Andrea Pardini)
To optimise sampling, we used tubes of different size for each set: two tubes of 2.5 (T1) and 3.5 cm in diameter (T2), tied together to form a pan-pipe were placed on the ground (Fig. 2B, D), whereas one tube 6 cm in diameter (T3) was horizontally fixed to a trunk at height ∼1.80 m from the ground (Suckling 1978; Pocock and Jennings 2006; Bertolino et al. 2009; Mortelliti et al. 2010; Chiron et al. 2018) (Fig. 2C, D).
The 50 sites were then subdivided in two groups of 25 each, to facilitate sampling and data collection logistics, and surveyed for four weeks in each period, from May 19 to June 18, and from June 23 to July 23, 2021. This sampling period was considered suitable for sampling all the target species (Boitani et al. 1985; Canova 1992; Pocock and Jennings 2006; Torre et al. 2010; Mortelliti et al. 2010; Chiron et al. 2018; Melcore et al. 2020).
During these sampling intervals, each tube was checked once weekly to renew the bait and replace the adhesive tapes. Consequently, each tube was checked four times. The sampling effort amounted to 12,600 days (28 days per tube × 9 tubes × 50 sites), during which 1800 tube stripes were collected (4 checks × 9 tubes × 50 sites).
Laboratory analyses
Hairs were identified morphologically under an optical microscope (250× – 400×). Several atlases (Debrot et al. 1982; Teerink 2003; Tóth 2017), and a hair library were used as references. Hair analysis, together with the known geographical distribution of the target species, enabled classification at the species level (Amori et al. 2008; Santini et al. 2012), although there were a few exceptions where only the genus could be identified (i.e., Crocidura spp. and Apodemus spp.).
In addition, although among Arvicolinae subfamily only Savi’s pine vole has been reported in the study area (Perfetti 2009; Santini et al. 2012), it is possible that one other common species, i.e., the bank vole Clethrionomys glareolus, have recently colonised the study area. For this reason, and because of the difficulty in distinguishing the hairs of these species, for these cases, we preferred refer to them as “voles”.
Key hair traits, as cuticular scales, medulla, length, and colour, were used in hair identification (Debrot et al. 1982; Teerink 2003). To minimise the likelihood of misclassification, assignments were only made when the different diagnostic characters for a given taxon matched exactly.
The hairs were put on a slide that was partially covered with a coating of transparent nail polish. When dry, the hairs were removed and then soaked in cedar oil. The imprints and hairs were examined under an optical microscope to analyse medulla types and the cuticular scale patterns. To analyse the cross-sections of the medulla, hairs were cut at the shield level and then examined under a microscope (Teerink 2003). Although species like insectivorous are not easily recognisable only by hairs microscopic analysis, we have been able to identify them with a deepened analysis of their hair diagnostic traits (De Marinis and Agnelli 1993; Teerink 2003).
Ideally, all hairs of the strips should be identified; however, this was not feasible considering the large number of hairs in many strips (up to hundreds). Apparently, this consideration has not been reported or discussed, except for Pocock and Bell (2011), who assessed just two hairs per strip. Therefore, we sought to determine the minimum number of hairs from each strip to adequately assess the composition of the small mammal assemblage. To address this objective, we built accumulation curves for each of the eight tube-habitat type combinations (the two tube types of the pan-pipes for the four habitats), with four strips chosen randomly, for a total of 32 strips. All hairs from these strips were analysed to build a presence-absence dataset of the species in this sub-sample. Using these data, eight accumulation curves were generated using the “vegan” package 2.6-2 (Oksanen et al. 2013) in R 4.2.1. By observing inflection points of the curves, it was determined that 12 was an adequate minimum number of hairs to analyse to estimate the entire species richness of the strips (Supplementary 2).
Hairs to be identified in the remaining hair-tubes, if more than 12, were selected randomly. To perform this selection, three rectangles of transparent plastic of the sizes matching the three types of strips were prepared. In each rectangle, 12 numbered points were randomly drawn using R software. When retrieving the hairs, plastic rectangles were superimposed on the corresponding strip and hairs closest to each of the points were selected.
Only tubes from one of the vertices of the sites were used, for a total of 600 strips. The chosen vertex was the one named V1 at the beginning of sampling. We chose the tubes of a single vertex because, in a preliminary analysis verified by a concordance test, it was sufficient in representing biodiversity of the entire site (Supplementary 3). To perform the test, we randomly chose ten sites, and of these we identified the hairs of all the tubes, for a total of 360 strips (3 tubes × 3 vertices × 4 checks × 10 sites). To this dataset, we applied the Fleiss’ K-test (Fleiss 1971; Hallgren 2012), by testing the concordance of the three vertices in determining the presence or absence of the different taxa, for each site and check. Furthermore, this concordance result could also be interpreted as a validation: as the concordance was significant for all taxa (k = 0.39–0.82, all p < 0.01), it is reasonable to state that the probability of misclassification was low. The analysis was performed using the “irr” R package 0.84.1 (Gamer et al. 2012). In addition, this sampling effort was comparable with that of literature (e.g., Suckling 1978; Green and Sanecki 2006; Fontúrbel 2010).
Environmental characterisation
In order to better assess the factors influencing the environmental suitability for small mammals in the burnt area, a number of environmental variables identified as potentially influential were recorded (e.g., Pardini et al. 2005; Greenberg et al. 2007; Lee et al. 2008; Mazzamuto et al. 2020; Hale et al. 2022). These included herbaceous, shrubby, and arboreal coverage (%), as well as deadwood, rock and litter (%), soil insolation (lux), soil hardness (cm), and number of trees (Table 2). These environmental characteristics were measured once in the field within four 5 × 5 m plots randomly placed on each site (within the 175 m2 triangular area defined by the three vertices) by three operators, and mean values of each variable were calculated. Phytosociological surveys followed the Braun-Blanquet (1932) method.
In addition, the site distance from burnt area edge (m) was also evaluated, through the QGIS 3.20.3 software (QGIS Development Team 2022), as it could be important in assessing the potential for external recolonisation (do Rosário and da Luz Mathias 2007; Puig-Gironès et al. 2018; Frock and Turner 2018) (Table 2).
Furthermore, a number of factors that may affect not the occurrence but only the detection of small mammal species were also recorded (e.g., McCafferty et al. 2003; Sassi et al. 2015; MacKenzie et al. 2017; Mori et al. 2020). These included the status of recovered tubes (i.e., displaced: yes/no) for each check, the check order (i.e., 1:4), and the site group number (i.e., I, II). Furthermore, daily temperature and rainfall data for the study area were retrieved from the Tuscany Region’s weather website (“Monte Serra” weather station), and weekly averages were calculated. Finally, the average weekly moon phase was calculated with R software 4.2.1 and “sunCalc” R package 0.5.0 (Thieurmel et al. 2019) (Table 2).
Data analyses
Variable selection
In order to include the environmental variables in subsequent statistical analyses while avoiding issues of collinearity, it was necessary to assess the correlations among the variables and to identify a set of independent variables (Zuur et al. 2007, 2009; Borcard et al. 2011). The selection process involved a series of steps.
To account for correlation among site-specific numerical variables, principal component analysis (PCA) was applied. To make our analysis more manageable, we identified a single variable for each principal component, i.e., the most representative one. This variable acted as a proxy, capturing the core information within the group. The first four components of the PCA accounted for 81% of total explained variance and were retained. Variables chosen as proxies were “arboreal cover”, “herbaceous cover”, “deadwood”, and “distance from burnt area edge” (Supplementary 4).
Subsequently, to assess the independence among each principal component and “fire” and “forest” variables, the four PCA proxy variables were used as dependent variables in Wilcoxon-Mann-Whitney tests, as they did not meet requirements for a Student’s t-test. In these tests, the two factors “fire” and “forest type” were treated as independent variables. From these tests, the only variables not associated to both “fire” and “forest type” were “deadwood” and “distance from burnt area edge”, and then were retained (Supplementary 5, 6).
In addition, we also used Pearson’s coefficient (R) to identify and select variables that had low or no correlation with each other among the numeric observation variables, namely, variables whose values may be changed during sampling (i.e., check order, weekly rain, temperature, and moon phase) (MacKenzie et al. 2017). In this case, PCA was not used due to its unsuitability for this analysis, as its Kaiser-Meyer-Olkin (KMO) value was too low (KMO = 0.34) (Zuur et al. 2007; Kassambara 2017). As weekly mean moon phase and rain variables were correlated (R = −0.78, p < 0.05), only weekly mean moon phase, temperature, and check order were retained as numeric observation variables. Between the weekly moon phase and rain, the former was chosen because of its greater documented ecological role (Maestri and Marinho 2014; Mori et al. 2020).
