Abstract
Stable isotope analysis of animal tissue samples is increasingly used to study the trophic ecology of target species. The isotopic signatures respond to the type of diet, but also to the environmental conditions of their habitat. In the case of omnivorous, seasonal or opportunistic feeding species, the interpretation of isotopic values is more complex, as it is largely determined by food selection, either due to individual choice or because of availability. We analysed C and N isotopes in brown bear (Ursus arctos) hair from four isolated populations of south-western Europe (Cantabrian, Pyrenees, Central Apennines and Alpine) accounting for the geographical and climatic differences among the four areas. We found inter-population differences in isotopic signatures that cannot be attributed to climatic differences alone, indicating that at least some bears from relatively higher altitude populations experiencing higher precipitation (Pyrenees) show a greater consumption of animal foods than those from lower altitudes (Cantabrian and Apennines). The quantification of isotopic niche space using Layman’s metrics identified significant similarities between the Cantabrian and Central Apennine samples that markedly differ from the Pyrenean and Alpine. Our study provides a baseline to allow further comparisons in isotopic niche spaces in a broad ranged omnivorous mammal, whose European distribution requires further conservation attention especially for southern isolated populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The brown bear (Ursus arctos) is the largest carnivore of Europe. Its geographical range includes the whole Holarctic realm and the IUCN currently considers this species as “Least Concern”. Within Europe, populations of brown bear are increasing in number although a great disparity in population size occurs between the south and the north of the continent (Chapron et al. 2014). Due to their small size, highly fragmented and human-impacted landscapes, bear populations from Italy and Spain/France are of a particular conservation concern (Penteriani et al. 2021). In the first half of the twentieth century, the bear population in the Cantabrian mountain range of northern Spain split into two subpopulations (western and eastern). Estimates of population size in the 1990s were no more than 60 individuals for the western subpopulation (Wiegand et al. 1998) and about 14 individuals in the eastern subpopulation (Clevenger and Purroy 1991). The most recent estimates from genetic sampling give a figure of 223 bears, most in the western subpopulation and only 19 in the eastern subpopulation of the Cantabrian Mountains (Pérez et al. 2014). In the Pyrenees, bears were almost extinct in 1995 with an alarmingly reduced population of only 5 individuals (Camarra and Dubarry 1997). After the introduction of 2 males and 6 females from Slovenia in 1996–1997 and 2006, 30 individuals were counted in 2015 (Piédallu et al. 2016) and a minimum of 46 detected in 2017 (Parres et al. 2020). Italian brown bears persist with two different and isolated populations. One, in the central Apennines, coincides with the relict population of Apennine brown bears (Ursus arctos marsicanus), numbering around 50 individuals (Ciucci et al. 2015) and critically endangered according to the regional IUCN criteria (Rondinini et al. 2013). The second, in the central Alps, originates from the successful re-introduction in Trentino and currently comprises a minimum of 43 individuals (Tosi et al. 2015) with no connectivity with the neighbouring Slovenian bear population.
Concerns related to the long-term future of these populations remain and major conservation efforts have been put in place, especially to protect the endemism of the Apennine (Thomsen et al. 2021) and Cantabrian Mountain populations (Rodríguez et al. 2007). Within this context, tools are now needed to understand differences in ecology and behaviour of these isolated populations to ensure their functional role within south-European landscapes. In addition, the particularities of each population must be known in order to adapt specific conservation policies (Martínez-Abraín et al. 2021).
Previous genetic evaluations suggested that these south-western bear populations reflect glacial refugia (Taberlet and Bouvet 1994); however, a recent genomic approach confirmed, at least for the Apennine population, an origin of about 1500 years which in turn resulted in the remarkable evolution of specific phenotypic and behavioural traits (Benazzo et al. 2017). Studies of ancient DNA also do not support the hypothesis of glacial refugia (Ersmark et al. 2019). In the north of the Iberian Peninsula, the Iberian Pleistocene lineage of bears was replaced by one coming from a cryptic Atlantic refuge that entered through the Pyrenees after the Late Glacial Maximum (García-Vázquez et al. 2019).
Available information on the feeding ecology of all these bear populations confirms a strong element of seasonality and opportunism, which is typical of the species (Swenson et al. 2021). Cantabrian and Pyrenean brown bears have a diet generally characterised by the major consumption of vegetable compared to animal matter (Couturier 1954; Clevenger et al. 1992; Braña et al. 1993; Naves et al. 2006; Rodríguez et al. 2007). In the Cantabrian Mountains, the food most frequently eaten in spring is grass, although carrion consumption also increases compared to the rest of the year. In summer, the dominant food is fleshy fruits, and in autumn and winter, hard mast (nuts). The season in which animal protein is most important is summer, although the volume of insects (ants, for example) in their diet is almost double that of mammals (Braña et al. 1993). Pyrenean brown bears have been reported to consume more animal-derived food in addition to tubers (such as Conopodium majus), roots and, occasionally, aquatic animals such as frogs and trout (Couturier 1954). The diet of the Apennine brown bears was also described as being characterised by major consumption of a seasonally diversified array of vegetables (Zunino and Herrero 1972; Ciucci et al. 2014). In terms of annual energetic contribution, the ranking for the Apennine bear population is as follows: (i) hard mast (beechnuts and acorns) and fleshy fruits, (ii) herbaceous vegetation and insects (mostly ants), (iii) wild ungulates and livestock, and (iv) roots (mainly carrots). Hard mast is particularly important in autumn during hyperphagia, with fleshy fruits (especially buckthorn berries) contributing more during late summer. Summer data support higher consumption of herbs, forbs and animal proteins (wild ungulates, particularly red deer as expected by their availability, Ciucci et al. 2014; Careddu et al. 2021).
For the re-introduced Alpine population, preliminary data based on DNA metabarcoding support a great consumption of vegetable matter (De Barba et al. 2014) with high frequency of occurrence for plants belonging to the Asteraceae, Apiaceae and Maleae families. Damage reports by individual bears also suggest that livestock consumption can be relatively high although a quantitative incidence of this phenomenon in the diet of the whole population is lacking (Groff et al. 2014, 2015). Altogether, these data on the south-western populations of brown bear support a highly flexible ecology in relation to food availability with predominant consumption of vegetable matter, as expected from brown bear geographic diet variation (Bojarska and Selva 2012).
Using methods proposed by Turner et al. (2010) and Jackson et al. (2011), we analyse the isotopic niche of these populations testing the hypothesis that isotopic niche should differ between geographically distinct populations. Stable isotope analysis (SIA) is an important complementary method to assess diet, feeding ecology and ecophysiology of mammals (Crawford et al. 2008; Ben-David and Flaherty 2012). For dietary studies, carbon and nitrogen isotopes are most often used. In terrestrial ecosystems, the carbon isotopic signature is primarily related to the proportion of C3 or C4 plants in the diet, as the two types of plants differ in the way they take up CO2 during photosynthesis and have divergent δ13C values (O’Leary 1988; Farquhar et al. 1989). In Europe, C4 plants are not common. Its percentage ranges from 1.1 in central Europe to 5.6 in south-western Europe, and is even lower in mountainous areas with temperate vegetation, where the arid conditions that favour its development do not exist (Collins and Jones 1986; Pyankov et al. 2010). However, in some regions there is extensive cultivation of maize (Zea mays), a C4 plant, which can be consumed by wildlife directly or indirectly (through the intake of livestock fed on this type of crop). If ingested regularly, its isotopic signature will be appreciably recorded in the tissues of the consumer. Inputs of marine protein in diet produce also a distinctive δ13C signature (Chisholm et al. 1982) but, unlike their North American relatives that consume seasonally migrating salmon, there is no evidence of marine protein intake in European brown bears (Swenson et al. 2007). Other sources of δ13C variation are related to climatic and geographical variables, such as rainfall, temperature, insolation, altitude or tree cover, with sometimes cumulative, sometimes opposing effects on isotopic values (Dawson et al. 2002; Körner et al. 1991; Diefendorf et al. 2010). In herbivores, the mean observed diet–hair offset in δ13C was + 3.2‰, with a range of + 2.7 to + 3.5‰ (Sponheimer et al. 2003a). Each step in the food chain, from the primary consumer onwards, produces an additional increase in δ13C up to 1‰ (Bocherens and Drucker, 2003).
