Abstract
Deforestation remains the most pervasive driver of biodiversity erosion across tropical forests. Understanding how species can cope with such habitat changes is particularly important along the rapidly expanding agricultural frontiers. To do so, we used a functional perspective examining small mammal responses to habitat loss, fragmentation, and degradation across the ‘Arc of Deforestation’ in the Southern Brazilian Amazon. Small mammals were surveyed using a combination of conventional and pitfall traps across 20 forest fragments—ranging from 42 to 4743 ha—in addition to two relatively continuous forest sites (> 7000 ha). These fragments lie isolated by a cattle pasture matrix of varying grazing intensity. We then analysed taxonomic and functional diversity patterns—represented by Simpson Diversity and Rao Quadratic entropy indices—in Generalised Linear Models containing local- to landscape-scale predictors of variation. Further, we used a functional trait composition approach based on community-weighted mean trait values to depict and predict small mammal functional variations across this degradation gradient. From a total of 847 individuals recorded belonging to 24 taxa, functional responses tended to follow the taxonomic diversity, both increasing with fragment area. The functional dimension further was promoted by low fire-related disturbance. Functional trait composition was mainly driven by habitat quality, represented by tree density, arthropod biomass, and fire-related disturbance. Our results reinforce that small forest fragments sustain depauperate small mammal assemblages both taxonomically and functionally. Accounting for habitat quality further allows for boosting the persistence across functional groups. Our findings can be used to improve the efficiency of management practices thereby maximising the multiple dimensions of small mammal diversity and their associated ecosystem services across tropical deforestation frontiers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical forests are among the most diverse habitats worldwide but are currently perishing in the face of the quickly expanding agricultural frontiers. The Amazon Forest, although still comprising the largest tract of tropical forest on the planet, is subject to the highest absolute deforestation rates globally (Hansen et al. 2020). Indeed, over the last five decades, the intensification of logging followed by forest conversion into cattle ranches and crop monocultures, road building, and human settlements resulted in the loss of more than 25% of the Amazon Forest (Peres et al. 2010). In Brazil, deforestation is especially exacerbated in the south of Pará state and the north of Mato Grosso state, configuring the so-called Arc of Deforestation (Laurance et al. 2018). Understanding how biodiversity persists and copes with such rapid habitat changes remains a cornerstone to proposing effective conservation guidelines.
Within fragmented landscapes, biological communities are likely to be affected by several multi-scale drivers including landscape, patch, and habitat quality features of any given site (Pardini et al. 2005; Arroyo-Rodríguez et al. 2013). As such, species diversity is typically lower in smaller and more isolated habitat fragments (Mendenhall et al. 2014), or in lower remaining habitat amounts (Fahrig 2013). The size of the habitat fragment is a limiting factor of the species population size, while the degree of isolation constrains species colonization rates (MacArthur and Wilson 1967). The habitat quality of the fragments is usually related to the intensity of the edge effects, being particularly marked at more irregularly shaped fragments (Laurance and Yensen 1991), as well as to the degree of additional human-induced disturbance, including previous fire and logging events (Barlow et al. 2016). Moreover, the surrounding matrix tends to limit dispersal according to the degree of matrix hostility (Prevedello and Vieira 2010). In the Amazon biome, this degree is commonly expressed by the structural complexity of vegetation surrounding forest fragments (Santos-Filho et al. 2012).
Several studies demonstrate the immediate and long-term detrimental effects of habitat loss, fragmentation, and consequent degradation on biodiversity (Sala et al. 2000; Haddad et al. 2015). However, only recently, multiple dimensions of biodiversity have started to be considered (e.g., Farneda et al. 2018; Palmeirim et al. 2021). Beyond taxonomic diversity, the functional dimension incorporates species trait variation that potentially affects species performance, fitness, and ecological functions (Weiss and Ray 2019). Both taxonomic and functional dimensions of diversity can be concurrently affected by habitat changes (Salgado‐Luarte et al. 2019; Aguirre-Gutiérrez et al. 2020). Moreover, as individual species traits reflect environmental preferences and associated behaviours (Violle et al. 2007), distinct functional groups may also respond differently to habitat loss, fragmentation, and consequent degradation (Wang et al. 2010; Farneda et al. 2015). Overall, traits conferring species with high dispersal ability, as well as habitat and diet generalist habits, are associated with success in thriving in fragmented landscapes (Devictor et al. 2008). For instance, in the Brazilian Amazon, small-bodied mammals (marsupials and rodents) were selectively extinct from smaller forest fragments according to their increasing body size and decreased ability to transverse the non-native matrix (Palmeirim et al. 2021). Species traits might therefore be critical determinants of the winners and losers of biodiversity (Filgueiras et al. 2021).
To improve our understanding of biodiversity responses to habitat loss, fragmentation, and consequent degradation across a deforestation frontier expanding towards the interior of the Amazon biome in Brazil, we surveyed small mammal assemblages across 20 forest fragments of different sizes, isolation, and remaining habitat quality, in addition to two relatively continuous forest sites. Small mammals are ecologically diversified in neotropical forests, spanning across the vertical forest strata, and occupying multiple trophic niches (Paglia et al. 2012). These mammals also play a variety of roles in natural ecosystems, including as predators, prey, grazers, seed dispersers, and commensal species, which makes them critical for ecosystem functioning and forest regeneration (Terborgh et al. 2001). Across a habitat disturbance gradient located at the Arc of Deforestation, we first examined patterns and predictors of taxonomic and functional diversity of small mammals, which we hypothesised to increase with fragment size and habitat quality (i.e., low-intensity edge effects and fire-related disturbance and higher canopy cover, insect biomass, and litter depth) and decrease on more isolated fragments (i.e., in terms of time since isolation and forest cover in the surrounding area; MacArthur and Wilson 1967; Meynard et al. 2011), surrounded by a lower quality matrix. We then analysed functional trait composition, expecting that traits allowing small mammal species to persist on small, isolated, and habitat-degraded islands are favoured over the gradient of fragmentation (Devictor et al. 2008). In particular, we expect those traits to be related to higher dispersal ability (e.g., larger body size and overall matrix tolerance) and generalist habits in terms of both habitat (e.g., ability to use open-habitat areas) and diet (Farneda et al. 2015; Wang et al. 2010).