Through this approach, a number of independent variables that may represent significant environmental drivers, in addition to “fire” and “forest”, were included in the following statistical analyses.
Assemblage analyses (db-RDA)
Statistical analyses were conducted only for highly sampled taxa (Legendre and Gallagher 2001; Baker et al. 2009; Borcard et al. 2011; MacKenzie et al. 2017), i.e., those for which the Hair Index (HI, Pocock and Jennings 2006; Chiron et al. 2018), calculated by dividing the number of sites where the taxa were recorded by the total number of sites, exceed 0.1. These included Crodicura spp., house mouse, Apodemus spp., voles, garden dormouse, and red squirrel (Supplementary 3).
For them, a site-detection dataset was built, in which 1 was used if the taxon was recorded in at least one tube per site, and 0 if it was not recorded in any tube.
Initially, a comprehensive survey was carried out to examine the overall relationships between the small mammal assemblage and the four selected environmental variables, and to minimise the number of variables to be tested in the taxa-specific statistical models. Distance-based Redundancy Analysis (db-RDA) was used; it is a constrained ordination technique that models linear relationships among environment predictors and community data (Borcard et al. 2011). Starting from the binary detection history dataset, a Jaccard dissimilarity matrix was built. This was done using the Jaccard dissimilarity index, a binary index suitable for our input data and commonly used by ecologists (e.g., Lekberg and Waller 2016; Ricotta et al. 2016; Stone and Jackson 2016).
The two independent environmental variables were standardised, and “fire” and “forest type” were added to them (fire: 0 = UB, 1 = B; forest type: 0 = C, 1 = P).
The significance of the model, axes, and variables were tested with a Monte Carlo permutation test (999 randomisations). The analysis was done with the “vegan” R package 2.6-1.
Single species response models
In addition to the assemblage analysis, the small mammal taxon-specific response was investigated. This approach was chosen because it provides a more in-depth perspective, allowing us to gain deeper insights into the post-fire ecological dynamics within the small mammal assemblage (Mata et al. 2017).
As with the assemblage analysis, poorly sampled taxa (HI ≤ 0.1) were excluded from these investigations (MacKenzie et al. 2017). Single-species occupancy models (Dorazio and Royle 2005; Kéry and Royle 2008) were used as they are suitable for presence-absence data, and they also detach estimation of detection probability (p) from that of true occupancy (ψ) (MacKenzie et al. 2002, 2017).
Since sites were far enough away from each other, in order to be considered independent for the target taxa, occupancy was interpreted as a relative abundance estimation (MacKenzie and Nichols 2004).
For each taxon, using both site and observation variables, best detection probability (p) model was identified in terms of Akaike’s information criterion corrected for small samples (AICc; Burnham and Anderson 2002) through backward selection. By setting the p-related part of the models, one-three specific hypotheses were tested per taxon on variables affecting the probability of occurrence (ψ). For all the taxa, we also tested a model with the best detection probability (p) and the only intercept for the occupancy (ψ), and the null model for p and ψ as a baseline comparison. Variables for the models of each taxon were chosen based on Hypotheses 1, 2, and 3 (fire, forest type) and on db-RDA output. Specifically, only those variables that were highly correlated with at least one taxon (angles between vectors close to 0° or 180°) were added. In particular, for the house mouse, Apodemus spp. and voles, we conducted only a test, with fire as the only explanatory variable. Likewise, for the red squirrel, we tested only a model with forest as the sole explanatory variable. In the case of the Crocidura spp., we tested three different models: one with fire, another with deadwood, a third with both variables together as explanatory variables. Similarly, for the garden dormouse, we tested three different models: one relying solely on forest as explanatory variable, another including deadwood, and a third considering both variables simultaneously. In total, 22 occupancy models were tested.
Model selection was performed through AICc. Only the most supported models were considered (ΔAICc < 2.00). In the case of equally supported models, the model with the highest Akaike weight was selected (Burnham and Anderson 2002). All analyses used the R software and R “unmarked” package 1.2.5 (Fiske and Chandler 2011).
Results
Of the 600 tubes examined, N = 329 (54.83%) tested positive for at least one small mammal taxon, and a total of N = 2104 hairs were identified. The number of tubes and hairs per habitat type are only indicative of sampling effort and may not reflect the true distribution and abundance of the species (Table 3). In fact, the number of tubes reported may refer to the same site, and the number of hairs per tube is not reliable as abundance index. However, these data can still provide some useful information. The 2104 hairs identified indicated a sufficient sample size for statistical analysis. On average, the ratio of hairs to tubes for each taxon was 5.08. This suggests that the likelihood of misidentification was generally low. However, there were few exceptions (e.g., Crocidura spp. in UC (ratio = 1), voles in UC (ratio = 1.67)). Black rat, hazel dormouse, and edible dormouse were poorly sampled overall, but 83.3–100% of the hair-tubes in which they were sampled belonged to the same habitat, suggesting a degree of habitat selection that should be addressed in further research (Table 3).
Small mammal assemblage analysis
Constrained variance explained by the db-RDA model amounted to 38.9%. Monte Carlo tests indicated the significance of the model (F = 6.68, p < 0.001), the first two axes (first: F = 16.98, p < 0.001; second: F = 8.16, p = 0.002), and four variables (fire: F = 12.71, p < 0.001; forest type: F = 9.98, p < 0.001; deadwood: F = 2.71, P = 0.038; and distance from burnt area edge: F = 1.34, P = 0.263) (Fig. 3).
Db-RDA triplot, based on Jaccard dissimilarity, indicating the small mammal assemblage sampled through hair-tubes. The black dots represent sites, arrows represent environmental variables, and labels represent taxa. Their positions in the triplot depends on their correlation with the two axes. The environmental variables were standardised, and the two main factors of the sampling design were added: “fire” (0 = UB, 1 = B) and “forest type” (0 = C, 1 = P). The graph was drawn with scaling: angles between the vectors (variables and taxa) express their correlation (0°: maximum positive correlation, 90°: no correlation, 180°: maximum negative correlation)
Crocidura spp. appeared to be mainly associated with fire and deadwood; house mouse and voles were linked to fire; garden dormouse was influenced by forest type and deadwood; and red squirrel was related just with forest type. However, Apodemus spp. had no associations (Fig. 3).
Single small mammal taxon response
Occupancy models were fitted for five of six taxa, as the red squirrel models did not converge. Consequently, our inferences for these taxa were based on db-RDA only. The occupancy of Crocidura spp. (mean estimated ψ = 0.67 ± 0.23 (SE), mean estimated p = 0.14 ± 0.06), the only ground insectivore modelled, was not significantly dependent on any factor tested (Table 4; Table 5).
Nonetheless, there were differences among ground granivores. Occupancy of Apodemus spp. (mean estimated ψ = 0.95 ± 0.034, mean estimated p = 0.77 ± 0.046) was not significantly associated with any factor (Table 4; Table 5). By contrast, house mouse (mean estimated ψ = 0.72 ± 0.09, mean estimated p = 0.40 ± 0.08) and voles (mean estimated ψ = 0.52 ± 0.16, mean estimated p = 0.20 ± 0.07) occupancy values were higher in the burnt area (house mouse: ψ = 0.95, CIs = 0.64–0.99, p = 0.02; voles : ψ = 0.86, Cis = 0.45–0.97, p < 0.01) (Table 4; Table 5; Fig. 4A, B).
Predicted occupancy probability (ψ) of small mammal’s taxa, sampled through hair-tube sampling in Monte Pisano (Tuscany, Italy) between May 19 and July 23 2021. Predictions were carried out on the best-supported model of each taxon. A House mouse occupancy probability (ψ) in relation to fire; B voles occupancy probability (ψ) in relation to fire; C garden dormouse occupancy probability (ψ) in relation to forest type. Values were back-transformed in probability scale. The dots and solid lines are predicted mean values, whereas the vertical bars are 95% confidence intervals (95% CIs)
Lastly, among arboreal species, for garden dormouse, occupancy (mean estimated ψ = 0.39 ± 0.12, mean estimated p = 0.48 ± 0.09) increased in pine forest (ψ = 0.54, Cis = 0.31–0.76, p < 0.05) (Table 4; Table 5; Fig. 4C).
Discussion
Overall, the small mammalian assemblage structure mainly depended on burnt status and forest type. Ground-foraging herbivorous/granivorous were more abundant in the recently burnt area, whereas insectivore abundance was not different among investigated variables. Arboreal species were mainly associated with forest type, albeit in different ways. Consequently, only Hypotheses 1 and 3 were supported, albeit partially.