The nitrogen isotopic signature in plants also depends on geographic and climatic factors related to temperature that influences the microbial activity in soils and thus the fixation of atmospheric N. To a lesser extent, it depends also on altitude and rainfall (Amundson et al. 2003). The dietary isotopic offset is more pronounced than for carbon. δ15N increases by 3.5 to 5‰ at each step of the trophic chain from primary up to tertiary consumers (Bocherens and Drucker 2003), although in various types of herbivores there are notable differences between species (Sponheimer et al. 2003b).
There have been a number of studies exploring isotopic variation in bears as a proxy to discriminate diet between species and populations (Hildebrand et al. 1996, 1999; Hobson et al. 2000). Isotopes vary considerably between different tissues, with hair being demonstrated to record bear’s diet during the hair growth period, without undergoing subsequent turnover, unlike, for example bone collagen (Hilderbrand et al. 1996). Meta-analyses of the North American grizzly (U. arctos horribilis) based on isotopes extracted from hair demonstrated the expected high incidence of salmon diet in populations closer to the coastline, while terrestrial prey (wild ungulates) represent a larger portion of grizzly diet from mountainous and more internal regions (Mowat and Heard 2006). For European brown bear, with the exception of Apennine bears (Careddu et al. 2021), there are currently no extensive isotopic data published based on hair even if this is relatively available in the field and has been targeted for non-invasive genetic analyses (Pérez et al. 2009; De Barba et al. 2014; Gervasi et al. 2012).
Vulla et al. (2009) proposed an ecogeographical gradient for representative omnivorous carnivores (including bears), suggesting that at higher latitudes omnivorous species should consume more animal matter. Accordingly, we hypothesised that the nitrogen isotope ratio should increase following a geographical gradient from the Apennines, through Pyrenees, then Cantabrian Mountains and finally central Alps. Other factors that may have an influence are geographical and climatological, as there are differences in altitude between the populations studied, and subtle climatic variations. Considering the high dependency of all these populations on plant food, we predict a geographical effect both in nitrogen and carbon isotopic signatures reflecting those variations.
Materials and methods
Specimen collection
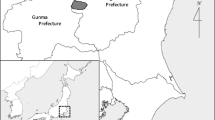
The data set comprises hair (n = 32) from 20 distinct locations opportunistically collected during genetic non-invasive surveys or from museum specimens (Fig. 1). Hairs from the Cantabrian population (n = 14) were collected in hair traps or rub trees between 2007 and 2014, aside from one female sample collected in 1991. The sex of nine individuals was known.
Map showing locations of sampled individuals from each of the four brown bear (Ursus arctos) populations: blue circles, Cantabrian; green triangles, Pyrenean; purple inverted triangles, Alpine; red squares, Apennine. The map was generated using Simplemappr (https://www.simplemappr.net/)
The sample from the Pyrenees included three specimens from the Museu de Ciènces Naturals de Barcelona of the early twentieth century (of which one was male, one female and one juvenile), and one bear re-introduced from Slovenia in 2011. The Italian samples were all collected from hair snag or rub trees sites both in the Apennines (2001, n = 7) and central Alps (2013, n = 7). Specimens from the Apennines were not individually recognised while all the hairs from Alpine population belonged to 6 adult males and 1 female that were constantly monitored in the Adamello Brenta National Park.
Sample preparation and isotopic extraction
We collected at least 0.5 g of guard hair for each specimen, excluding the scalp. The use of whole hairs makes it possible to obtain an isotopic signal averaged over the entire period of hair growth, avoiding the difficulty of assigning a specific time interval for each section of hair, since hairs from different parts of the body do not grow at the same rate or reach the same length.
SIA was carried out in three laboratories: Institute of Geology of the University of A Coruña, Spain (IUX-UDC; n = 27), NERC National Environmental Isotope Facility, British Geological Survey (Nottingham, UK, NEIF; n = 4, © UK Research and Innovation 2022) and the University of Liverpool, UK (n = 2).
At IUX-UDC, dirt, natural oils and museum preservatives were removed from the samples following a standard protocol: the hair was sonicated for 1 h in a 2:1 methanol:dichloromethane solution (O'Connell et al. 2001), after which the procedure was repeated. This was followed by two 20-min rinse in Millipore water, also in a sonicator. The hair was then left to dry in a desiccator chamber. Since the hairs grow over several months during which the diet may vary, the samples were homogenised by cutting the entire length into segments of less than 1 mm and mixing them for analysis. Two sub-samples of approx. 200 mg of guard hair from each animal were weighed in tin capsules using a UMX-2 balance (Mettler Toledo). The determination of δ15N and δ13C was carried out by combustion in an EA1108 elemental analyser (Carlo Erba Instruments) linked via a ConFlo III interface to a MAT253 isotope ratio mass spectrometer (ThermoFinnigan). In each analytical sequence, USGS 40 (− 4.52‰), USGS41a (+ 47.55‰), IAEA-N-1 (+ 0.4‰), IAEA-N-2 (+ 20.3‰) and USGS-25 (− 30.4‰) were used as secondary standards for δ15N. For δ13C, USGS 40 (− 26.39‰), USGS41a (+ 36.55‰), NBS 22 (− 30.031‰) and USGS 24 (− 16.049‰) were used. Replicates (n = 10) of an internal standard (acetanilide) evaluated the precision of the measurement (standard deviation), resulting in ± 0.15‰ for both C and N. The preservation of hair keratin was measured by its atomic C:N ratio, which should be between 2.9 and 3.8 (O’Connell et al. 2001).
At the NEIF, each analysis was performed on a composite of one to six whole hairs (equivalent to 0.6 mg for combined δ13C and δ15N isotope ratio measurement). Isotope ratios of carbon and nitrogen were measured by continuous flow-elemental analysis-isotope ratio mass spectrometry (CF-EA-IRMS). The instrumentation was a ThermoFinnigan continuous flow system, comprising a Delta Plus XL isotope ratio mass spectrometer interfaced with a “Flash/EA” elemental analyser via a ConFlo III interface. All samples were analysed in triplicate. Hair carbon and nitrogen isotope ratios were calibrated using an in-house reference material Hair-2 (calibrated against CH7, USGS40, USGS41, N-1 and N-2, IAEA) and using additional check standards of nylon and Hair-1 (calibrated against CH7, USGS40, USGS41, N-1 and N-2, IAEA). Replicates (n = 17) of an internal standard (Hair-2) evaluated the precision of the measurement (standard deviation), resulting in ± 0.03‰ for carbon and ± 0.12‰ for nitrogen.