Material and Methods
Study area
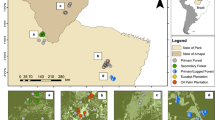
Small mammals were sampled across 20 forest fragments—ranging from 42 to 4743 ha—in addition to two relatively continuous forest sites (> 7000 ha). Sampling took place in a 3609-km2 landscape of Southwestern Mato Grosso state, Brazil, within the watersheds of two tributaries of the Paraguay River, the Jaurú and Cabaçal rivers (between 15° 15′ S/58° 42′ W and 15° 33′ S/58° 00′ W, Fig. 1). The vegetation of the study area is classified as Amazon rain forest, more specifically the forest type is submontane semi-deciduous seasonal forest (Amaral and Fonzar 1982). This type of forest occurs in places where the altitude varies from 100 to 500 m.a.s.l. Eutrophic red-yellow podzolic soils predominate in the region (Oliveira et al. 1982). The climate is Aw, following the Köppen climatic classification. The mean annual precipitation is 1330 mm, mostly concentrated from November to April (wet season), and there is virtually no rain during the dry season (Resende et al. 1994). High-temperature peaks (> 25 °C) persist throughout the year, with a maximum average temperature of 25 °C between December and January (Resende et al. 1994).
Location of the 20 forest fragments and two continuous forest sites where small mammal assemblages were surveyed in Southwestern Brazilian Amazonia. Information on each of the environmental variables characterising the fragments can be found in Table S1. Landcover map were derived from MapBiomas project (https://brasil.mapbiomas.org/en/colecoes-mapbiomas-1?cama_set_language=en) and correspond to the 2002 year of collection 7.1. The classes of land cover are available online at https://mapbiomas-br-site.s3.amazonaws.com/Legenda/C%C3%B3digos_Classes_Legenda_Cole%C3%A7%C3%A3o_7_-__PT__.docx__1___1_.pdf
Small mammal sampling
In sampling sites F1 to F9, small mammals were surveyed twice, one time in the wet season and another time in the dry season; in sites, F10 to F13 as well as C1 and C2 were surveyed once in the wet season, and sites F14 to F20 were surveyed once in the dry season (Table S1). Despite sampling covering different seasons, sampling season was not related to any of the response variables considered in subsequent analysis (P > 0.05 in all instances). Sampling was carried out between January 2002 and January 2004, using a combination of conventional and pitfall traps (Fig. 2). At each forest fragment and continuous forest site, we established six 135-m long parallel transects of conventional traps, placed 50 m apart from each other. The first transect was placed along the edge of fragments, and the remaining ones towards the fragment interior. A repeated spatial configuration of one Sherman (8 × 9 × 23 cm), one Tomahawk (14.5 × 14.5 × 41 cm), and one snap-trap (9 × 15 cm) were placed sequentially every 15 m along each transect, with one trap per station, yielding a total of 10 trapping stations per transect. All trapping transects were operated for 10 consecutive days, yielding a total of 700 trap nights per forest site sampled. In addition, the different trap types were alternated along consecutive trap stations with traps placed either on the ground or at ~ 2 m in height (see Fig. 2). Consecutive trapping transects started with a different trap type so that the same number of traps from the same type would be used in the sampling site. As such, each sampling site was surveyed by 20 Sherman, 20 Tomahawk, and 20 snap-traps, and overall trapping effort along either terrestrial or arboreal stations was allocated equally at these two forest strata. Traps were baited with a mixture of banana and peanut butter and subsequently checked daily during both the dry and wet season trapping periods. In this study, the total trapping effort amounted to 15,400 conventional trap nights.
Schematic representation of the sampling design applied in each forest site. In the central panel, continuous lines depict the live-trapping transects and the dashed lines depict the pitfall-trapping transects, amounting to seven and five live- and pitfall-trapping transects per site, respectively. On the left panel, we indicate the design of each live-trapping transect, including ten trapping stations (denoted by “x”), each of which sampled by a trap ((Sh) Sherman, (Tw) Tomahawk, and (ST) snap trap) which position changed along the transect (forest floor and understory, as represented). On the right panel, we indicate the design of each pitfall-trapping transect, including five trapping stations (denoted by a representation of a bucket), each of which sampled by a bucket, connected by the other buckets through a plastic fence. Distances between transects and trap stations are indicated in the schematic representation
Additionally, we installed four transects of pitfall traps at each fragment surveyed. These were interspersed with the first five parallel conventional trapping transects. The first pitfall transect was located along the fragment edge, whereas the others were placed every 50 m apart farther into the forest fragment up to 200 m from the edge. The distance between adjacent conventional and pitfall trapping transects was therefore 25 m, except along the forest edge, where it was only 10 m. Each pitfall trapping transect consisted of a 50-m length of plastic fencing (80 cm in height), along which one 24-L plastic bucket (37 cm in depth) was placed every 10 m, totalling five pitfall traps per transect. Buckets were cone-shaped, measuring 30 cm in diameter at the mouth, and 26 cm at the bottom. These buckets were buried flush to the ground and joined at ground level by the plastic fence, which was buried along the entire fence line 5 cm deep into the soil and attached by heavy-duty staples to wooden stakes. During the rainy season, a piece of polystyrene foam was placed into the buckets, to prevent animals from drowning, and buckets were regularly emptied. Pitfall traps were checked on a daily basis, and this additional effort amounted to a total of 4400 pitfall trap nights over the entire study.