Ground-foraging herbivorous/granivorous taxa
In general, ground-foraging herbivorous/granivorous were more abundant in burnt versus unburnt area; the former had denser undergrowth, where ground-foraging herbivorous/granivorous could hide better from predators and had more food availability (Torre and Díaz 2004; Swan et al. 2015; Puig-Gironès et al. 2018; Torre et al. 2023). This preference for burnt area was evident for house mouse and voles. These two taxa colonised the recently burnt area with a large number of individuals and fulfilled the predictions of HAM for ground-foraging herbivorous/granivorous species (Fox 1982; Monamy and Fox 2010). In fact, according to the HAM, the availability of post-fire resources, such as dense vegetation cover and increased seed abundance, likely facilitated the recovery observed in these two taxa. These findings regarding house mouse corroborate several studies from Australia, where this species is relevant to fire-related research (e.g., Kelly et al. 2010, 2012; Fox 2022; Hale et al. 2022), as well as several studies about other Mus species, as the Algerian mouse M. spretus (Puig-Gironès et al. 2018; Torre et al. 2023) and the Macedonian mouse M. macedonicus (Izhaki et al. 1993; Haim and Izhaki 1994). In fact, Mus sp. are considered to be well adapted to xeric habitats, and their opportunistic foraging made them optimal pioneer species (Haim and Izhaki 1994; Puig-Gironès and Pons 2023).
However, studies on voles in Mediterranean ecosystems are scarce; although, according to Arrizabalaga et al. (1993), the Mediterranean pine vole Microtus duodecimcostatus occupied a large part of the burnt area 3 years after a fire in Catalonia (Spain). Do Rosário and da Luz Mathias (2007) supported the role of vegetation structure for the Cabrera vole M. cabrerae, which recolonised the burnt area 1 year after the fire. Likewise, Puig-Gironès et al. (2020) found the common vole M. arvalis abundant in post-fire logged areas, where the cover was dense and food resources were abundant. Therefore, our results appeared consistent with the literature.
The observed consistency of responses to fire among different Mus species and voles, together with their relevance to both Australian and European Mediterranean ecosystems, underscores their importance as key agents in early post-fire community dynamics. This convergence of results reinforces the ecological principles underlying the HAM, and its applicability to a wide range of fire-prone Mediterranean ecosystems. In view of their presumed elevated mortality during fires (Griffiths and Brook 2014; Jolly et al. 2022), the recovery of these species probably occurred through recolonisation from unburnt areas (Puig-Gironès et al. 2018).
By contrast, Apodemus spp. were widespread throughout the study area. In the Mediterranean basin, the wood mouse usually occurs in a burnt area starting soon after a fire (Torre and Díaz 2004; Puig-Gironès et al. 2018), such as the yellow-necked wood mouse (Izhaki et al. 1993). Our results, in line with literature, can be attributed to the highly ecological plasticity and generalist habits of Apodemus spp., which allow them to adapt to even the least advantageous conditions (Sainz-Elipe et al. 2012; Torre et al. 2023).
In turn, the entering of Apodemus spp. in the post-fire succession may have influenced the other early species through competitive relationships (Monamy and Fox 2010; Fox 2022). The potential for competition for resources should be reduced with voles due to different ecological requirements (Amori et al. 2008; Puig-Gironès et al. 2020). However, competition is known to occur with the house mouse (Boitani et al. 1985) and other Mus sp. (Haim and Izhaki 1994; Bauduin et al. 2013). As Apodemus spp. is regarded as dominant over the house mouse and is known to be highly adaptable, we predict that as post-fire succession progresses, the house mouse population is likely to be gradually displaced by Apodemus spp., in line with the HAM.
Arboreal-foraging species
Fires have the capability to destroy mature forests, with potentially detrimental effects for forest species such as arboreal-foraging ones (Zwolak and Foresman 2007; Chia et al. 2015). However, these species are able to survive fires (Koprowski et al. 2006; Mazzamuto et al. 2020; Mazzella and Koprowski 2020).
Both arboreal species investigated depended more on the forest type than on fire occurrence. For the red squirrel, however, this is easily explained by taking fire severity into account.
This species was more abundant in chestnut forest, a habitat characterised by structural complexity, high connectivity among branches, and dense canopy even in the burnt part, as it burnt only at low severity. It is known that the red squirrel prefers this type of habitat over more open ones, such as burnt and unburnt pine forests, which offer it better opportunities for feeding, nesting and hiding (Gurnell et al. 2002; Amori et al. 2008; Flaherty et al. 2012). In the chestnut forest, most of the red squirrels may have survived the fire, mainly thanks to their arboreal locomotion (Amori et al. 2008; Flaherty et al. 2012; Jolly et al. 2022). In addition, their recovery in the severely burnt habitat, with scarcity of food resources and nesting sites, is likely to be very slow (Lee and Rhim 2012; Linnell et al. 2018).
On the other hand, the garden dormouse exhibited higher abundance in the pine forest. This was an unexpected finding that suggests a complex scenario. In the burnt pine forest, the occurrence of the garden dormouse could be attributed to the survival to the fire of at least part of its population, which could have started the post-fire recovery process (Fons et al. 1993, 1996). In fact, although small mammals typically face high mortality rates during fires, in situ survival can be an effective mechanism for recovery (Banks et al. 2011; Hale et al. 2022). The survival to the fire by this species may have been facilitated by its ability to store food, its capacity to hibernation and preference for rocky terrain (Fons et al. 1993; Bertolino et al. 2003; Bertolino and Cordero di Montezemolo 2007). In fact, the burnt pine forest was rich of rocky habitats, in whose crevices the garden dormouse might have survived (Fons et al. 1993).
However, external recolonisation cannot be ruled out, especially considering the relatively small size of the burnt area and the dispersal ability of the garden dormouse (Bertolino and Cordero di Montezemolo 2007). These two hypotheses are not mutually exclusive (Banks et al. 2011; Puig-Gironès et al. 2018). Furthermore, this partially ground-foraging species (Bertolino et al. 2003) may have opportunistically exploited the dense undergrowth cover provided by the burnt canopy-lacking pine forest. However, its absence from the chestnut forest was unexpected and warrants further investigation.
Ground-foraging insectivorous taxa
Ground-foraging insectivorous were the least represented group, among the three identified, in terms of specific richness. Indeed, Crocidura spp. was the only sampled and analysed taxon. For Crocidura spp., abundance was not significantly different between burnt and unburnt areas.
In the immediate post-fire period, relative abundances of insectivorous are usually higher in unburnt versus burnt areas (Buech 1977; Zwolak and Foresman 2007). Regardless of this, microclimate is decisive, as in burnt litter-free areas, the soil would be too dry to meet a insectivorous high water requirements (Kirkland Jr 1991; Greenberg et al. 2007; Zwolak and Foresman 2007). In the forests of Mediterranean basin, there was evidence supporting this fact for the greater white-toothed shrew Crocidura russula (Arrizabalaga et al. 1993; Fons et al. 1993; Torre et al. 2023) and for the lesser white-toothed shrew C. suaveolens (Haim et al. 1997). However, the hypothesis of a lower abundance of ground-foraging insectivorous in the burnt area was not supported by the data. This could be due to ongoing recolonisation, perhaps accelerated by the relatively small size of the burnt portion of the study area, as reported by Haim (2002) for Mount Carmel (Israel). This interpretation was consistent with pilot sampling that we conducted in the burnt part of the study area, 1 year after the fire. Live-trapping sampling was performed with Sherman and Heslinga trap models, targeting ground-foraging herbivorous, granivorous, and insectivorous. Of 137 small mammals captured, not a single insectivorous was caught (Tomassini et al., unpubl. data), suggesting their lack from the early successional phases, in accordance with HAM (Fox 1982; Monamy and Fox 2010; Torre and Díaz 2004). In addition, it must be acknowledged that burnt habitat may not be still so unsuitable, 3 years after the fire. Factors such as increased vegetation cover and the accumulation of deadwood on the ground can potentially compensate for other unfavourable microhabitat characteristics, such as moisture, that may have initially posed a challenge to recolonisation (Kirkland Jr 1991; Greenberg et al. 2007). Moreover, recolonisation by arthropods, an essential component of the shrew’s diet, may have occurred at an enhanced rate, likely influenced by the relatively small size of the fire (Radea and Arianoutsou 2012; Ferrenberg et al. 2019).