At University of Liverpool, washed (Milli-Q water 18 MΩ cm−1) de-fatted (2:1 methanol:dichloromethane) dry hair samples were ground (liquid N2) and weighed into tin cups. They were then analysed in duplicate using an elemental analyser (Costech) coupled to a Delta V Advance mass spectrometer (Thermoquest). Samples were combusted at 1000 °C and diluted with N2 prior to entering ECS 4010 CH/N/CN reaction tube and the mass spectrometer. USGS24, USGS40 and USGS41 were used to determine the accuracy (δ13C < 0.1‰, δ15N < 0.5‰) and precision (δ13C < 0.05‰, δ15N < 0.7‰) of the EA/IRMS and to correct the isotopic values obtained from the bear samples.
Isotopic values were extracted at least twice for each specimen and to ensure consistency across the labs, six hairs collected from the same locations were analysed by at least two labs separately and values compared. For carbon values, standard deviation ranged for the same specimens between 0.065 and 0.71 (mean st.dev = 0.41) while for nitrogen the range was between 0.18 and 0.63 (mean st.dev = 0.30). These data were within the range of intra-population variation and were smaller than inter-population standard deviation (mean st.dev δ13C = 0.91; mean st.dev δ15N = 1.65). The whole raw data is available in the Electronic Supplementary Material.
Calibration curve
Due to samples being collected in different time periods, we accounted for the Suess effect caused by the contribution of CO2 depleted in δ13C due to the burning of fossil fuels (Keeling 1979) using a calibration curve. We used data from Friedli et al. (1986) (covering years 1744 to 1953 aD), Keeling et al. (1989) (years 1978 to 1988 aD), Leuenberger et al. (1992) (40,000 BP to 1270 aD), Francey et al. (1999) (years 1006 to 1997 aD) and Graven et al. (2017) (years 1851 to 2015 aD). All these data cover the last 40,000 years, and specially the last 100 years, across the range of our samples. The data were fitted to an exponential curve with the statistical programme PAST (Hammer et al. 2001). The line obtained was as follows:
where x is the year of collection before 2019, being 2019 = 0. This curve showed a good fit (R2 = 0.97) and allowed us to calibrate all the carbon isotopic values to the year 2014, which corresponds to the most recent date of our samples.
Data analysis
The final dataset consisted of 32 individual samples from 16 distinct locations for which latitude and longitude were available (Supporting Information 1). To test the hypothesis that isotopic signature differs among populations, we first computed Layman metrics in the R environment using packages “SIBER” and “siar” (Layman et al. 2007; Turner et al. 2010). These include total isotopic niche area (TA, used as a measure of the total foraging width of a population, Layman and Allgeier 2012), mean distance to the centroid of each population (CD), and the eccentricity of the scatter of isotopic values in the isoscape (E). Eccentricity is used to determine whether the points scatter similarly in all direction, in which case it will be close to 0.0, or if the points scatter in a linear fashion, with this value approaching 1.0 (Turner et al. 2010).
To compare niche area and overlap among the brown bear populations, a Bayesian statistical model [Stable Isotope Bayesian Ellipses in R (SIBER)] was applied to the isotopic data to compute and delimit stable isotope Bayesian ellipses, correcting ellipses areas (SEAB) for sample size in each group, and measure the level of overlap between populations (Turner et al. 2010).
To test the hypothesis of geographical/environmental gradient in the isotopic values, each geographic location was characterised by latitude, longitude, altitude and additional climatic data (precipitation and temperature) combined into 19 bioclimatic variables as described by Fick and Hijmans (2017). These climatic parameters are representative of historical climatic conditions that covers 30 years between 1970 and 2000 and have been proved to effectively characterise eco-physiological adaptations of small (e.g. Moreno-García and Baiser 2021) and large (e.g. Di Marco et al. 2021) mammals. Localities with the same bioclimatic parameters were combined into one data point and this reduced the sample to n = 14 distinct geographic samples. Carbon and nitrogen values were equally averaged for each unique location.
The 19 bioclimatic variables together with altitude were first reduced through a principal component analysis (named ENV PCA) of the correlation matrix (recommended when variables have different magnitudes, Hair 2010), in order to account for the high level of correlation and reduce data dimensionality (Jiang 2018) using PAST (Hammer et al. 2001). Latitude and longitude data were transformed into a truncated geographic distance matrix subjected to a principal coordinates of neighbourhood matrix (PCNM) using the package vegan (Oksanen et al. 2022). This procedure generates PCNM eigenfunctions that represent a spectral decomposition of the spatial relationships among our 14 sampled locations and accounts for spatial bias in statistical models (Borcard and Legendre 2002).
Linear models were built in order to identify the best predictors (ENV PC scores and PCNMs) of carbon and nitrogen values across the 14 unique geographical samples. Dependent variables were initially identified using a forward selection procedure through the function “forward.sel”, package ade.spatial (version 03–16, Dray et al. 2022). This was applied in two separate instances using carbon or nitrogen as separate independent variables. Multiple linear models of several complexities were compared using Akaike information criteria for small samples (AICc) and delta AICc (Brewer et al. 2016) to identify the best combination of ENV PCs and PCNMs (independent variables) that predicts variation into carbon or nitrogen isotopic data (dependent variables).
Results
The preservation of hair keratin was adequate in all samples, even those from museum preserved pelts, with a mean atomic C/N ratio of 3.4 (sd = 0.1, range between 3.2 and 3.6). The δ13C values extracted from the bear hair sample ranged from − 23.15 to − 20.15‰ (mean ± SD: − 22.33 ± 0.89‰, n = 32) while δ15N values for the bears ranged from 1.1 to 9.2‰ (mean ± SD: 5.56 ± 1.65‰, n = 32).
The isotopic values averaged by populations show a large disparity especially for the Alpine and Pyrenean sets. The standard ellipses of these two populations are also large. This is to be expected in the Pyrenean population due to the scarcity of data and their different chronology (Fig. 2, Table 1).
The Bayesian estimate for SEA (= SEAb) differed significantly between populations (one-way ANOVA, F = 32.678, df = 3, 431, p = 0001). However, post hoc multiple comparisons, based on Tukey, identified overlap between the Pyrenean and Alpine populations and between the Cantabrian and the Apennine (Table 2). These results concur with the density plot (Fig. 3), which shows the modes for standard ellipse area of the Pyrenean and Alpine populations at similar levels and the Cantabrian and Apennine populations at similar levels on the y axis.
Density plots showing the measures of uncertainty and central tendency (red symbols = mode) of Bayesian standard ellipse areas (SEAC, corrected for small samples in a bivariate distribution for 2 df) based on 100 posterior draws of parameters showing 95, 75 and 50% credibility intervals from light to dark grey respectively for Central Apennine (n = 7), Cantabrian (n = 14), Pyrenean (n = 4) and Alpine (n = 7) populations of brown bear (Ursus arctos). BGS data Copyright UKRI 2022
The polar histograms and the polar density plots (Fig. 4) show that the Cantabrian and Apennine populations occupy the most similar isotopic niche space. This matches the isotope biplot, which showed the Apennine standard ellipse and convex hull fitting within those of the Cantabrian. The Pyrenean population has the highest position on the δ15N axis of all the populations, and along the δ13C axis was higher than the Cantabrian and the Apennines. Generally, the Alpine population has slightly lower δ15N and larger δ13C than the Cantabrian and Apennine populations.
The estimated area of overlap between each pair of ellipses was calculated independently for 1000 draws. When comparing the Pyrenean and Alpine populations and the Apennine and Cantabrian populations, most of the estimates for the overlaps occupy the 20–30% range; however, some estimates range up to 80% (Table 3).