Animal trapping and handling followed the guidelines of the American Society of Mammalogists (Sikes 2016) and was approved by the Instituto Chico Mendes, the appropriate Brazilian government agency (SISBIO license numbers: 033/02, 004/03 and 057/04). Since none of the fragments yielded more than 20 individuals per species—the number of individuals per site expected to be collected as vouchers in each site—all individuals in this study were collected, rendering a mark-recapture approach impossible. Voucher specimens were euthanized in the field using anaesthetics and taxidermized, and subsequently deposited at the Zoological Collection of the State University of Mato Grosso (UNEMAT), in Caceres, Brazil.
Landscape, fragment, and local variables
To understand the drivers of both taxonomic and functional aspects of small mammal diversity, we considered a set of variables at multiple scales. At the landscape scale, considering the 1-km buffer from the perimeter of each forest site, we included forest cover and matrix quality; at the fragment scale, fragment area, age since isolation, and a shape index; at the local scale, fire-related disturbance, arthropod biomass, litter depth, tree density, and forest canopy cover. The 1-km buffer size allowed us to characterise the fragment surroundings while minimising overlaps with adjacent sampling fragments. Landscape (cover) and fragment variables (area and shape) were extracted from a georeferenced Landsat ETM (2004) satellite image using Fragstats v. 3.3 (McGarigal et al. 2012) and ArcView 3.2 (ESRI 2018). All other variables were measured in situ. A detailed description of each variable can be found in Table 1, and the corresponding values for each fragment surveyed in Table S1 (for further information on methods used to measure each variable, see Santos-Filho et al. 2012).
Species traits
Functional traits were selected based on their potential to be related to the species’ persistence in the fragmented landscape (Dreiss et al. 2015). Small mammal traits––body mass, trophic level, locomotion habit, and degree of matrix tolerance––were obtained from both field measurement and an available database (i.e., Paglia et al. 2012). Small mammal locomotion habit was the only categorical variable that was further transformed into a discrete variable reflecting the degree of arboreality in all subsequent analyses (i.e., the locomotion habits—terrestrial, scansorial, and arboreal—were converted to [1], [2], and [3], correspondingly). Information on individual traits is detailed in Table 2 (see also Table S2 for trait values of each small mammal species).
Data analysis
In subsequent analysis, species abundance was standardized according to sampling effort (Table S1). As such, we doubled the number of individuals recorded in the sampling sites surveyed only once (e.g., Silva et al. 2022). Following Ricotta et al. (2016), taxonomic and functional diversities were correspondingly given by the Simpson diversity and Rao’s quadratic entropy functional indices, considering observed species abundances. Higher values of the Simpson index—ranging between 0 and 1—indicate higher species richness and evenness. Rao’s quadratic entropy index considers the trait-based variance between species pairs. Both Simpson's and Rao’s indices are based on the same species pairwise distances and relative species abundances so Rao’s is considered a generalization of Simpson's (Botta-Dukát 2005). For this, we used the SYNCSA R-package (Debastiani and Pillar 2012). Then, we analysed the combined effects of all uncorrelated landscape (matrix), fragment (size, age, and shape), and local variables (fire, arthropods, litter, trees, and canopy) on each of the diversity metrics. Given that our response variables were normally distributed (Shapiro–Wilk test, P > 0.10 in both instances), we then applied Linear Models (LMs), using a Gaussian error structure with an identity link function, for each of the response variables. A candidate model set was further constructed, using all combinations of the nine explanatory variables retained (including the null model with only intercept and residual variance, without explanatory variables), and models were ranked based on their Akaike Information Criteria values corrected for small sample sizes (AICc, Burnham and Anderson 2002), using the MuMIn R-package (Barton 2016). A model-averaging approach was then performed, to account for model uncertainty in multi-model inference, considering all alternative models (Burnham and Anderson 2002).
Patterns of trait functional composition were analysed individually considering each small mammal trait and calculating its community-weighted mean value (CWM, Lavorel et al. 2008), which was obtained considering observed species abundance using the ‘functcomp’ function of the FD R-package (Laliberté and Legendre 2010). By incorporating species abundance, CWM ensures assessments of shifts in mean trait values across the range of spatial and habitat-related variables considered (Lavorel et al. 2008). As before, we analysed the effect of all non-correlated landscape, fragment, and local variables on each of the CWM values of each species trait by applying GLMs using a Gaussian error structure. A model-averaging approach was then performed as above, again using all alternative models (Burnham and Anderson 2002). Explanatory variables were previously standardized (\(x\) = 0, σ = 1) to place coefficient estimates onto the same scale. Small-bodied mammal sampling effort sufficiency was previously assessed for each taxon and considered satisfactory (the corresponding rarefaction curves are available in Santos-Filho et al. 2012) and the observed species abundance corresponded to the sum of the number of small mammal individuals recorded at each sampling site during the two field seasons (Santos-Filho et al. 2012). Assumptions about the normal distribution of the variables and their residuals were verified using the R package Dharma (Hartig 2019). All aforementioned data analyses were performed in R (R Core Team 2015).