Small mammal assemblage
Results obtained from habitats investigated indicated almost the same taxa composition. Nevertheless, we detected differences in the abundances of the assemblage, and, in some cases, even within functional groups. Therefore, the community structure of small mammals was differentiated in the four habitats. Differences of abundances in burnt and unburnt areas have been reported, and they usually reflect secondary successional stages (Fox 1982; Torre and Díaz 2004; Monamy and Fox 2010). However, the extent of these differences across the Mediterranean basin is poorly known, and may depend on fire regime features (Diffendorfer et al. 2012; Culhane et al. 2022), post-fire interval (Kelly et al. 2011; Torre et al. 2022), and features of local fauna (Griffiths and Brook 2014; Chia et al. 2015).
That the fire was relatively small may have favoured recovery phenomena in all habitats (Haim 2002; van Mantgem et al. 2015), even if we cannot exclude species in situ that survived in underground shelters (Arrizabalaga et al. 1993; Puig-Gironès et al. 2018; Hale et al. 2022). The four investigated habitats differed significantly in terms of undergrowth and tree cover; this difference may have had a key role in small mammals’ succession and abundance divergences (Fox 1982; Torre and Díaz 2004; Monamy and Fox 2010; Culhane et al. 2022; Torre et al. 2023).
From an ecological perspective, these differences in abundance have notable implications. Arboreal species and most of the ground-foraging herbivorous/granivorous exhibited greater differences in abundance among habitats, indicating potential sensitivity to microhabitat variation. In contrast, ground-foraging insectivorous species and Apodemus spp. showed a more homogeneous distribution. These results suggest that post-fire habitat heterogeneity may exert selective pressures on small mammal assemblages, potentially driving shifts in their composition and structure (Haim and Izhaki 1994; Fox 2022; Torre et al. 2022). Such insights are essential for understanding the complex interactions among fire regimes, habitat characteristics, and small mammal population dynamics, ultimately contributing to more informed conservation and management strategies in fire-prone areas (Whelan et al. 2002; Syphard et al. 2009; Van Wagtendonk 2009; Puig-Gironès and Pons 2023).
Detectability effects
The statistical models used revealed interesting effects of different variables on the detection of several taxa. Fire also played a key role in the detection of four of the five taxa tested, with three of them showing increased detectability in burnt areas (i.e., Crocidura spp., house mouse, Apodemus spp.). It is reasonable to assume that in post-fire environments with denser undergrowth, individuals of these species may perceive reduced predation risk (Mandelik et al. 2003), resulting in increased locomotory activity and leading to more frequent visits to the hair-tubes. Conversely, the garden dormouse showed increased detectability in unburnt areas, may be due to increased tree connectivity (Mazzamuto et al. 2020), potentially favouring locomotion and visits to the hair-tubes. Similarly, detectability for Crocidura spp., Apodemus spp. and voles increased with increasing moon phase, indicating greater nighttime brightness. This effect was unexpected, but could be explained by improved visual acuity resulting in increased foraging activity, leading individuals into the baited hair-tubes (Prugh and Brashares 2010; Maestri and Marinho 2014). However, lunar phases could mask another effect related to rainfall. Indeed, periods with brighter nights coincided with more rainfall. The hypothesised increase in activity could therefore be due to a reduced predation risk, which is typically lower during rainy periods (McCafferty et al. 2003). Furthermore, some studies support an increase in invertebrates activity during these periods (Naxara et al. 2009; Maestri and Marinho 2014), possibly leading to synchronisation with small mammals, especially insectivores such as Crocidura spp. Unfortunately, due to the high correlation between lunar phases and rainfall during our study period, it was not possible to disentangle their effects. Further research is therefore needed. Finally, the house mouse and voles showed higher detectability at higher environmental temperatures, which could be due to an increase of locomotory activity levels. The opposite effect is more commonly observed in rodents (Sassi et al. 2015; Wróbel and Bogdziewicz 2015); however, the effect found could be due to an anti-predatory strategy, to reduce the temporal overlap with predators (Cavallini and Lovari 1991; Zalewski 2000).
In conclusions, several variables, both environmental and meteorological, showed an effect on the detection probability (p) of small mammals sampled with hair-tubes. Therefore, a statistical approach that takes this into account (e.g., occupancy models; MacKenzie et al. 2002, 2017) should be used, also in future studies.
Limitations of the study
To test the hypotheses related to the HAM, our study focused exclusively on a single post-fire period, nearly 3 years after the fire, and no data were available prior to this period. Consequently, it is crucial to acknowledge that our findings could potentially be affected by population trends. Indeed, elucidating the role of population dynamics in shaping these associations remains a major challenge. Some taxa may have survived the fire event, starting an in situ recovery, whereas others may have colonised the burnt area at a later stage (do Rosário and da Luz Mathias 2007; Banks et al. 2011; Puig-Gironès et al. 2018; Hale et al. 2022). In addition, unsampled species may have occupied earlier post-fire successional stages and potentially influenced the small mammal assemblage, for example through competitive interactions (Monamy and Fox 2010; Fox 2022). Hence, the main limitation of the study was to not investigate also earlier post-fire intervals.
Likewise, we encourage future investigations to broaden the focus to encompass later successional stages over both medium and long-term periods (Kelly et al. 2011; Torre et al. 2022). Combining these analyses with evaluations of the role of environmental variables (e.g., habitat structure), and with an exploration of post-fire successional dynamics across diverse ecosystems around the world, represents, in our view, the most comprehensive approach for advancing our understanding of the intricate interplay between wildfire events and small mammal assemblages.
Concluding remarks
The HAM, supported in the Mediterranean ecosystems of Australia, California (USA), and South Africa (Fox et al. 1985; Monamy and Fox 2010; Culhane et al. 2022; Torre et al. 2022), was also supported in a Mediterranean basin area, probably due to the shared temperate climate.
In Mediterranean-type ecosystems (MTEs), which are characterised by a strong climatic seasonality with rainy winters and dry summers (Aschmann 1973; Keeley et al. 2011), the key to understanding the support for the HAM may lie in the complex interplay between climatic conditions, vegetation dynamics and ecological interactions. These ecosystems exhibit a unique ecological pattern in which the winter-spring vegetation period is particularly prolonged, resulting in exceptionally high primary productivity during the first springs after fire (Trabaud 1994; Keeley et al. 2011). As a consequence, there is usually a marked increase in the early post-fire understorey cover, which is shared by the different MTEs (e.g., Kayes et al. 2010; Tessler et al. 2016; Nalliah et al. 2022). This increased understorey cover should, in turn, affect microhabitat features and resource availability, which in turn might affect the secondary succession of specific ecological groups (e.g., ground-foraging herbivorous, insectivorous, and arboreal species). Confirming this, HAM did not find support in semi-arid ecosystems, which are different in terms of climate (Bradstock and Cohn 2002; Letnic et al. 2004; Kelly et al. 2011). Other theoretical frameworks may therefore be more appropriate in these circumstances (Letnic et al. 2004; Kelly et al. 2011).
However, unexpected patterns emerged related to divergences from HAM, e.g., for Crocidura spp. and garden dormouse. Clearly, there are still knowledge gaps regarding the effects of fires on animal communities. For instance, the role of recovery type (i.e., through in situ survival or external recolonisation; Banks et al. 2011; Puig-Gironès et al. 2018; Hale et al. 2022) and its interactions with microhabitat features (Bertolino and Cordero di Montezemolo 2007; Greenberg et al. 2007; Frock and Turner 2018), resources availability (Radea and Arianoutsou 2012; Ferrenberg et al. 2019) and population dynamics (Haim and Izhaki 1994; Bauduin et al. 2013; Fox 2022) remain to be disentangled.
To our knowledge, this was the first study conducted in Italy that evaluated the post-fire successional status of an assemblage of small mammals, and one of the few conducted in the Mediterranean basin (Torre and Díaz 2004; Torre et al. 2022; Puig-Gironès and Pons 2023). By shedding light on the dynamics of post-fire recovery in this region, our study provides valuable insights into fire management practices and biodiversity conservation strategies.
The urgency of this research is highlighted by the increasing frequency and severity of wildfires worldwide, especially in the Mediterranean area (Bowman et al. 2020; Flannigan et al. 2009; Turco et al. 2018). With climate change exacerbating these trends, the effects of fire on animal communities are expected to escalate.
