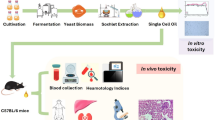
Abstract
Sepsis represents a complex clinical syndrome that results from a harmful host response to infection. The infections most associated with sepsis are pneumonia, intra-abdominal infection, and urinary tract infection. Tea tree oil (TTO) has shown high antibacterial activity; however, it exhibits low aqueous solubility and high volatility, which have motivated its nanoencapsulation. In this study, the performance of nanoemulsions (NE) and nanocapsules (NC) loaded with TTO was compared. These systems were prepared by spontaneous emulsification and nanoprecipitation methods, respectively. Poly-ε-caprolactone or Eudragit® RS100 were tested as polymers for NCs whereas Tween® 80 or Pluronic® F68 as surfactants in NE preparation. Pluronic® F68 and Eudragit® RS100 resulted in more homogeneous and stable nanoparticles. In accelerated stability studies at 4 and 25 °C, both colloidal suspensions (NC and NE) were kinetically stable. NCs showed to be more stable to photodegradation and less cytotoxic than NEs. After sepsis induction by the cecal ligation and puncture (CLP) model, both NE and NC reduced neutrophil infiltration into peritoneal lavage (PL) and kidneys. Moreover, the systems increased group thiols in the kidney and lung tissue and reduced bacterial growth in PL. Taken together, both systems showed to be effective against injury induced by sepsis; however, NCs should be prioritized due to advantages in terms of cytotoxicity and physicochemical stability.
Graphical abstract








Similar content being viewed by others
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Cao C, Yu M, Chai Y. Pathological alteration and therapeutic implications of sepsis-induced immune cell apoptosis. Cell Death Dis. 2019;1–14. Nature Publishing Group.
Milioli MVM, Burger H, Olivieri R, Michels M, Ávila P, Abatti M, et al. The impact of age on long-term behavioral and neurochemical parameters in an animal model of severe sepsis. Neurosci Lett. 2019;708:134339.
Uppu DSSM, Ghosh C, Haldar J. Surviving sepsis in the era of antibiotic resistance: are there any alternative approaches to antibiotic therapy? Microb Pathog. 2015;7–13. Academic Press.
Wang H, Xu T, Lewin MR. Future possibilities for the treatment of septic shock with herbal components. Am J Emerg Med. 2009;107–12.
Hammer KA. Treatment of acne with tea tree oil (melaleuca) products: a review of efficacy, tolerability and potential modes of action. Int J Antimicrob Agents. 2015;106–10. Elsevier.
Quintans JSS, Shanmugam S, Heimfarth L, Araújo AAS, Almeida JRG, Picot L, et al. Monoterpenes modulating cytokines - a review. Food Chem Toxicol. 2019;123:233–57.
Ramage G, Milligan S, Lappin DF, Sherry L, Sweeney P, Williams C, et al. Antifungal, cytotoxic, and immunomodulatory properties of tea tree oil and its derivative components: potential role in management of oral candidosis in cancer patients. Front Microbiol. 2012;3: 1-8.
Brophy JJ, Davies NW, Southwell IA, Stiff IA, Williams LR. Gas chromatographic quality control for oil of Melaleuca terpinen-4-ol type (Australian tea tree). J Agric Food Chem. 1989;37:1330–5.
Halcón L, Milkus K. Staphylococcus aureus and wounds: a review of tea tree oil as a promising antimicrobial. Am J Infect Control. 2004;32:402–8.
Dryden M, Dailly S, Crouch M. A randomized, controlled trial of tea tree topical preparations versus a standard topical regimen for the clearance of MRSA colonization. J Hosp Infect. 2004;56:283–6.
Garozzo A, Timpanaro R, Stivala A, Bisignano G, Castro A. Activity of Melaleuca alternifolia (tea tree) oil on influenza virus A/PR/8: study on the mechanism of action. Antiviral Res. 2011;89:83–8.