The standard ellipses for the Cantabrian and Alpine populations are likely distinct and occupy different niches. Similarly, for the Pyrenean and Apennine populations, the highest frequencies occupy the 5–10% range, and none of the estimates reaches higher than 50% (Table 3).
The smallest overlap estimates were between the Apennine and Alpine populations with the highest frequency ranging between 0 and 2%, supporting significantly different isotopic niches. The Cantabrian and Pyrenean populations’ histogram had a frequency range of 0–60% and the highest frequencies were for 15–20%, showing some cross-over (Table 3). Overall, these overlap estimates suggest that there is some level of distinction between the standard ellipses of all the populations, the least being between Pyrenean and Alpine and Cantabrian and Apennine and the highest being between Apennine vs Alpine. The 14 unique isotopic sampling locations could be clearly separated through ENV PCA that extracted 13 PC vectors of which the first two explained cumulatively 81.80% of variance (Fig. 5A, Supplementary Information 1). ENV PC1 (45.49% var.) was positively loaded on altitude (r = 0.78) as well as multiple bioclimatic parameters associated with precipitation such as BIO12 (r = 0.93), BIO13 (r = 0.90), BIO14 (r = 0.86) and BIO16 (r = 0.96) (see also Supplementary Information 1 for additional loading values). This axis separates alpine locations (negative ENV PC1 scores) from the rest due to their relatively lower rainy precipitations and some samples from low altitudes (Fig. 5A). ENV PC2 exhibit an opposite trend being negatively loaded on altitude (r = − 0.54) and positively on temperature parameters (BIO1 r = 0.76, BIO2 r = 0.76, BIO3 r = 0.81, BIO6 r = 0.96, BIO9 r = 0.82, BIO11 r = 0.96, see also Supplementary Information 1) and it distinguished Alpine and Pyrenean locations (negative scores, lower temperatures) from the Apennine and Cantabrian mountains range (positive scores).
The forward selection identified ENV PC1 to correlate positively with δ15N (r = 0.708, p = 0.0044, Fig. 5B) while ENV PC2 negatively with δ13C (r = − 0.745, p = 0.0022; but see below for predictive model selection). None of the other ENV PCs could be identified as potential predictors of isotopic values while the PCNM1, 3, 4 and 5 were all selected to generate linear models. Based on AICc criteria, the best predictive model for δ13C included only PCNM3, PCNM4 and PCNM5 (without the inclusion of ENV PC2) as predictors while for δ15N both ENV PC1 and PCNM5 combined resulted in the top model selection (Table 4, Supplementary Information 2).
Discussion
Isotopic niche analysis
We identified significant geographical differences in isotopic signature of south-western European brown bear populations. The geographical isolation appears to have impacted ecological habits in the brown bear that historically managed to survive even in ecosystems heavily impacted by human activity, such as the one we sampled in the central Apennine. Our samples incorporate a large range of environments including mountainous ecosystems from an altitude of 590 m (in Cantabrian Mountains) up to ca 2200 m (Pyrenees).
Cantabrian brown bears inhabit steep highlands with sparse tree cover and feed predominantly on vegetable matter (García-Vázquez et al. 2018). Similarly, Apennine brown bears feed on predominantly vegetable matter such as herbs and fleshy fruits. They have an overall low consumption of large mammals, which peaks in the spring/early summer. During summer and autumn, males have higher δ15N values than females (Careddu et al. 2021).
We found that the Apennine and Cantabrian populations are likely to have similar isotopic niches. This may be due to their predominantly herbivorous diets. Field data has shown that both Cantabrian and Apennine brown bear faeces contain predominantly plant material with scavenged animal protein supplementing their diet (Clevenger et al. 1992; Ciucci et al. 2014). A more recent isotopic analysis also noted a relatively lower degree of meat consumption for the Apennine bears compared to other populations (Careddu et al. 2021).
Pyrenean brown bears have been found to have δ13C values slightly more positive than ungulates inhabiting the same area, and δ15N values more positive than ancient Mont Ventoux brown bears, suggesting a greater consumption of meat (Bocherens et al. 2004). The Pyrenean and Alpine populations both had similar isotopic values, which is likely due to the higher prevalence of animal protein throughout the year. Recent studies indicate that the Pyrenean reintroduced bear population uses the same habitat as the extinct native bears (Palazón 2017), which had already been described as feeding on more animal protein than the Cantabrian bears (Couturier 1954). Still, the results from the Pyrenean bears should be taken with caution, for several reasons: they are only 4 individuals, 2 of them adults from the beginning of the twentieth century. A third individual is a juvenile, also from the beginning of the twentieth century, whose high δ15N value seems to indicate that its isotopic signature could be affected by lactation. The fourth bear is current, reintroduced from Slovenia and a larger sample size is required to better interpret the feeding ecology of the current population.
Influence of geography and bioclimatic parameters
For mammalian herbivores, variation in soil δ15N, root depth of dietary plant and the composition of woody plants in the diet can all influence the δ15N value. Generally, δ13C in herbivorous mammals is influenced by the composition of C3 plants in their diet. However, the canopy effect also has an influence (Cormie and Schwartz, 1994; Vogel et al. 1990; Ambrose and DeNiro 1986). Other studies have also shown an interaction between δ13C and climatic variables, including hours of sunshine, precipitation amount, humidity and temperature (Van Klinken et al. 1994).
Our data for the first time allowed to disentangle the geographical signal (that was strong especially on the brown bear hair δ13C values) from the climatic one that influence δ15N values through temperature and altitudinal parameters summarised by ENV PC1. In previous research, the impact of altitude on isotopic signatures was demonstrated for plants, and subsequently for domestic and wild ungulates, with a negative altitudinal gradient for δ15N (Männel et al. 2007; Hofman-Kamińska et al. 2018). The same negative correlation was observed in European Pleistocene cave bears (Krajcarz et al. 2016), whose diet was shown to be primarily plant-based (Bocherens 2019). This is mainly due to the differences in precipitation and temperature. The relationship found between δ15N and ENV PC1 is consistent with the observation of a more carnivorous diet in the Pyrenean and some individual Alpine bears. Higher precipitation parameters imply more snowfall that has been demonstrated to impact significantly diet of brown bear at large spatial scale (Bojarska and Selva 2012). It is important to note that the relationship we identified between δ15N and altitude + precipitation parameters is not impacted by geographical bias that still occurs in this data as supported by the inclusion of PCNM5 in the best predictive model. This is possibly a limitation related to our sample and such relationship should be better tested on a larger database.
The δ13C values in our sample showed a stronger bias in geographical sampling with none of the ENV PCs being selected as best predictors. Generally, for plants, there is a positive relationship between temperature and δ13C (Liu et al. 2017); however, this association has never been tested accounting for geographical bias. Plant δ13C are expected to show latitudinal and altitudinal trends (Körner et al. 1991; Kohn 2010), related to climatic and insolation conditions in each area. This is mirrored by some herbivorous species such as white-tailed deer (Cormie and Schwartz, 1994), but this trend does not extend to all herbivores. For instance, there was no systematic association between δ13C values in bone collagen and Pleistocene cave bears from various European regions (Krajcarz et al. 2016) or different chronology during MIS 3 (Grandal-d’Anglade et al. 2019).
Our data suggests that a possible strong spatial fidelity in levels of herbivory occurs in the diet of the sampled brown bears; however, this would again require a larger spatial variation. Mowat and Heard (2006) analysed isotopic variation in 81 distinct populations of North American grizzly bears and identified a strong link between level of salmon in the diet and carbon/nitrogen isotopic variation. Still, no spatial filter was included in their diet and link towards levels of herbivory have not been identified yet on a large spatial scale.