Results
We recorded a total of 847 small-bodied mammals belonging to 24 taxa (12 marsupials and 12 rodents). From these, three species were particularly abundant: the marsupials Marmosa demerarae (N = 226 individuals) and Marmosops noctivagus (N = 181) and the rodent Hylaeamys megacephalus (N = 114), accounting for 61.5% of all individuals recorded. On the other hand, six species were only recorded either once or twice throughout the study: the marsupials Caluromys philander, Glironia venusta, Metachirus nudicaudatus, and Monodelphis brevicaudata and the rodents Akodon toba and Mesomys hispidus (Table S3).
Taxonomic diversity of small mammals, as indicated by the Simpson index, was slightly affected by the fragment area (CImin = − 0.005, CImax = 0.082) (Fig. 3a). The functional dimension, given by the Rao Q index, was positively affected by the fragment area (CImin = 0.003, CImax = 0.055) and, to a lesser extent, negatively affected by fire-related disturbance (CImin = − 0.047, CImax = 0.002) (Fig. 3b, Table 3 and S4). Both dimensions of diversity were highly intercorrelated (r = 0.86), and the variance explained (given by the R2) in each of the averaged models was 26.2% and 42.6% when considering the Simpson and Rao indices, respectively.
Relationships between a the Simpson D index denoting the taxonomic diversity and b the Rao Q index indicating the functional diversity with fragment area (log10 x). Data points denote the diversity of small mammals across 20 forest fragments and two continuous forest sites in the Southern Brazilian Amazonia. Data points are identified according to sampling site identity (Table S1) and scaled according to their area
Changes in functional trait composition of small mammals were partly associated with at least one of the environmental variables considered, except for body mass which was not driven by any of the predictors analysed (Fig. 4a). Small mammal assemblages characterised by a higher trophic level were found under higher tree densities (CImin = 0.018, CImax = 0.312) and lower arthropod biomass (CImin = − 0.341, CImax = − 0.028) (Fig. 4b). In addition, more arboreal small mammal assemblages were more commonly found in fire-disturbed fragments (CImin = 0.143, CImax = 0.530) (Fig. 4c), whereas assemblages with higher matrix tolerance were more commonly found in fragments not previously fire-disturbed (CImin = − 0.047, CImax = − 0.001) (Fig. 4d; Table S5). In terms of variance explained by each of the averaged models, we noted 15.1% for the CWM regarding body mass, 43.7% for the trophic level, 52.8% for the locomotion habitat, and 24.9% for the matrix tolerance species traits.
Estimates of averaged models and their 95% confidence intervals for predictors of the community-weighted mean (CWM) trait values for (a) body mass, (b) trophic level, (c) locomotion habit, and (d) matrix tolerance. Predictors included matrix complexity (matrix), age since isolation (age), shape index (shape), litter depth (litter), tree density (trees), arthropod biomass (arthropod), canopy openness (canopy), and fire-related disturbance (fire). Statistically significant negative and positive coefficients are colour-coded in red and blue, respectively
Discussion
Arc of Deforestation in the southern Brazilian Amazon, currently the world’s largest deforestation frontier (Abreu et al. 2013), comprises ever-fragmented landscapes from where widespread species extinctions have been commonly reported (Michalski and Peres 2007; Lees and Peres 2008; Palmeirim et al. 2020). In the Mato Grosso state, the landscape comprised between the Jaurú and Cabaçal rivers is no exception to this pattern, as observed for small mammals (Santos-Filho et al. 2012) and lizards (Silva et al. 2022). Here, we extend the impacts of habitat loss, fragmentation, and consequent degradation to the functional dimension of the small mammal diversity, which followed a similar pattern to the taxonomic dimension. Yet, functional diversity was further sensitive to fire-induced disturbance. In addition, the distribution of the species traits within the small mammal assemblages was mainly driven by habitat quality factors, namely in terms of tree density, arthropod biomass, and fire-related disturbance.
Functional diversity of small mammals was predicted by forest fragment area, while the taxonomic dimension showed the same trend to some extent. These results are in general consistent with our expectations (Petchey et al. 2007; Meynard et al. 2011; Rurangwa et al. 2021). Yet, in a previous study using the same data, Santos-Filho et al. (2012) found that small mammal abundance, species richness, and composition were primarily affected by the quality of the open-habitat matrix of cattle pastures, rather than by patch metrics such as fragment size. The stronger relationship observed between fragment size and functional diversity might be related to the higher habitat variability in larger forest fragments (August 1983), including higher complexity of the vertical forest strata (Laurance 1994), allowing the persistence of species further characterised by different ecomorphological attributes.
Indeed, despite the presence of selective logging in the study area, especially of trees with high economic value, large trees persist in forest fragments larger than 100 ha in our study area (M. Santos-Filho, Pers. Obs.). The structure and complexity of the vegetation are important in the supply of resources such as food, shelter, and other dimensions of the ecological niche of a species, being of fundamental importance for a species to persist in a certain area (Alho 1981; August 1983). This more complex tridimensionality of larger forest fragments is likely to contribute to decreasing the interspecific competition, minimising, therefore, species niche overlap (Borges-Matos et al. 2016; Galetti et al. 2016). Nevertheless, the effect of tree density or canopy cover on the overall functional diversity remained undetected in this study. Meanwhile, it is possible that these local habitat variables did not capture the complexity of the vertical forest strata that could be further better represented in terms of aggregated tree biomass and height (Benchimol and Peres 2015).