However, there is a significant geographical gap in this area of research, with many studies conducted primarily in Australia and North America (Geary et al. 2020; Jolly et al. 2022). While these regions have provided useful information into the effects of wildfires on wildlife, it is crucial to broaden our understanding by focusing on less studied, but ecologically relevant, high-fire-risk ecosystems such as those of the Mediterranean basin. Studying the effects of fire on wildlife in the Mediterranean basin can provide important insights into adaptation, conservation, and management strategies specific to this increasingly fire-prone region.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Amori G, Contoli L, Nappi A (2008) Fauna d’Italia: Mammalia II, Edizioni Calderini de Il Sole
Arrizabalaga A, Montagud E, Fons R (1993) Post-fire succession in small mammal communities in the Montserrat Massif (Catalonia, Spain). Fire Mediter Ecosyst 5:281–291
Aschmann H (1973) Distribution and peculiarity of Mediterranean ecosystems. In: Di Castri F, Mooney HA (eds) Mediterranean type ecosystems: origin and structure. Springer, pp 11–19
Baker KL, Langenheder S, Nicol GW et al (2009) Environmental and spatial characterisation of bacterial community composition in soil to inform sampling strategies. Soil Biol Biochem 41:2292–2298
Banks SC, Dujardin M, McBurney L et al (2011) Starting points for small mammal population recovery after wildfire: recolonisation or residual populations? Oikos 120:26–37. https://doi.org/10.1111/j.1600-0706.2010.18765.x
Banks SC, McBurney L, Blair D et al (2017) Where do animals come from during post-fire population recovery? Implications for ecological and genetic patterns in post-fire landscapes. Ecography 40:1325–1338
Bauduin S, Cassaing J, Issam M, Martin C (2013) Interactions between the short-tailed mouse (Mus spretus) and the wood mouse (Apodemus sylvaticus): diet overlap revealed by stable isotopes. Can J Zool 91:102–109
Bertacchi A, Sani A, Tomei PE (2004) La vegetazione del Monte Pisano. Felici Editore, Pisa
Bertolino S, Cordero di Montezemolo N (2007) Garden dormouse (Eliomys quercinus) nest site selection in an alpine habitat. Ethol Ecol Evol 19:51–60
Bertolino S, Cordero N, Currado I (2003) Home ranges and habitat use of the garden dormouse (Eliomys quercinus) in a mountain habitat in summer. Acta Zool Acad Sci Hungaricae 49:11–18
Bertolino S, Wauters L, Pizzul A et al (2009) A general approach of using hair-tubes to monitor the European red squirrel: a method applicable at regional and national scales. Mamm Biology 74:210–219. https://doi.org/10.1016/j.mambio.2009.02.003
Boitani L, Loy A, Molinari P (1985) Temporal and spatial displacement of two sympatric rodents (Apodemus sylvaticus and Mus musculus) in a Mediterranean coastal habitat. Oikos 45:246–252. https://doi.org/10.2307/3565711
Borcard D, Gillet F, Legendre P (2011) Numerical ecology with R. Springer, New York
Bowman DM, Kolden CA, Abatzoglou JT et al (2020) Vegetation fires in the Anthropocene. Nat Rev Earth Environ 1:500–515
Bowman DMJS, Balch JK, Artaxo P et al (2009) Fire in the Earth system. Science 324:481–484. https://doi.org/10.1126/science.1163886
Bradstock RA, Cohn JS (2002) Fire regimes and biodiversity in semi-arid mallee ecosystems. In: Bradstock RA, Williams JE, Gill AM (eds) Flammable Australia: the fire regimes and biodiversity of a continent. Cambridge University Press, Cambridge, pp 238–258
Braun-Blanquet J (1932) Plant sociology. The study of plant communities. McGraw-Hill Book Co
Buech RR (1977) Small mammal populations after a wildfire in northeast Minnesota. Department of Agriculture, Forest Service, North Central Forest Experiment Station
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Canova L (1992) Distribution and habitat preference of small mammals in a biotope of the north Italian plain. Ital J Zool 59:417–420
Canova L, Fasola M (1993) Food habits and trophic relationships of small mammals in six habitats of the northern Po plain (Italy). Mammalia 57:189–199
Catling PC (1991) Ecological effects of prescribed burning practices on the mammals of southeastern Australia. In: Lunney D (ed) Conservation of Australia’s forest fauna. Royal Zoological Society of New South Wales, Mosman, pp 353–363
Cavallini P, Lovari S (1991) Environmental factors influencing the use of habitat in the red fox, Vulpes vulpes. J Zool 223:323–339. https://doi.org/10.1111/j.1469-7998.1991.tb04768.x
Chia EK, Bassett M, Nimmo DG et al (2015) Fire severity and fire-induced landscape heterogeneity affect arboreal mammals in fire-prone forests. Ecosphere 6:1–14
Chiron F, Hein S, Chargé R et al (2018) Validation of hair tubes for small mammal population studies. J Mammal 99:478–485. https://doi.org/10.1093/jmammal/gyx178
Culhane K, Sollmann R, White AM et al (2022) Small mammal responses to fire severity mediated by vegetation characteristics and species traits. Ecol Evol 12:e8918
De Marinis AM, Agnelli P (1993) Guide to the microscope analysis of Italian mammals hairs: Insectivora, Rodentia and Lagomorpha. Boll Zool 60:225–232. https://doi.org/10.1080/11250009309355815
Debrot S, Fivaz G, Mermod C, Weber J-M (1982) Atlas des poils de mammifères d’Europe. Institut de Zoologie de l’Université de Neuchàtel, Paris, France
Diffendorfer J, Fleming GM, Tremor S et al (2012) The role of fire severity, distance from fire perimeter and vegetation on post-fire recovery of small-mammal communities in chaparral. Int J Wildland Fire 21:436–448. https://doi.org/10.1071/WF10060
do Rosário IT, da Luz Mathias M (2007) Post-fire recolonisation of a montado area by the endangered Cabrera vole (Microtus cabrerae). Int J Wildland Fire 16:450–457
Doherty TS, Geary WL, Jolly CJ et al (2022) Fire as a driver and mediator of predator–prey interactions. Biol Rev 97:1539–1558. https://doi.org/10.1111/brv.12853
Dorazio RM, Royle JA (2005) Estimating size and composition of biological communities by modeling the occurrence of species. J Am Stat Assoc 100:389–398. https://doi.org/10.1198/016214505000000015
Eby S, Mosser A, Swanson A et al (2013) The impact of burning on lion Panthera leo habitat choice in an African savanna. Curr Zool 59:335–339
Engstrom RT (2010) First-order fire effects on animals: review and recommendations. Fire Ecol 6:115–130. https://doi.org/10.4996/fireecology.0601115
Fernandes PM, Davies GM, Ascoli D et al (2013) Prescribed burning in southern Europe: developing fire management in a dynamic landscape. Front Ecol Environ 11:e4–e14. https://doi.org/10.1890/120298
Ferrenberg S, Wickey P, Coop JD (2019) Ground-dwelling arthropod community responses to recent and repeated wildfires in conifer forests of northern New Mexico, USA. Forests 10:667
Fiske I, Chandler R (2011) Unmarked package for fitting hierarchical models of wildlife occurrence and abundance. J Stat Softw 43:1–23. https://doi.org/10.18637/jss.v043.i10
Flaherty S, Patenaude G, Close A, Lurz PWW (2012) The impact of forest stand structure on red squirrel habitat use. Forestry 85:437–444
Flannigan MD, Krawchuk MA, de Groot WJ et al (2009) Implications of changing climate for global wildland fire. Int J Wildland Fire 18:483–507. https://doi.org/10.1071/WF08187
Fleiss JL (1971) Measuring nominal scale agreement among many raters. Psychol Bull 76:378
Fons R, Grabulosa I, Feliu C et al (1993) Postfire dynamics of a small mammal community in a Mediterranean forest (Quercus suber). In: Trabaud L, Prodon R (eds) Fire in Mediterranean ecosystems, pp 259–270
Fons R, Grabulosa I, Marchand B et al (1996) Mammiferes et incendie en milieu mediterraneen reponses de l’insectivore Crocidura russula (Soricidae) et du rongeur Eliomys quercinus (Gliridae) en foret de chenes-lieges brulee. Vie Milieu 46:313–318
Fontúrbel FE (2010) A methodological approach to assess the small mammal community diversity in the temperate rainforest of Patagonia. Mamm 75:294–301. https://doi.org/10.1016/j.mambio.2009.03.012