The recent work of Careddu et al. (2021) on Apennine bears revealed for δ13C variation to be linked with individual preferences. Managed bears generally exhibited 0.9‰ higher in δ13C than non-managed bears and estimated herb consumption has been detected to change from 15.5 up to 73% within the same season. Consequently, it is possible that the climatic pattern observed in our data reflects proportional differences within the sampled populations of individuals that consume more anthropogenic sources of food or show highly distinct preference in herb consumption.
Bear impact on livestock and crops
Data on livestock predation suggest a relatively higher level of damage for the Alpine population than for the Cantabrian, with livestock damage ratios of 0.26 ± 0.045 for Western Cantabrian population, 0.070 ± 0.043 for the Eastern one and 0.47 ± 0.23 for Trento (Bautista et al. 2017). Across the Pyrenees, there is a strong dissymmetry (Catalonia 0.47 ± 0.23 and France 6.8 ± 1.8) in the damage ratio to livestock, and our Pyrenean bears, with the highest values of δ15N being on the Catalonian side.
Tosi et al. (2015) found that for bears inhabiting Trentino between 2000 and 2012, their number was correlated with the number of damage events. Considering that we sampled mostly males some of which were directly responsible of livestock damages in 2013 (i.e. M11, M6 and MJ2G1, Groff et al. 2014, 2015), we would expect to find elevated δ15N values in these bears, which is not always the case. In summary, the data seem to indicate that although the damage to livestock is apparently significant, it is not systematically reflected in the isotopic values, calling into question the true impact of this type of feeding on the individuals involved.
The impact on crops is more difficult to identify from isotopic values, except in the case of maize. Maize is a C4 plant with a distinctive high δ13C value. It is cultivated in both the northern Iberian and Italian peninsula, including the highland regions, where, on the other hand, wild C4 plants are very rare (Collins and Jones 1986). Specifically in Apennine bears, no direct or indirect contribution of C4 plants in the diet was detected (Careddu et al. 2021). The δ13C values in the four populations studied are consistent with a food chain based on C3 plants. Some alpine specimens show carbon signatures somewhat biased towards positive values, which could have been acquired by feeding on maize or by consumption of maize-fed cattle. However, the δ13C values (which do not exceed − 20‰ in any case) are not high enough to point to an appreciable influence of this type of crop on their diet.
Evidently, variation within and between the population sampled can be particularly high in isotopic values. This suggests that livestock/crop consumption can be difficult to detect in brown bears and more controlled experimental data might be needed to elucidate this issue. Our data suggest relatively similar (lower) rate of livestock and crop consumption in Cantabrian and Apennine bears compared to (higher) rate in the sampled populations of central Alps and the Pyreneans.
Conclusion
Isotopic analyses might provide additional insights on the ecological adaptations of brown bear populations. We identified potential overlap for the Cantabrian and Apennine bears while individuals sampled in central Alps and Pyreneans were possibly impacted by altitudinal gradients and greater consumption in animal protein. Our data set the scene for merging databases from different locations across Europe to obtain further information on the variability in brown bear feeding behaviour that might become critical to plan for the future of the species.
Data availability
All data obtained during the development of the work are attached as supplementary information along with the submission of the manuscript.
References
Ambrose SH, DeNiro MJ (1986) The isotopic ecology of East African mammals. Oecologia 69:395–406
Amundson R, Austin AT, Schuur EAG, Yoo K, Matzek V, Kendall C, Uebersax A, Brenner D, Baisden WT (2003) Global patterns of the isotopic composition of soil and plant nitrogen. Global Biogeochem Cycles 17:1031. https://doi.org/10.1029/2002GB001903
Bautista C, Naves J, Revilla E, Fernández N, Albrecht J, Scharf AK, Rigg R, Karamanlidis AA, Jerina K, Huber D, Palazón S, Kont R, Ciucci P, Groff C, Dutsov A, Seijas J, Quenette P-I, Olszańska A, Shkvyria M, Adamec M, Ozolins J, Jonozovič M, Selva N (2017) Patterns and correlates of claims for brown bear damage on a continental scale. J Appl Ecol 54:282–292. https://doi.org/10.1111/1365-2664.12708
Ben-David M, Flaherty EA (2012) Stable isotopes in mammalian research: a beginner’s guide. J Mammal 93(2):312–328. https://doi.org/10.1644/11-MAMM-S-166.1
Benazzo A, Trucchi E, Cahill JA, Delser PM, Mona S, Fumagalli M, Bunnefeldh L, Cornetti L, Ghirotto S, Girardik M, Ometto L, Panziera A, Rota-Stabelli O, Zanetti E, Karamanlidis A, Groff C, Paule L, Gentile L, Vilà C, Vicario S, Boitani L, Orlando L, Fuselli S, Vernesi C, Shapiro B, Ciucci P, Bertorelle G (2017) Survival and divergence in a small group: the extraordinary genomic history of the endangered Apennine brown bear stragglers. Proc Nat Ac Sci 114(45):E9589–E9597. https://doi.org/10.1073/pnas.170727911
Bocherens H (2019) Isotopic insights on cave bear palaeodiet. Hist Biol 31(4):410–421. https://doi.org/10.1080/08912963.2018.1465419
Bocherens H, Argant A, Argant J, Billiou D, Crégut-Bonnoure E, Donat-Ayache B, Philippe M, Thinon M (2004) Diet reconstruction of ancient brown bears (Ursus arctos) from Mont Ventoux (France) using bone collagen stable isotope biogeochemistry (13C, 15N). Canadian J Zool 82:576–586. https://doi.org/10.1139/z04-017
Bocherens H, Drucker D (2003) Trophic level isotopic enrichment of carbon and nitrogen in bone collagen: case studies from recent and ancient terrestrial ecosystems. Int J Osteoarch 13(1–2):46–53. https://doi.org/10.1002/oa.662
Bojarska K, Selva N (2012) Spatial patterns in brown bear Ursus arctos diet: the role of geographical and environmental factors. Mamm Rev 42(2):120–143. https://doi.org/10.1111/j.1365-2907.2011.00192.x
Borcard D, Legendre P (2002) All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol Model 153(1–2):51–68. https://doi.org/10.1016/S0304-3800(01)00501-4
Braña F, Naves J, Palomero G (1993) Hábitos alimenticios y configuración de la dieta del oso pardo en la cordillera cantábrica. EL oso pardo (Ursus arctos) en España, Colección Técnica, ICONA, Madrid, pp 81–103