Over and above the stronger relationship between functional diversity and fragment size than that of taxonomic diversity, both dimensions were highly correlated. Following the Island Biogeography Theory, larger forest areas are expected to sustain higher species richness (MacArthur and Wilson 1967), which, under relatively low functional redundancy (i.e., functional traits are rapidly lost from the assemblage as taxonomic diversity declines), would contribute to higher functional diversity (Tucker et al. 2018). The correlation between these two dimensions of diversity was therefore expected, as local species extinctions in fragmented landscapes are typically not random processes, with some species with traits being more prone to undergo extinction in the first place (Newbold et al. 2014). Based on that principle, our results suggest that considering either taxonomic or functional diversity may to some extent be sufficient to predict the other (Safi et al. 2011; Dreiss et al. 2015). A consistently positive relationship between taxonomic and functional diversities with the fragment area has been observed for small mammals elsewhere in the Amazon (Palmeirim et al. 2021), as well as in the Atlantic Forest biomes (Bovendorp et al. 2019). Moreover, the steeper (and stronger) relationship observed between functional diversity and the fragment size, when compared to that observed for the taxonomic dimensions, further hints at low functional redundancy characterising the small mammal assemblages across the fragmented landscape. In fact, as larger forest fragments typically tend to harbour more species-rich assemblages, those fragments end up accumulating more species traits (Dainese et al. 2015; Farneda et al. 2018). We would then expect some functional redundancy of those assemblages which would be also more resilient to disturbance (Petchey et al. 2007; Ricotta et al. 2016). Although functional redundancy has been noted for some small mammal assemblages in either the Amazon (Palmeirim et al. 2021) or the Atlantic Forest biomes (Bovendorp et al. 2019), this does not seem to be the case for this mammal group in Southern Brazilian Amazon.
Furthermore, functional diversity was also marginally affected by the incidence of previous fire events in the forest fragment, implying that the effect of fire has a particular effect on species harbouring certain traits. Overall, natural fires are known to induce changes in the small mammal assemblages (Briani et al. 2004), with wildfires generally having negative effects on species diversity (Mendes-Oliveira et al. 2012 but see Berlinck et al. 2021). In a global review, body size and habitat preference were most important in explaining variation in small mammal species’ responses to fire (Griffiths and Brook 2014). Our analysis of the functional composition of small mammal assemblages using community-weighted mean trait values allowed us to unveil the identity of those traits: locomotion habit and matrix tolerance. Fragments subject to previous fire disturbance were characterised by either less terrestrial or more arboreal small mammal assemblages (but see Figueiredo and Fernandez 2004). From the 24 taxa recorded, four species (16.7% of all species; Table S2) were classified as arboreal and eight (33.3%; Table S2) as scansorial, including the two most abundant species. In addition to the arboreal species, our results are also likely to reflect an increase in the abundance of scansorial species in the fire-disturbed fragments. Forest fires typically occur at low heights, so those tend to affect mostly terrestrial and fossorial species (Mendes-Oliveira et al. 2012), while scansorial and arboreal species can persist using the canopy of the trees (Griffiths and Brook 2014). In the aftermath of forest fires, trophic resource availability is further expected to be low at the forest floor level, and again species that can use the upper understorey and the forest canopies would be expected to do better under higher fire-related disturbance (Griffiths and Brook 2014). This same reasoning might further explain why small mammal assemblages with higher matrix tolerance were unexpectedly found in not previously fire-disturbed fragments. Indeed, terrestrial species—the most negatively affected by fire events—also are characterised by a higher matrix tolerance (mean ± SD = 0.376 ± 0.948), when compared to both scansorial and arboreal species (0.054 ± 0.038).
The averaged trophic level of small mammal assemblages persisting in the fragmented landscape was particularly affected by local habitat variables: a higher averaged trophic level was associated with higher tree densities and lower arthropod biomass. Higher tree density reflects a less disturbed habitat (Laurance et al. 2006; Benchimol and Peres 2015), which would be in accordance with expectations given that higher trophic levels also tend to be more sensible to disturbance given that they would need more productive habitats in comparison to lower trophic levels (Wang et al. 2010; Farneda et al. 2015). However, the finding that assemblages characterised by higher trophic levels were associated with lower arthropod biomass was at first unexpected. Indeed, we were expecting to find assemblages composed by an overall higher trophic level, i.e., with the predominance of insectivorous, in habitats where arthropods are widely available (Lambert et al. 2005, 2006). The opposite relationship observed in which a higher predominance in insectivorous/carnivorous small mammals is associated with lower arthropod biomass suggests that small mammals are instead controlling arthropod biomass through top-down mechanisms as commonly reported to be the case of higher trophic levels (Terborgh et al. 2001; Estes et al. 2011). At least part of the arthropod biomass recorded in this study consists of non-native dung-beetles that are bought by farmers and released in the nearby cattle pastures to speed up the decomposition of the cattle faeces, as a strategy adopted in more intensively grazed areas (M. Santos-Filho, pers. obs.). As the mechanisms acting to shape species diversity typically depend on the availability of trophic resources (Terborgh 2015), it is possible that an augmented arthropod availability led top-down forces to act in this case. In any case, this is a speculative interpretation of our results, given the absence of any data to support it. Furthermore, some of the models in our study were not very strong particularly when predicting taxonomic diversity (R2 = 0.262), as well as some of the CWM trait values, namely body mass (R2 = 0.151) and matrix tolerance (R2 = 0.248). As such, we urge caution when interpreting those results, given that other unaccounted variables might also play a role in dictating small mammal diversity in the fragmented landscape.