Fox BJ (1982) Fire and mammalian secondary succession in an Australian coastal heath. Ecology 63:1332–1341. https://doi.org/10.2307/1938861
Fox BJ (1990) Changes in the structure of mammal communities over successional time scales. Oikos 59:321–329
Fox BJ (2022) How habitat selection, succession, and assembly rules can influence landscape ecology in natural and disturbed areas. Therya 13:5–15
Fox BJ, Quinn RD, Breytenbach GJ (1985) A comparison of small-mammal succession following fire in shrublands of Australia, California and South Africa. Proc Ecol Soc Aust 14:179–197
Fox BJ, Taylor JE, Thompson PT (2003) Experimental manipulation of habitat structure: a retrogression of the small mammal succession. J Anim Ecol 72:927–940
Frock CF, Turner MG (2018) Microhabitat conditions and landscape pattern explain nocturnal rodent activity, but not seed removal, in burned and unburned lodgepole pine forests. Landsc Ecol 33:1895–1909. https://doi.org/10.1007/s10980-018-0717-x
Gamer M, Lemon J, Gamer MM, et al (2012) Package ‘irr.’ Various coefficients of interrater reliability and agreement 22:1–32
Geary WL, Doherty TS, Nimmo DG et al (2020) Predator responses to fire: a global systematic review and meta-analysis. J Anim Ecol 89:955–971. https://doi.org/10.1111/1365-2656.13153
Gigliotti L, Curveira-Santos G, Slotow R et al (2022) Community-level responses of African carnivores to prescribed burning. J Appl Ecol 59:251–262. https://doi.org/10.1111/1365-2664.14050
González TM, González-Trujillo JD, Muñoz A, Armenteras D (2022) Effects of fire history on animal communities: a systematic review. Ecol Process 11:1–11
Grant PR (1972) Interspecific competition among rodents. Annu Rev Ecol Syst 3:79–106. https://doi.org/10.1146/annurev.es.03.110172.000455
Green K, Sanecki G (2006) Immediate and short-term responses of bird and mammal assemblages to a subalpine wildfire in the Snowy Mountains, Australia. Austral Ecol 31:673–681. https://doi.org/10.1111/j.1442-9993.2006.01629.x
Greenberg CH, Miller S, Waldrop TA (2007) Short-term response of shrews to prescribed fire and mechanical fuel reduction in a Southern Appalachian upland hardwood forest. For Ecol Manage 243:231–236. https://doi.org/10.1016/j.foreco.2007.03.003
Griffiths AD, Brook BW (2014) Effect of fire on small mammals: a systematic review. Int J Wildland Fire 23:1034–1043. https://doi.org/10.1071/WF14026
Gurnell J, Clark MJ, Lurz PW et al (2002) Conserving red squirrels (Sciurus vulgaris): mapping and forecasting habitat suitability using a Geographic Information Systems Approach. Biol Conserv 105:53–64
Haim A (2002) Fire size and location in forest restoration: the use of small mammal community structure for bioindication. In: Trabaud L, Prodon R (eds) Fire and biological processes. Bachuys, Brussels-Luxembourg, pp 249–254
Haim A, Izhaki I (1994) Changes in rodent community during recovery from fire: relevance to conservation. Biodivers Conserv 3:573–585. https://doi.org/10.1007/BF00114202
Haim A, Izhaki I (2000) The effect of different treatments on the community composition of small mammals in a post-fire pine forest. J Mediterr Ecol 1:249–257
Haim A, Rozenfeld A, Izhaki I (1997) Post-fire response of shrews (Crocidura suaveolens) on Mount Carmel, Israel. Mammalia 61:527–536
Hale S, Mendoza L, Yeatman T et al (2022) Evidence that post-fire recovery of small mammals occurs primarily via in situ survival. Divers Distrib 28(3):404–416. https://doi.org/10.1111/ddi.13283
Hallgren KA (2012) Computing inter-rater reliability for observational data: an overview and tutorial. TQMP 8:23–34. https://doi.org/10.20982/tqmp.08.1.p023
Izhaki I, Haim A, Zohar O (1993) Rodent populations recovering from fire in an east Mediterranean woodland. Water Sci Technol 27:539–545. https://doi.org/10.2166/wst.1993.0593
Jolly CJ, Dickman CR, Doherty TS et al (2022) Animal mortality during fire. Glob Chang Biol 28:2053–2065. https://doi.org/10.1111/gcb.16044
Jolly WM, Cochrane MA, Freeborn PH et al (2015) Climate-induced variations in global wildfire danger from 1979 to 2013. Nat Commun 6:1–11
Jones MW, Smith A, Betts R, et al (2020) Climate change increases the risk of wildfires. ScienceBrief Review 116:117
Kassambara A (2017) Practical guide to principal component methods in R: PCA, M (CA), FAMD, MFA, HCPC, factoextra. Sthda
Kayes LJ, Anderson PD, Puettmann KJ (2010) Vegetation succession among and within structural layers following wildfire in managed forests. J Veg Sci 21:233–247
Keeley J, Babr-Keeley M (1999) Role of charred wood, heat-shock, and light in germination of postfire phrygana species from the eastern Mediterranean basin. Israel J Plant Sci 47:11–16
Keeley JE, Bond WJ, Bradstock RA et al (2011) Fire in Mediterranean ecosystems: ecology, evolution and management. Cambridge University Press, Cambridge
Keeley JE, Pausas JG (2018) Evolution of ‘smoke’ induced seed germination in pyroendemic plants. S Afr J Bot 115:251–255. https://doi.org/10.1016/j.sajb.2016.07.012
Kelly LT, Giljohann KM, Duane A, Aquilué N, Archibald S, Batllori E, Bennett AF, Buckland ST, Canelles Q, Clarke MF et al (2020) Fire and biodiversity in the Anthropocene. Science 370:1–10
Kelly LT, Nimmo DG, Spence-Bailey LM et al (2010) The short-term responses of small mammals to wildfire in semiarid mallee shrubland, Australia. Wildl Res 37:293–300. https://doi.org/10.1071/WR10016
Kelly LT, Nimmo DG, Spence-Bailey LM et al (2011) Influence of fire history on small mammal distributions: insights from a 100-year post-fire chronosequence. Divers Distrib 17:462–473
Kelly LT, Nimmo DG, Spence-Bailey LM et al (2012) Managing fire mosaics for small mammal conservation: a landscape perspective. J Appl Ecol 49:412–421
Kéry M, Royle JA (2008) Hierarchical Bayes estimation of species richness and occupancy in spatially replicated surveys. J Appl Ecol 45:589–598
Kirkland GL Jr (1991) Competition and coexistence in shrews (Insectivora: Soricidae). In: Findley JS, Yates TL (eds) The biology of the Soricidae, University of New Mexico, Albuquerque, pp 15–22
Koprowski JL, Leonard KM, Zugmeyer CA, Jolley JL (2006) Direct effects of fire on endangered Mount Graham red squirrels. Southwestern Nat 51:59–63. https://doi.org/10.1894/0038-4909(2006)51[59:DEOFOE]2.0.CO;2
Krebs CJ (1989) Ecological methodology. Harper–Collins Publishers
Leahy L, Legge SM, Tuft K et al (2016) Amplified predation after fire suppresses rodent populations in Australia’s tropical savannas. Wildl Res 42:705–716. https://doi.org/10.1071/WR15011
Lee E, Rhim S (2012) Differences in mammal abundance of post-fire silvicultural management stands within the South Korean pine forest. J Anim Vet Adv 11:3350–3354
Lee E-J, Lee W-S, Rhim S-J (2008) Characteristics of small rodent populations in post-fire silvicultural management stands within pine forest. For Ecol Manage 255:1418–1422. https://doi.org/10.1016/j.foreco.2007.10.055
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280
Lekberg Y, Waller LP (2016) What drives differences in arbuscular mycorrhizal fungal communities among plant species? Fungal Ecol 24:135–138
Letnic M, Dickman CR, Tischler MK et al (2004) The responses of small mammals and lizards to post-fire succession and rainfall in arid Australia. J Arid Environ 59:85–114
Letnic M, Tamayo B, Dickman CR (2005) The responses of mammals to La Niña (El Niño Southern Oscillation)–associated rainfall, predation, and wildfire in central Australia. J Mammal 86:689–703
Lindenmayer DB, Incoll RD, Cunningham RB et al (1999) Comparison of hairtube types for the detection of mammals. Wildl Res 26:745–753. https://doi.org/10.1071/WR99009
Linnell MA, Lesmeister DB, Bailey JD et al (2018) Response of arboreal rodents to increased availability of nest substrates in young forests. J Mammal 99:1174–1182. https://doi.org/10.1093/jmammal/gyy111
Luna F, Antinuchi CD (2006) Cost of foraging in the subterranean rodent Ctenomys talarum: effect of soil hardness. Can J Zool 84:661–667
MacKenzie DI, Nichols JD (2004) Occupancy as a surrogate for abundance estimation. Anim Biodivers Conserv 27:461–467