Brewer MJ, Butler A, Cooksley SL (2016) The relative performance of AIC, AICC and BIC in the presence of unobserved heterogeneity. Meth Ecol Evol 7(6):679–692. https://doi.org/10.1111/2041-210X.12541
Camarra JJ, Dubarry E (1997) The brown bear in the French Pyrenees: distribution, size, and dynamics of the population from 1988 to 1992. Bears 9(2):31–35
Careddu G, Ciucci P, Mondovì S, Calizza E, Rossi L, Costantini ML (2021) Gaining insight into the assimilated diet of small bear populations by stable isotopeanalysis. Sci Rep 11(14118):1–16. https://doi.org/10.1038/s41598-021-93507-y
Chisholm BS, Nelson DE, Schwarcz HP (1982) Stable-carbon isotope ratios as a measure of marine versus terrestrial protein in ancient diets. Sci 216(4550):1131–1132. https://doi.org/10.1126/science.1257553
Ciucci P, Gervasi V, Boitani L, Boulanger J, Paetkau D, Prive R, Tosoni E (2015) Estimating abundance of the remnant Apennine brown bear population using multiple noninvasive genetic data sources. J Mammal 96(1):206–220. https://doi.org/10.1093/jmammal/gyu029
Ciucci P, Tosoni E, Di Domenico G, Quattrociocchi F, Boitani L (2014) Seasonal and annual variation in the food habits of Apennine brown bears, central Italy. J Mammal 95:572–586. https://doi.org/10.1644/13-MAMM-A-218
Clevenger AP, Purroy FJ (1991) Demografía del oso pardo (Ursus arctos) en la Cordillera Cantábrica. Ecología 5:243–256
Clevenger AP, Purroy FJ, Pelton MR (1992) Food habits of brown bears (Ursus arctos) in the Cantabrian Mountains, Spain. J Mammal 73:415–421. https://doi.org/10.2307/1382077
Collins RP, Jones MB (1986) The influence of climatic factors on the distribution of C4 species in Europe. Vegetatio 64(2):121–129
Cormie AB, Schwarcz HP (1994) Effects of climate on deer bone δ15N and δ13C: lack of precipitation effects on δ15N for animals consuming low amounts of C4 plants. Geochim Cosmochim Acta 60:4161–4166
Couturier MAJ (1954) L’Ours Brun. Ursus arctos, L. (Grenoble), Isére, France, p 905
Crawford K, Mcdonald RA, Bearhop S (2008) Applications of stable isotope techniques to the ecology of mammals. Mamm Rev 38(1):87–107. https://doi.org/10.1111/j.1365-2907.2008.00120.x
Chapron G, Kaczensky P, Linnell JD, Von Arx M, Huber D, Andrén H et al (2014) Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346(6216):1517–1519. https://doi.org/10.1126/science.1257553
Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP (2002) Stable isotopes in plant ecology. Ann Rev Ecol Syst 33:507–559. https://doi.org/10.1146/annurev.ecolsys.33.020602.095451
De Barba M, Miquel C, Boyer F, Mercier C, Rioux D, Coissac E, Taberlet P (2014) DNA metabarcoding multiplexing and validation of data accuracy for diet assessment: application to omnivorous diet. Mol Ecol Res 14(2):306–323. https://doi.org/10.1111/1755-0998.12188
Diefendorf AF, Mueller KE, Wing SL, Koch PL, Freeman KH (2010) Global patterns in leaf 13C discrimination and implications for studies of past and future climate. Proc Nat Acad Sci 107(13):5738–5743. https://doi.org/10.1073/pnas.091051310
Di Marco M, Pacifici M, Maiorano L, Rondinini C (2021) Drivers of change in the realised climatic niche of terrestrial mammals. Ecography 44(8):1180–1190. https://doi.org/10.1111/ecog.05414
Dray S, Bauman D, Blanchet G, Borcard D, Clappe S, Guenard G, Jombart T, Larocque G, Legendre P, Madi N, Wagner HH (2022) adespatial: multivariate multiscale spatial analysis. R package version 0.3–16, https://CRAN.R-project.org/package=adespatial.
Ersmark E, Baryshnikov G, Higham T, Argant A, Castaños P, Döppes D, Gasparik M, Germonpré M, Lidén K, Lipecki G, Marciszak A, Miller T, Moreno-García M, Pacher M, Robu M, Rodriguez-Varela R, Rojo Guerra M, Sabol M, Spassov N, Storå J, Valdiosera C, Villaluenga A, Stewart JR, Dalén L (2019) Genetic turnovers and northern survival during the last glacial maximum in European brown bears. Ecol Evol 9:5891–5905. https://doi.org/10.1002/ece3.5172
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Ann Rev Plant Phys Plant Mol Biol 40:503–537
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37(12):4302–4315. https://doi.org/10.1002/joc.5086
Francey RJ, Allison CE, Etheridge DM, Trudinger CM, Enting IG, Leuenberger M, Langenfelds RL, Michel E, Steele LP (1999) A 1000-year high precision record of δ13C in atmospheric CO2. Tellus 51B(2):170–193. https://doi.org/10.3402/tellusb.v51i2.16269
Friedli H, Lötscher H, Oeschger H, Siegenthaler U, Stauffer B (1986) Ice core record of the 13C/12C ratio of atmospheric col in the past two centuries. Nature 324:237–238. https://doi.org/10.1038/324237A0
García-Vázquez A, Pinto-Llona AC, Grandal-d’Anglade A (2018) Brown bear (Ursus arctos L.) palaeoecology and diet in the Late Pleistocene and Holocene of the NW of the Iberian Peninsula: a study on stable isotopes. Quat Int 481:42–51. https://doi.org/10.1016/j.quaint.2017.08.063
García-Vázquez A, Pinto Llona AC, Grandal-d’Anglade A (2019) Post-glacial colonization of Western Europe brown bears from a cryptic Atlantic refugium out of the Iberian Peninsula. Hist Biol 31(5):618–630. https://doi.org/10.1080/08912963.2017.1384473
Gervasi V, Ciucci P, Boulanger J, Randi E, Boitani L (2012) A multiple data source approach to improve abundance estimates of small populations: the brown bear in the Apennines, Italy. Biol Cons 152:10–20. https://doi.org/10.1016/j.biocon.2012.04.005
Grandal-d’Anglade A, Pérez-Rama M, García-Vázquez A, González-Fortes GM (2019) The cave bear’s hibernation: reconstructing the physiology and behaviour of an extinct animal. Hist Biol 31(4):429–441. https://doi.org/10.1080/08912963.2018.1468441
Graven H, Allison CE, Etheridge DM, Hammer S, Keeling RF, Levin I, Meijer HAJ, Rubino M, Tans PP, Trudinger CM, Vaughn BH, White JWC (2017) Compiled records of carbon isotopes in atmospheric CO2 for historical simulations in CMIP6. Geosci Model Dev 10:4405–4417. https://doi.org/10.5194/gmd-10-4405-2017
Groff C, Bragalanti N, Rizzoli R, Zanghellini P (2014) Rapporto Orso 2013 del Servizio Foreste e fauna della Provincia Autonoma di Trento. Ufficio Faunistico PAT - Publistampa Arti grafiche
Groff C, Bragalanti N, Rizzoli R, Zanghellini P (2015) Rapporto Orso 2014 del Servizio Foreste e fauna della Provincia Autonoma di Trento. Ufficio Faunistico PAT - Publistampa Arti grafiche
Hair JF (2010) Multivariate data analysis: a global perspective. Upper Saddle River, N.J. : Pearson Education, 7th ed..
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeont Electr 4(1):9. http://palaeo-electronica.org/2001_1/past/issue1_01.htm.