Conservation implications
Given the surrogacy in the relative predictability of taxonomic and functional diversity by fragment area, either of these two dimensions of diversity can be used to assess the co-effects of habitat loss, fragmentation, and consequent degradation on small mammal assemblages (Safi et al. 2011; Dreiss et al. 2015). Nevertheless, the use of individual functional traits allowed a more general predictive ability of the different trajectories of each of the functional groups in the aftermath of fragmentation. Indeed, fire disturbance is expected to promote an increase in the abundance of scansorial and arboreal species (yet, not necessarily matrix-tolerant ones), while higher trophic levels will depend on reasonable tree density and enough arthropod abundance. Moreover, small mammals seem to be exerting top-down pressures on the arthropod community. Supporting the functional diversity of small non-flying mammal communities is essential because it boosts the stability, endurance, and resilience of ecosystems (Wood et al. 2017). To maintain the taxonomic and functional integrity of small mammal assemblages, forest fragment size should be maximised and its habitat quality maintained mostly through preventing fire disturbance. We argue that properly incorporating such guidelines within country-level legislation would be a major policy advantage, yet challenging to put into practice, to preclude regional-scale biodiversity collapse and associated losses in ecosystem services.
Data availability
The data used in this study has been made available in the corresponding supplementary material.
References
Abreu MJP, Pinheiro M, Lederman MR (2013) Mosaico Da Amazônia Meridional: Vencendo Limites Geográficos e Integrando Gestão. 1a edição. Brasília, Brazil: WWF-Brasil. Available in: https://d3nehc6yl9qzo4.cloudfront.net/downloads/mam_livro_vencendo_limites_geograficos_final.pdf
Aguirre-Gutiérrez J, Malhi Y, Lewis SL, Fauset S, Adu-Bredu S, Affum-Baffoe K, Baker TR, Gvozdevaite A, Hubau W, Moore S, Peprah T, Ziemińska K, Phillips OL, Oliveras I (2020) Long-term droughts may drive drier tropical forests towards increased functional, taxonomic and phylogenetic homogeneity. Nat Commun 11:3346
Alho CJR (1981) Small mammal populations of Brazilian Cerrado: the dependence of abundance and diversity on habitat complexity. Rev Bras Biol 41:223–230
Amaral DL, Fonzar BC (1982) Levantamento de recursos naturais. In: RADAMBRASIL. Folha SD 21. Cuiabá. Rio de Janeiro, MME, 540 pp
Arroyo-Rodríguez V, González-Perez IM, Garmendia A, Solà M, Estrada A (2013) The relative impact of forest patch and landscape attributes on black howler monkey populations in the fragmented Lacandona rainforest, Mexico. Landsc Ecol 28:1717–1727
August PV (1983) The role of habitat complexity and heterogeneity in structuring tropical mammal communities. Ecology 64:1495–1507
Barlow J, Lennox GD, Ferreira J, Berenguer E, Lees AC, Nally RM, Gardner TA (2016) Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature 535(7610):144–147
Barton K (2016) MuMIn: multi-model inference. R Package Version 1(15):6
Benchimol M, Peres CA (2015) Edge-mediated compositional and functional decay of tree assemblages in Amazonian forest islands after 26 years of isolation. J Ecol 103:408–420
Berlinck CN, Lima LHA, Carvalho Junior EARD (2021) Historical survey of research related to fire management and fauna conservation in the world and in Brazil. Biota Neotrop 21:e20201144
Borges-Matos C, Aragón S, Da Silva MNF, Fortin MJ, Magnusson WE (2016) Importance of the matrix in determining small-mammal assemblages in an Amazonia forest-savanna mosaic. Biol Conserv 204:417–425
Botta-Dukát Z (2005) Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. J Veg Sci 16:533–540
Bovendorp RS, Brum FT, McCleery RA, Baiser B, Loyola R, Cianciaruso MV, Galetti M (2019) Defaunation and fragmentation erode small mammal diversity dimensions in tropical forests. Ecography 42:23–35
Briani DC, Palma AR, Vieira EM, Henriques RP (2004) Post-fire succession of small mammals in the Cerrado of central Brazil. Biodivers Conserv 13:1023–1037
Burnham KP, Anderson DR (2002) A practical information-theoretic approach. Model selection and multimodel inference, 2nd edn. Springer, New York
Dainese M, Leps J, de Bello F (2015) Different effects of elevation, habitat fragmentation and grazing management on the functional, phylogenetic and taxonomic structure of mountain grasslands. Perspect Plant Ecol Evol Syst 17:44–53
Debastiani VJ, Pillar VD (2012) SYNCSA—R tool for analysis of metacommunities based on functional traits and phylogeny of the community components. Bioinformatics 28:2067–2068
Devictor V, Julliard R, Jiguet F (2008) Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 117:507–514
Dreiss LM, Burgio KR, Cisneros LM, Klingbeil BT, Patterson BD, Presley SJ, Willig MR (2015) Taxonomic, functional, and phylogenetic dimensions of rodent biodiversity along an extensive tropical elevational gradient. Ecography 38:876–888
ESRI (2018) ArcMap 10.1. Environmental Systems Research Institute Inc., Redlands
Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, Carpenter SR, Essington TE, Holt RD, Jackson JBC, Marquis RJ, Oksanen L, Oksanen T, Paine RT, Pikitch EK, Ripple WJ, Sandin SA, Scheffer M, Schoener TW, Shurin JB, Sinclair ARE, Soulé ME, Virtanen R, Wardle DA (2011) Trophic downgrading of planet Earth. Science 333:301–306
Fahrig L (2013) Rethinking patch size and isolation effects: the habitat amount hypothesis. J Biogeogr 40:1649–1663
Farneda FZ, Rocha R, López-Baucells A, Groenenberg M, Silva I, Palmeirim JM, Bobrowiec PED, Meyer CF (2015) Trait-related responses to habitat fragmentation in Amazonian bats. J Appl Ecol 52:1381–1391
Farneda FZ, Rocha R, López-Baucells A, Sampaio EM, Palmeirim JM, Bobrowiec PE, Bobrowiec PED, Meyer CF (2018) Functional recovery of Amazonian bat assemblages following secondary forest succession. Biol Conserv 218:192–199
Figueiredo MDSL, Fernandez FAS (2004) Contrasting effects of fire on populations of two small rodent species in fragments of Atlantic Forest in Brazil. J Trop Ecol 20:225–228
Filgueiras BK, Peres CA, Melo FP, Leal IR, Tabarelli M (2021) Winner–loser species replacements in human-modified landscapes. Trends Ecol Evol 36:545–555
Galetti M, Rodarte RR, Neves CL, Moreira M, Costa-Pereira R (2016) Trophic niche differentiation in rodents and marsupials by stable isotopes. PLoS One 11:e0152494
Griffiths AD, Brook BW (2014) Effect of fire on small mammals: a systematic review. Int J Wildland Fire 23:1034–1043
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Lovejoy TE, Sexton JO, Austin MP, Collins CD, Cook WM, Damschen EI, Ewers RM, Foster BL, Jenkins CN, King AJ, Laurance WF, Levey DJ, Margules CR, Melbourne BA, Nicholls AO, Orrock JL, Song DX, Townshend JR (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1:e1500052
Hansen MC, Wang L, Song XP, Tyukavina A, Turubanova S, Potapov PV, Stehman SV (2020) The fate of tropical forest fragments. Sci Adv 6:eaax8574
Hartig MF (2019) Package ‘DHARMa’. R package version 0.2.3. https://cran.r-hub.io/web/packages/DHARMa/DHARMa.pdf
Laliberté E, Legendre P (2010) A distance-based framework for measuring functional diversity from multiple traits. Ecology 91:299–305
Lambert TD, Malcolm JR, Zimmerman BL (2005) Effects of mahogany (Swietenia macrophylla) logging on small mammal communities, habitat structure, and seed predation in the southeastern Amazon Basin. For Ecol Manag 206:381–398
Lambert TD, Malcolm JR, Zimmerman BL (2006) Amazonian small mammal abundances in relation to habitat structure and resource abundance. J Mammal 87:766–776
Laurance WF (1994) Rainforest fragmentation and the structure of small mammal communities in tropical Queensland. Biol Conserv 57:205–219
Laurance WF, Yensen E (1991) Predicting the impacts of edge effects in fragmented habitats. Biol Conserv 55:77–92
Laurance WF, Nascimento HEM, Laurance SG, Andrade AC, Fearnside PM, Ribeiro JEL, Capretz RL (2006) Rain forest fragmentation and the proliferation of successional trees. Ecology 87:469–482
Laurance WF, Camargo JL, Fearnside PM, Lovejoy TE, Williamson GB, Mesquita RC, Meyer CFJ, Bobrowiec PED, Laurance SG (2018) An Amazonian rainforest and its fragments as a laboratory of global change. Biol Rev 93:223–247
Lavorel S, Grigulis K, McIntyre S, Williams NSG, Garden D, Dorrough J, Berman S, Quétier F, Thébault A, Bonis A (2008) Assessing functional diversity in the field – methodology matters! Funct Ecol 22:134–147
Lees AC, Peres CA (2008) Avian life-history determinants of local extinction risk in a hyper-fragmented neotropical forest landscape. Anim Conserv 11:128–137
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Press Princeton, Princeton
McGarigal K, Cushman SA, Ene E (2012) FRAGSTATS v4: spatial pattern analysis program for categorical and continuous maps. https://www.umass.edu/landeco/research/fragstats/fragstats.html. Accessed 01 Jan 2022
Mendenhall CD, Karp DS, Meyer CF, Hadly EA, Daily GC (2014) Predicting biodiversity change and averting collapse in agricultural landscapes. Nature 509:213
Mendes-Oliveira AC, Santos PGPD, Carvalho-Júnior OD, Montag LFDA, Lima RCSD, Maria SLSD, Rossi RV (2012) Edge effects and the impact of wildfires on populations of small non-volant mammals in the forest-savanna transition zone in Southern Amazonia. Biota Neotrop 12:57–63
Meynard CN, Devictor V, Mouillot D, Thuiller W, Jiguet F, Mouquet N (2011) Beyond taxonomic diversity patterns: how do α, β and γ components of bird functional and phylogenetic diversity respond to environmental gradients across France? Glob Ecol Biogeogr 20:893–903
Michalski F, Peres CA (2007) Disturbance-mediated mammal persistence and abundance-area relationships in Amazonian forest fragments. Conserv Biol 21:1626–1640
Newbold T, Scharlemann JP, Butchart SH, Şekercioğlu ÇH, Joppa L, Alkemade R, Purves DW (2014) Functional traits, land-use change and the structure of present and future bird communities in tropical forests. Glob Ecol Biogeogr 23:1073–1084