MacKenzie DI, Nichols JD, Lachman GB et al (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255
MacKenzie DI, Nichols JD, Royle JA et al (2017) Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence. Elsevier
MacKenzie DI, Royle JA (2005) Designing occupancy studies: general advice and allocating survey effort: designing occupancy studies. J Appl Ecol 42:1105–1114. https://doi.org/10.1111/j.1365-2664.2005.01098.x
Maestri R, Marinho JR (2014) Singing in the rain. Rainfall and moonlight affect daily activity patterns of rodents in a Neotropical forest. Acta Theriol 59:427–433
Mandelik Y, Jones M, Dayan T (2003) Structurally complex habitat and sensory adaptations mediate the behavioural responses of a desert rodent to an indirect cue for increased predation risk. Evol Ecol Res 5:501–515
Mata C, Ruiz-Capillas P, Malo JE (2017) Small-scale alterations in carnivore activity patterns close to motorways. Eur J Wildl Res 63:64. https://doi.org/10.1007/s10344-017-1118-1
Mazzamuto MV, Mazzella MN, Merrick MJ, Koprowski JL (2020) Fire impacts on a forest obligate: Western gray squirrel response to burn severity. Mamm Biol 100:295–303
Mazzella MN, Koprowski JL (2020) Response to fire by a forest specialist in isolated montane forest. For Ecol Manage 462:117996. https://doi.org/10.1016/j.foreco.2020.117996
McCafferty DJ, Moncrieff JB, Taylor IR (2003) Winter microclimate of field voles (Microtus agrestis) in SW Scotland. J Therm Biol 28:397–401
McGregor HW, Legge S, Jones ME, Johnson CN (2014) Landscape management of fire and grazing regimes alters the fine-scale habitat utilisation by feral cats. PloS One 9:e109097
Melcore I, Ferrari G, Bertolino S (2020) Footprint tunnels are effective for detecting dormouse species. Mammal Rev 50:226–230
Monamy V, Fox BJ (2000) Small mammal succession is determined by vegetation density rather than time elapsed since disturbance. Austral Ecol 25:580–587
Monamy V, Fox BJ (2010) Responses of two species of heathland rodents to habitat manipulation: vegetation density thresholds and the habitat accommodation model. Austral Ecol 35:334–347. https://doi.org/10.1111/j.1442-9993.2009.02042.x
Moreira F, Russo D (2007) Modelling the impact of agricultural abandonment and wildfires on vertebrate diversity in Mediterranean Europe. Landsc Ecol 22:1461–1476. https://doi.org/10.1007/s10980-007-9125-3
Mori E, Sangiovanni G, Corlatti L (2020) Gimme shelter: the effect of rocks and moonlight on occupancy and activity pattern of an endangered rodent, the garden dormouse Eliomys quercinus. Behav Processes 170:103999. https://doi.org/10.1016/j.beproc.2019.103999
Morris CF, McLean D, Engleson JA et al (2012) Some observations on the granivorous feeding behavior preferences of the house mouse (Mus musculus L.). Mammalia 76:209–218
Morris G, Hostetler JA, Oli MK, Conner LM (2011) Effects of predation, fire, and supplemental feeding on populations of two species of Peromyscus mice. J Mammal 92:934–944. https://doi.org/10.1644/10-MAMM-A-419.1
Mortelliti A, Cervone C, Amori G, Boitani L (2010) The effect of non-target species in presence-absence distribution surveys: a case study with hair-tubes. Ital J Zool 77:211–215. https://doi.org/10.1080/11250000903373771
Nalliah R, Sitters H, Smith A, Di Stefano J (2022) Untangling the influences of fire, habitat and introduced predators on the endangered heath mouse. Anim Conserv 25:208–220. https://doi.org/10.1111/acv.12731
Naxara L, Pinotti BT, Pardini R (2009) Seasonal microhabitat selection by terrestrial rodents in an old-growth Atlantic Forest. J Mammal 90:404–415
Ne’eman G, Lahav H, Izhaki I (1993) The resilience of vegetation to fire in an east Mediterranean pine forest on Mount Carmel, Israel: the effects of post-fire management. In: Trabaud L, Prodon R (eds) Fire in Mediterranean ecosystems, pp 127–140
Nimmo DG, Avitabile S, Banks SC et al (2019) Animal movements in fire-prone landscapes. Biol Rev 94:981–998. https://doi.org/10.1111/brv.12486
Nimmo DG, Carthey AJ, Jolly CJ, Blumstein DT (2021) Welcome to the Pyrocene: animal survival in the age of megafire. Glob Chang Biol 27:5684–5693
Oksanen J, Blanchet FG, Kindt R, et al (2013) Package ‘vegan.’ Community ecology package, version 2:1–295
Pardini R, de Souza SM, Braga-Neto R, Metzger JP (2005) The role of forest structure, fragment size and corridors in maintaining small mammal abundance and diversity in an Atlantic forest landscape. Biol Conserv 124:253–266
Pausas JG, Bradstock RA, Keith DA, Keeley JE (2004) Plant functional traits in relation to fire in crown-fire ecosystems. Ecology 85:1085–1100
Pausas JG, Keeley JE (2009) A burning story: the role of fire in the history of life. BioScience 59:593–601. https://doi.org/10.1525/bio.2009.59.7.10
Pausas JG, Parr CL (2018) Towards an understanding of the evolutionary role of fire in animals. Evol Ecol 32:113–125. https://doi.org/10.1007/s10682-018-9927-6
Perfetti A (2009) La fauna del Monte Pisano e la Rete Natura 2000. Edizioni ETS, Pisa
Pocock MJO, Bell SC (2011) Hair tubes for estimating site occupancy and activity-density of Sorex minutus. Mamm 76:445–450. https://doi.org/10.1016/j.mambio.2011.02.002
Pocock MJO, Jennings N (2006) Use of hair tubes to survey for shrews: new methods for identification and quantification of abundance. Mamm Rev 36:299–308. https://doi.org/10.1111/j.1365-2907.2006.00092.x
Prodon R (1987) The impact of fire on animal communities in Mediterranean area. In: Trabaud L (ed) The role of fire in ecological systems. SPB Academic Publishers, The Hague, pp 121–157
Prugh L, Brashares J (2010) Basking in the moonlight? Effect of illumination on capture success of the endangered giant kangaroo rat. J Mammal 91:1205–1212
Puig-Gironès R, Brotons L, Pons P (2017) Aridity influences the recovery of vegetation and shrubland birds after wildfire. PloS One 12:e0173599
Puig-Gironès R, Clavero M, Pons P (2018) Importance of internal refuges and the external unburnt area in the recovery of rodent populations after wildfire. Int J Wildland Fire 27:425–436. https://doi.org/10.1071/WF17102
Puig-Gironès R, Imbeau L, Clavero M et al (2020) Does post-fire salvage logging affect foraging activity by rodents? Eur J Forest Res. https://doi.org/10.1007/s10342-020-01285-5
Puig-Gironès R, Pons P (2020) Mice and habitat complexity attract carnivorans to recently burnt forests. Forests 11:855. https://doi.org/10.3390/f11080855
Puig-Gironès R, Pons P (2023) Mice population dynamics and structure over time and space after wildfires. J Zool 321:128–141
QGIS Development Team AE (2022) QGIS geographic information system. Open source geospatial foundation project
Radea C, Arianoutsou M (2012) Soil arthropod communities and population dynamics following wildfires in pine forests of the Mediterranean Basin: a review. Israel J Ecol Evol 58:137–149
Rapetti F, Vittorini S (1994) I caratteri del clima. In: Mazzanti M (ed) La pianura di Pisa e i rilievi contermini. Memorie della società geologica italiana, Roma 50:103–132
Ricotta C, Podani J, Pavoine S (2016) A family of functional dissimilarity measures for presence and absence data. Ecol Evol 6:5383–5389
Rost J, Clavero M, Brotons L, Pons P (2012) The effect of postfire salvage logging on bird communities in Mediterranean pine forests: the benefits for declining species. J Appl Ecol 49:644–651. https://doi.org/10.1111/j.1365-2664.2012.02127.x
Sainz-Elipe S, Sáez-Durán S, Galán-Puchades MT, Fuentes MV (2012) Small mammal (Soricomorpha and Rodentia) dynamics after a wildfire in a Mediterranean ecosystem. Mammalia 76:251–259
Salbitano F, Foderi C, Bertacchi A (2020) Indirizzi operativi per la realizzazione di interventi di ripristino dei soprassuoli boscati interessati dagli incendi di Calci 2018 e Vicopisano 2019. http://www.agroecologiacalci.it/wp-content/uploads/2020/12/INDIRIZZI_OPERATIVI_V17_FINAL.pdf
Santini L, Canale A, Giannotti P, Mastrobuoni G (2012) Micromammiferi delle aree protette del comune di San Giuliano Terme. Felici Editore, Pisa