Hilderbrand GV, Farley SD, Robbins CT, Hanley TA, Titus K, Servheen C (1996) Use of stable isotopes to determine diets of living and extinct bears. Can J Zool 74:2080–2088. https://doi.org/10.1139/z96-236
Hildebrand GV, Jenkins SG, Schwartz CC, Hanley TA, Robbins CT (1999) Effect of seasonal differences in dietary meat intake on changes in body mass and composition in wild and captive brown bears. Can J Zool 77:1623–1630. https://doi.org/10.1139/z99-133
Hobson KA, McLellan BN, Woods JG (2000) Using stable carbon (d13C) and nitrogen (d15N) isotopes to infer trophic relationships among black and grizzly bears in the upper Columbia River basin, British Columbia. Can J Zool 78:1332–1339. https://doi.org/10.1139/z00-069
Hofman-Kamińska E, Bocherens H, Borowik T, Drucker DG, Kowalczyk R (2018) Stable isotope signatures of large herbivore foraging habitats across Europe. PLoS ONE 13(1):e0190723. https://doi.org/10.1371/journal.pone.0190723
Jiang F (2018) Bioclimatic and altitudinal variables influence the potential distribution of canine parvovirus type 2 worldwide. Ecol Evol 8(9):4534–4543. https://doi.org/10.1002/ece3.3994
Keeling CD, Bacastow RB, Carter AF, Piper SC, Whorf TP, Heimann M, Mook WG, Roeloffzen, H (1989) A three-dimensional model of atmospheric CO2 transport based on observed winds: 1. Analysis of observational data. In: D. H. Peterson (Ed.), Aspects of climate variability in the Pacific and the Western Americas: Vol. Geophysica, 165–236. American Geophysical Union.
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J Anim Ecol 80(3):595–602. https://doi.org/10.1111/j.1365-2656.2011.01806.x
Keeling CD (1979) The Suess effect: 13carbon-14carbon interrelations. Environ Int 2(4–6):229–300
Körner C, Farquhar GD, Wong SC (1991) Carbon isotope discrimination by plants follows latitudinal and altitudinal trends. Oecologia 88:30–40
Kohn MJ (2010) Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. Proc Natl Acad Sci U S A 107(46):19691–19695. https://doi.org/10.1073/pnas.100493310
Krajcarz MP, Krajcarz MT, Laughlan L, Rabeder G, Sabol M, Wojtal P, Bocherens H (2016) Isotopic variability of cave bears (δ15N, δ13C) across Europe during MIS 3. Quat Sci Rev 131:51. https://doi.org/10.1016/j.quascirev.2015.10.028
Layman CA, Allgeier JE (2012) Characterizing trophic ecology of generalist consumers: a case study of the invasive lionfish in The Bahamas. Mar Ecol Progr Ser 448:131–141. https://doi.org/10.3354/MEPS09511
Layman CA, Arrington DA, Montana CG, Post DM (2007) Can stable isotope ratios provide for community wide measures of trophic structure? Ecol 88:42–48. https://doi.org/10.1890/0012-9658(2007)88[42:csirpf]2.0.co;2
Leuenberger M, Siegenthaler U, Langway CC (1992) Carbon isotope composition of atmospheric CO2 during the last ice age from an Antarctic ice core. Lett Nat 357:488–490
Liu XZ, Li YZ, Feng T, Su Q, Song Y (2017) Carbon isotopes of C3 herbs correlate with temperature on removing the influence of precipitation across a temperature transect in the agro-pastoral ecotone of northern China. Ecol Evol 7:10582–10591. https://doi.org/10.1002/ece3.3548
Männel TT, Auerswald K, Schnyder H (2007) Altitudinal gradients of grassland carbon and nitrogen isotope composition are recorded in the hair of grazers. Glob Ecol Biog 16(5):583–592. https://doi.org/10.1111/j.1466-8238.2007.00322.x
Martínez-Abrain A, Llaneza L, Ballesteros F, Grandal-d’Anglade A (2021) Do apex predators need to regulate prey populations to be a right conservation target? Biol Conserv 261:109281. https://doi.org/10.1016/j.biocon.2021.109281
Moreno-García P, Baiser B (2021) Assessing functional redundancy in Eurasian small mammal assemblages across multiple traits and biogeographic extents. Ecography 44(2):320–333. https://doi.org/10.1111/ecog.05312
Mowat G, Heard DC (2006) Major components of grizzly bear diet across North America. Can J Zool 84:473–489. https://doi.org/10.1139/z06-016
Naves J, Wiegand T, Revilla E, Delibes M (2006) Endangered species constrained by natural and human factors: the case of brown bears in Northern Spain. Cons Biol 17(5):1276–1289
O’Connell TC, Hedges REM, Healey MA, Simpson AHRW (2001) Isotopic comparison of hair, nail and bone: modern analyses. J Archaeol Sci 28:1247–1255
O’Leary MH (1988) Carbon isotopes in photosynthesis. Biosci 38(5):328–336. https://doi.org/10.1006/jasc.2001.0698
Oksanen J, Simpson G, Blanchet F, Kindt R, Legendre P, Minchin P, O'Hara R, Solymos P, Stevens M, Szoecs E, Wagner H, Barbour M, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, De Caceres M, Durand S, Evangelista H, FitzJohn R, Friendly M, Furneaux B, Hannigan G, Hill M, Lahti L, McGlinn D, Ouellette M, Ribeiro Cunha E, Smith T, Stier A, Ter Braak C, Weedon J (2022) vegan: community ecology package. R package version 2.6–2, https://CRAN.R-project.org/package=vegan.
Palazón S (2017) The importance of reintroducing large carnivores: the brown bear in the Pyrenees. Pp. 231–249, in: (J. Catalan, J. Ninot, M. Aniz, eds): "High mountain conservation in a changing world". Advances in global change research, vol 62. Springer, Cham. https://doi.org/10.1007/978-3-319-55982-7_10
Parres A, Palazón S, Afonso I, Quenette P-Y, Batet A, Camarra J-J, Garreta X, Gonçalves S, Guillén J, Mir S, Jato R, Rodríguez J, Sentilles J, Xicola L, Melero Y (2020) Activity patterns in the reintroduced Pyrenean brown bear population. Mamm Res 65:435–444. https://doi.org/10.1007/s13364-020-00507-w
Penteriani V, Karamanlidis AA, Ordiz A, Ciucci P, Boitani L, Bertorelle G, Zarzo-Arias A, Bombieri G, González-Bernardo E, Morini P, Pinchera F, Fernández N, Mateo-Sánchez MC, Revilla E, de Gabriel Hernando M, Mertzanis Y, Melletti M (2021) Bears in human-modified landscapes: the case studies of the Cantabrian, Apennine, and Pindos Mountains. Pp. 260–272, in: (V. Penteriani, M. Melletti, eds): “Bears of the world. Ecology, conservation and management”. Cambridge University Press, Cambridge, U.K. ISBN: 9781108483520.
Pérez T, Naves J, Vázquez JF, Fernández-Gil A, Seijas J, Albornoz J, Revilla E, Delibes M, Domínguez A (2014) Estimating the population size of the endangered Cantabrian brown bear through genetic sampling. Wild Biol 20(5):300–309. https://doi.org/10.2981/wlb.00069
Pérez T, Vázquez F, Naves J, Fernández A, Corao A, Albornoz J, Domínguez A (2009) Non-invasive genetic study of the endangered Cantabrian brown bear (Ursus arctos). Conserv Genet 10(2):291–301. https://doi.org/10.1007/s10592-008-9578-1
Piédallu B, Quenette P-Y, Mounet C, Lescureux N, Borelli-Massines M, Dubarry E, Camarra J-J, Gimenez O (2016) Spatial variation in public attitudes towards brown bears in the French Pyrenees. Biol Cons 197:90–97. https://doi.org/10.1016/j.biocon.2016.02.027
Pyankov VI, Ziegler H, Akhani H, Deigele C, Lüttge U (2010) European plants with C4 photosynthesis: geographical and taxonomic distribution and relations to climate parameters. Bot J Linn Soc 163:283–304. https://doi.org/10.1111/j.1095-8339.2010.01062.x
Rodríguez C, Naves J, Fernández-Gil A, Obeso JR, Delibes M (2007) Long-term trends in food habits of a relict brown bear population in northern Spain: the influence of climate and local factors. Env Cons 34(1):36–44. https://doi.org/10.1017/S0376892906003535
Rondinini C, Battistoni A, Peronace V, Teofili C (2013) Lista Rossa IUCN dei vertebrati Italiani. Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare, Roma, Italy.