Oliveira VA, Amaral-Filho ZP, Vieira PC (1982) Levantamento de recursos naturais. In: RADAMBRASIL. Folha SD 21. Cuiabá. Rio de Janeiro: MME, 540 pp
Paglia AP, Fonseca GD, Rylands AB, Herrmann G, Aguiar LM, Chiarello AG, Patton JL (2012) Lista Anotada dos Mamíferos do Brasil. Occasional Papers in Conservation biology 6, 2nd ed. Conservation International, Arlington, 76p
Palmeirim AF, Santos-Filho M, Peres AC (2020) Marked decline in forest-dependent small mammals following habitat loss and fragmentation in an Amazonian deforestation frontier. PLoS One 15:e0230209
Palmeirim AF, Farneda FZ, Vieira MV, Peres CA (2021) Forest area predicts all dimensions of small mammal and lizard diversity in Amazonian insular forest fragments. Landsc Ecol 36:3401–3418
Pardini R, de Souza SM, Braga-Neto R, Metzger JP (2005) The role of forest structure, fragment size and corridors in maintaining small mammal abundance and diversity in an Atlantic forest landscape. Biol Conserv 124:253–266
Peres CA, Gardner TA, Barlow J, Zuanon J, Michalski F, Lees AC, Vieira ICG, Moreira FMS, Feeley KJ (2010) Biodiversity conservation in human-modified Amazonian forest landscapes. Biol Conserv 143:2314–2327
Petchey OL, Evans KL, Fishburn IS, Gaston KJ (2007) Low functional diversity and no redundancy in British avian assemblages. J Anim Ecol 76:977–985
Prevedello JA, Vieira MV (2010) Does the type of matrix matter? A quantitative review of the evidence. Biodivers Conserv 19:1205–1223
R Core Team (2015) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Resende MS, Sandanielo A, Couto EG (1994) Zoneamento agroecológico do sudoeste do Estado de Mato Grosso. Documento 4. EMPAER/EMBRAPA
Ricotta C, de Bello F, Moretti M, Caccianiga M, Cerabolini BE, Pavoine S (2016) Measuring the functional redundancy of biological communities: a quantitative guide. Methods Ecol Evol 7:1386–1395
Rurangwa ML, Aguirre‐Gutiérrez J, Matthews TJ, Niyigaba P, Wayman JP, Tobias JA, Whittaker RJ (2021) Effects of land‐use change on avian taxonomic, functional and phylogenetic diversity in a tropical montane rainforest. Divers Distrib 27:1732–1746
Safi K, Cianciaruso MV, Loyola RD, Brito D, Armour-Marshall K, Diniz-Filho JAF (2011) Understanding global patterns of mammalian functional and phylogenetic diversity. Philos. Trans R Soc Lond B Biol Sci 3662:536–2544
Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774
Salgado-Luarte C, Escobedo VM, Stotz GC, Rios RS, Arancio G, Gianoli E (2019) Goat grazing reduces diversity and leads to functional, taxonomic, and phylogenetic homogenization in an arid shrubland. Land Degrad Dev 30:178–189
Santos-Filho M, Peres CA, Da Silva DJ, Sanaiotti TM (2012) Habitat patch and matrix effects on small-mammal persistence in Amazonian forest fragments. Biodivers Conserv 21:1127–1147
Sikes RS (2016) Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J Mammal 97:663–688
Silva DJ, Palmeirim AF, Santos-Filho M, Sanaiotti TM, Peres CA (2022) Habitat quality, not patch size, modulates lizard responses to habitat loss and fragmentation in the southwestern Amazon. J Herpetol 56:75–83
Terborgh JW (2015) Toward a trophic theory of species diversity. PNAS 112:11415–11422
Terborgh J, Lopez L, Nuñez P, Rao M, Shahabuddin G, Orihuela G, Riveros M, Ascanio R, Adler GH, Lambert TD, Balbas L (2001) Ecological meltdown in predator-free forest fragments. Science 294:1923–1926
Tucker CM, Davies TJ, Cadotte MW, Pearse WD (2018) On the relationship between phylogenetic diversity and trait diversity. Ecology 99:1473–1479
Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E (2007) Let the concept of trait be functional! Oikos 116:882–892
Wang Y, Bao Y, Yu M, Xu G, Ding P (2010) Nestedness for different reasons: the distributions of birds, lizards and small mammals on islands of an inundated lake. Divers Distrib 16:862–873
Weiss KC, Ray CA (2019) Unifying functional trait approaches to understand the assemblage of ecological communities: synthesizing taxonomic divides. Ecography 42:2012–2020
Wood CM, Mckinney ST, Loftin CS (2017) Intraspecific functional diversity of common species enhances community stability. Ecol Evol 7:1553–1560
Funding
Open access funding provided by FCT|FCCN (b-on). This study was funded by a Brazilian Ministry of Education (CNPq) studentship to JVMC and (FAPEMAT) to TR at the Universidade do Estado de Mato Grosso (UNEMAT). We further acknowledge the financial support from the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement no. 854248 (TROPIBIO).
Author information
Authors and Affiliations
Contributions
MSF and AFP conceived the ideas; MSF defined the sampling design; MSF collected the data; AFP analysed the data and wrote the first draft of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Andrzej Zalewski.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Santos-Filho, M., Ribeiro, T., da Silva, D.J. et al. Drivers of functional diversity in small-bodied mammals across a deforestation frontier in the Southern Brazilian Amazon. Mamm Res 69, 271–282 (2024). https://doi.org/10.1007/s13364-024-00740-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-024-00740-7