Santos X, Bros V, Miño À (2009) Recolonization of a burned Mediterranean area by terrestrial gastropods. Biodivers Conserv 18:3153–3165. https://doi.org/10.1007/s10531-009-9634-2
Santos X, Cheylan M (2013) Taxonomic and functional response of a Mediterranean reptile assemblage to a repeated fire regime. Biol Conserv 168:90–98. https://doi.org/10.1016/j.biocon.2013.09.008
Santos X, Poquet JM (2010) Ecological succession and habitat attributes affect the postfire response of a Mediterranean reptile community. Eur J Wildl Res 56:895–905. https://doi.org/10.1007/s10344-010-0387-8
Sassi PL, Taraborelli P, Albanese S, Gutierrez A (2015) Effect of temperature on activity patterns in a small andean rodent: behavioral plasticity and intraspecific variation. Ethology 121:840–849
Senf C, Seidl R (2022) Post-disturbance canopy recovery and the resilience of Europe’s forests. Glob Ecol Biogeogr 31:25–36
Sgardelis SP, Pantis JD, Argyropoulou MD, Stamou GP (1995) Effects of fire on soil macroinvertebrates in a Mediterranean phryganic ecosystem. Int J Wildland Fire 5:113–121
Simons LH (1991) Rodent dynamics in relation to fire in the Sonoran Desert. J Mammal 72:518–524
Soyumert A, Ertürk A, Tavşanoğlu Ç (2020) Fire-created habitats support large mammal community in a Mediterranean landscape. Mamm Res 65:323–330. https://doi.org/10.1007/s13364-019-00473-y
Soyumert A, Tavsanoglu C, Macar O et al (2010) Presence of large and medium-sized mammals in a burned pine forest in southwestern Turkey. Hystrix 21:97–102
Stone BW, Jackson CR (2016) Biogeographic patterns between bacterial phyllosphere communities of the Southern Magnolia (Magnolia grandiflora) in a small forest. Microb Ecol 71:954–961
Suckling G (1978) A hair sampling tube for the detection of small mammals in trees. Wildl Res 5:249–252. https://doi.org/10.1071/WR9780249
Sutherland WJ (2006) Ecological census techniques: a handbook. Cambridge University Press, Cambridge
Swan M, Christie F, Sitters H et al (2015) Predicting faunal fire responses in heterogeneous landscapes: the role of habitat structure. Ecol Appl 25:2293–2305. https://doi.org/10.1890/14-1533.1
Swan M, Galindez-Silva C, Christie F et al (2016) Contrasting responses of small mammals to fire and topographic refugia: small mammal responses to patchy fire. Austral Ecol 41:437–445. https://doi.org/10.1111/aec.12331
Syphard AD, Radeloff VC, Hawbaker TJ, Stewart SI (2009) Conservation threats due to human-caused increases in fire frequency in Mediterranean-climate ecosystems. Conserv Biol 23:758–769
Tann CR, Singleton GR, Coman BJ (1991) Diet of the house mouse, Mus domesticus, in the mallee wheatlands of north-western Victoria. Wildl Res 18:1–12
Teerink BJ (2003) Hair of West European mammals: atlas and identification key. Cambridge University Press, Cambridge
Tessler N, Wittenberg L, Greenbaum N (2016) Vegetation cover and species richness after recurrent forest fires in the Eastern Mediterranean ecosystem of Mount Carmel, Israel. Sci Total Environ 572:1395–1402
Thieurmel B, Elmarhraoui A, Thieurmel MB (2019) Package ‘suncalc.’ See https://cranr-projectorg/web/packages/suncalc/suncalc.pdf
Torre I, Díaz M (2004) Small mammal abundance in Mediterranean post-fire habitats: a role for predators? Acta Oecol 25:137–142. https://doi.org/10.1016/j.actao.2003.10.007
Torre I, Guixé D, Sort F (2010) Comparing three live trapping methods for small mammal sampling in cultivated areas of NE Spain. Hystrix 21:147–155
Torre I, Jaime-González C, Díaz M (2022) Habitat suitability for small mammals in Mediterranean landscapes: how and why shrubs matter. Sustainability 14:1562. https://doi.org/10.3390/su14031562
Torre I, Ribas A, Puig-Gironès R (2023) Effects of post-fire management on a Mediterranean small mammal community. Fire 6:34. https://doi.org/10.3390/fire6010034
Tóth M (2017) Hair and fur atlas of Central European mammals. Pars Limited
Trabaud L (1994) Postfire plant community dynamics in the Mediterranean Basin. In: Moreno JM and Oechel WC (eds) The role of fire in Mediterranean-type ecosystems. Springer, pp 1–15
Turco M, Rosa-Cánovas JJ, Bedia J et al (2018) Exacerbated fires in Mediterranean Europe due to anthropogenic warming projected with non-stationary climate-fire models. Nat Commun 9:1–9
van Mantgem EF, Keeley JE, Witter M (2015) Faunal responses to fire in chaparral and sage scrub in California, USA. Fire Ecol 11:128–148
Van Wagtendonk JW (2009) Fires and landscape conservation in Mediterranean ecosystems. . In: Chuvieco E (ed) Earth Observation of Wildland Fires in Mediterranean Ecosystems Berlin: Springer, pp 27–39
Vernes K (2000) Immediate effects of fire on survivorship of the northern bettong (Bettongia tropica): an endangered Australian marsupial. Biol Conserv 96:305–309
Vickery WL, Bider JR (1981) The influence of weather on rodent activity. J Mammal 62:140–145
Wauters LA, Lurz PW, Gurnell J (2000) Interspecific effects of grey squirrels (Sciurus carolinensis) on the space use and population demography of red squirrels (Sciurus vulgaris) in conifer plantations. Ecol Res 15:271–284
Whelan RJ, Rodgerson L, Dickman CR (2002) Critical life processes of plants and animals: developing a process-based understanding of population changes in fire-prone landscapes. Cambidge University Press
Wróbel A, Bogdziewicz M (2015) It is raining mice and voles: which weather conditions influence the activity of Apodemus flavicollis and Myodes glareolus? Eur J Wildl Res 61:475–478. https://doi.org/10.1007/s10344-014-0892-2
Zalewski A (2000) Factors affecting the duration of activity by pine martens (Martes martes) in the Bialowieża National Park, Poland. J Zool 251:439–447
Zozzoli R, Menchetti M, Mori E (2018) Spatial behaviour of an overlooked alien squirrel: the case of Siberian chipmunks Eutamias sibiricus. Behav Processes 153:107–111
Zuur AF, Ieno EN, Smith GM (2007) Analysing ecological data. Springer, New York, London
Zuur AF, Ieno EN, Walker N et al (2009) Mixed effects models and extensions in ecology with R. Springer, New York, New York, NY
Zwolak R, Foresman KR (2007) Effects of a stand-replacing fire on small-mammal communities in montane forest. Can J Zool 85:815–822. https://doi.org/10.1139/Z07-065
Acknowledgements
The authors would like to thank the University of Pisa Working group (Tavolo Tecnico) for the Monte Pisano fires, which is dedicated to support the local community to study the effects of fires and to promote restoration of burnt areas. Moreover, we acknowledged the recommendation provided by the Tavolo Tecnico. In addition, we would like to thank the University of Pisa Museum of Natural History and Farina S. for providing us with hair samples to use as references, Favilla A. and Pardini A. for their help with data collection in the field, Kastelic J. for his writing assistance, and Mori E. and Bisi F. for the review of the advanced draft of the manuscript.
Funding
Open access funding provided by Università di Pisa within the CRUI-CARE Agreement. This work was supported by the University of Pisa and Regione Toscana, and the Department of Biology.
Author information
Authors and Affiliations
Contributions
O. Tomassini developed the sampling design; conducted the field work, laboratory, and statistical analyses; and wrote the manuscript. A. Aghemo and B. Baldeschi contributed to the fieldwork and laboratory analyses. A. Massolo developed the sampling design together with O. Tomassini and supervised the overall study. D. Giunchi contributed to the statistical analyses of the study and with G. Petroni and G. Bedini supported the study financially, and revised the manuscript. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by: Jan M. Wójcik
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tomassini, O., Aghemo, A., Baldeschi, B. et al. Some like it burnt: species differences in small mammal assemblage in a Mediterranean basin nearly 3 years after a major fire. Mamm Res 69, 283–302 (2024). https://doi.org/10.1007/s13364-024-00742-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-024-00742-5