Sponheimer M, Robinson T, Ayliffe L, Passey B, Roeder B, Shipley L, Lopez E, Certling T, Dearing D, Ehleringer J (2003a) An experimental study of carbon-isotope fractionation between diet, hair, and feces of mammalian herbivores. Can J Zool 81(5):871–876. https://doi.org/10.1139/z03-066
Sponheimer M, Robinson T, Ayliffe L, Roeder B, Hammer J, Passey B, West A, Certling T, Dearing D, Ehleringer J (2003b) Nitrogen isotopes in mammalian herbivores: hair δ15N values from a controlled feeding study. Int J Osteoarch 13(1–2):80–87. https://doi.org/10.1002/oa.655
Swenson JE, Adamič M, Huber D, Stokke S (2007) Brown bear body mass and growth in northern and southern Europe. Oecologia 153:37–47. https://doi.org/10.1007/s00442-007-0715-1
Swenson JE, Ambarli H, ArnemoJM, Baskin L, Ciucci P, Danilov PI, Delibes M, Elfström E, Evans AL, Groff C, Hertel AG, Huber D, Jerina K, Karamanlidis AA, Kindberg J, Kojola I, Krofel M, Kusak J, Mano T, Melletti M, Mertzanis Y, Ordiz A, Palazón S, Parchizadeh J, Penteriani V, Quenette P-Y, Sergiel A, Selva N, Seryodkin I, Skuban M, Steyaert S, Støen O-G, Tirronen KF, Zedrosser A (2021) Brown bear (Ursus arctos; Eurasia). Pp. 139–161, in: (V. Penteriani, M. Melletti, eds): “Bears of the world. Ecology, conservation and management”. Cambridge University Press, Cambridge, U.K. ISBN: 9781108483520.
Taberlet P, Bouvet J (1994) Mitochondrial DNA polymorphism, phylogeography, and conservation genetics of the brown bear Ursus arctos in Europe. Proc Roy Soc Lond Series b: Biol Sci 255(1344):195–200. https://doi.org/10.1098/rspb.1994.0028
Thomsen B, Thomsen J, Cipollone M, Coose S (2021) Let’s save the bear: a multispecies livelihoods approach to wildlife conservation and achieving the SDGs. J Int Counc Small Bus 2(2):114–124. https://doi.org/10.1080/26437015.2021.1881934
Tosi G, Chirichella R, Zibordi F, Mustoni A, Giovannini R, Groff C, Zanin M, Apollonio M (2015) Brown bear reintroduction in the Southern Alps: to what extent are expectations being met? J Nat Cons 26:9–19. https://doi.org/10.1016/j.jnc.2015.03.007
Turner TF, Collyer MF, Krabbenhoft TJ (2010) A general hypothesis-testing framework for stable isotope ratios in ecological studies. Ecol 91(8):2277–2233. https://doi.org/10.1890/09-1454.1
Van Klinken GJ, van der Plicht H, Hedges REM (1994) Bond 13C/12C ratios reflect (palaeo-) climatic variations. Geophys Res Lett 21(6):445–448. https://doi.org/10.1029/94GL00177
Vogel JC, Eglington B, Auret JM (1990) Isotope fingerprints in elephant bone and ivory. Nature 346(6286):747–749
Vulla E, Hobson KA, Korsten M, Leht M, Martin AJ, Lind A, Männil P, Valdmann H, Saarma U (2009) Carnivory is positively correlated with latitude among omnivorous mammals: evidence from brown bears, badgers and pine martens. Annal Zool Fenn 46(6):395–415. https://doi.org/10.5735/086.046.0601
Wiegand T, Naves J, Stephan T, Fernandez A (1998) Assessing the risk of extinction for the brown bear (Ursus arctos) in the Cordillera Cantabrica, Spain. Ecol Monogr 68(4):539–570. https://doi.org/10.1890/0012-9615(1998)068[0539:ATROEF]2.0.CO;2
Zunino F, Herrero S (1972) The status of the brown bear (Ursus arctos) in Abruzzo National Park. Italy. Biol Cons 4(4):263–272. https://doi.org/10.1016/0006-3207(72)90123-1
Acknowledgements
We are grateful to R. Hartasánchez and D. Pando of the Fund for the Protection of Wild Animals (FAPAS) for providing bear hair samples from the Cantabrian Mountains, to P. Solé Castellarnau, V. García García and the Environment agents of the Conselh Generau d'Aran for the modern sample from the Pyrenees, and to J. Quesada of the Museum of Natural Sciences of Barcelona for samples of naturalised bear pelts from the Pyrenees. Also E. Valderrábamo, veterinarian of Marcelle Natureza Park (Lugo, Spain) for providing samples to improve the isotopic analysis technique on bear hairs. The staff from Parco Nazionale d’Abruzzo Lazio e Molise (PNALM) and Parco Naturale Adamello Brenta (PNAB) supported hair sample data collection in Italy. James Ball helped isotope analyses at University of Liverpool. Karol Zub, Vincenzo Penteriani and one anonymous reviewer provided useful support to improve the quality of this manuscript.
Funding
Financial support for isotopic analyses in Spain was provided by Xunta de Galicia (grant ID: ED431B 2021/17), while G. Guidarelli was supported by ERASMUS + programme (2013/2014) for research visit at Liverpool John Moores University. Isotope analyses performed at the British Geological Survey were funded by the NERC National Environmental Isotope Facility.
Author information
Authors and Affiliations
Contributions
Conceptualisation: Carlo Meloro, Ana García-Vázquez, Aurora Grandal-d’Anglade, Angela L. Lamb, Claudio Groff, Paolo Ciucci, Anna Loy; methodology: Ana García-Vázquez, Denise A. Crampton, Angela L. Lamb, George A. Wolff, Kostas Kiriakoulakis, Giulia Guidarelli, Ana C. Pinto-Llona; formal analysis and investigation: Ana García-Vázquez, DC, AL, George A. Wolff, Kostas Kiriakoulakis, Giulia Guidarelli, Ana C. Pinto-Llona, Aurora Grandal-d’Anglade, Carlo Meloro; writing — original draft preparation: Ana García-Vázquez, Denise A. Crampton, Aurora Grandal-d’Anglade, Carlo Meloro; writing — review and editing: Ana García-Vázquez, DC, Anna Loy, George A. Wolff, Kostas Kiriakoulakis, Giulia Guidarelli, Angela L. Lamb, Paolo Ciucci, CG, Ana C. Pinto-Llona, Aurora Grandal-d’Anglade, Carlo Meloro; funding acquisition: Aurora Grandal-d’Anglade, Giulia Guidarelli; resources: Angela L. Lamb, George A. Wolff, Aurora Grandal-d’Anglade; supervision: Carlo Meloro.
Corresponding author
Ethics declarations
Ethics approval
The authors declare that the study followed the institutional and national ethical guidelines for scientific research in the sites where data were collected.
Consent to participate
All the authors consent to participate in the development of the investigation.
Consent for publication
All the authors consent for the publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by: Karol Zub.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
García-Vázquez, A., Crampton, D.A., Lamb, A.L. et al. Isotopic signature in isolated south-western populations of European brown bear (Ursus arctos). Mamm Res 68, 63–76 (2023). https://doi.org/10.1007/s13364-022-00654-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-022-00654-2