Hammer KA, Carson CF, Riley TV. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J Appl Microbiol. 2003;95:853–60.
Hart PH, Brand C, Carson CF, Riley TV, Prager RH, Finlay-Jones JJ. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm Res. 2000;49:619–26.
Zhang X, Guo Y, Guo L, Jiang H, Ji Q. In vitro evaluation of antioxidant and antimicrobial activities of Melaleuca alternifolia essential oil. Biomed Res Int. 2018;2018:1–8.
Yadav E, Kumar S, Mahant S, Khatkar S, Rao R. Tea tree oil: a promising essential oil. Journal of Essential Oil Research. 2017;29:201–213.
Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (Tea Tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50–62.
Battisti MA, Caon T, Machado Campos A. A short review on the antimicrobial micro- and nanoparticles loaded with Melaleuca alternifolia essential oil. J Drug Deliv Sci Technol. 2021;63:102283.
Guideline FDAICHHT. Validation of analytical procedures: text and methodology, Q2 (R1). 1996. 2021.
Surh J, Decker EA, McClements DJ. Utilisation of spontaneous emulsification to fabricate lutein-loaded nanoemulsion-based delivery systems: factors influencing particle size and colour. Int J Food Sci Technol. 2017;52:1408–16.
Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–6.
Wang JJ, Liu KS, Sung KC, Tsai CY, Fang JY. Lipid nanoparticles with different oil/fatty ester ratios as carriers of buprenorphine and its prodrugs for injection. Eur J Pharm Sci. 2009;38:138–46.
Zhang J, Chen XG, Li YY, Liu CS. Self-assembled nanoparticles based on hydrophobically modified chitosan as carriers for doxorubicin. Nanomed Nanotechnol Biol Med. 2007;3:258–65.
Ritter C, Andrades M, Frota MLC, Bonatto F, Pinho RA, Polydoro M, et al. Oxidative parameters and mortality in sepsis induced by cecal ligation and perforation. Intensive Care Med. 2003;29:1782–9.
Young LM, Kheifets JB, Ballaron SJ, Young JM. Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions. 1989;26:335–41.
Aksenov MY, Markesbery WR. Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett. 2001;302:141–5.
Yadav E, Kumar S, Mahant S, Khatkar S, Rao R. Tea tree oil: a promising essential oil. J Essent Oil Res. 2017;201–13. Taylor and Francis Inc.
Bruxel F, Laux M, Wild LB, Fraga M, Koester LS, Teixeira HF. Nanoemulsões como sistemas de liberação parenteral de fármacos. Quim Nova. 2012;35:1827–40.
Santos LP, Caon T, Battisti MA, da Silva CHB, Simões CMO, Reginatto FH, et al. Antioxidant polymeric nanoparticles containing standardized extract of Ilex paraguariensis A. St.-Hil. for topical use. Ind Crops Prod. 2017;108:738–47.
Mirković D, Ibrić S, Balanč B, Knez Ž, Bugarski B. Evaluation of the impact of critical quality attributes and critical process parameters on quality and stability of parenteral nutrition nanoemulsions. J Drug Deliv Sci Technol. 2017;39:341–7.
Flores FC, Ribeiro F, Ferreira Ourique A, Madalena C, Rolim B, De Bona Da Silva C, et al. Nanostructured systems containing an essential oil: protection against volatilization. Quim Nova. 2011.
Joshi AS, Patil CC, Shiralashetti SS, Kalyane NV. Design, characterization and evaluation of Eudragit microspheres containing glipizide. Drug Invent Today. 2013;5:229–34.
Chernysheva YV, Babak VG, Kildeeva NR, Boury F, Benoit JP, Ubrich N, et al. Effect of the type of hydrophobic polymers on the size of nanoparticles obtained by emulsification-solvent evaporation. Mendeleev Commun. 2003;13:65–7.
Schaffazick SR, Pohlmann AR, Mezzaliraa G, Guterres SS. Development of nanocapsule suspensions and nanocapsule spray-dried powders containing melatonin. J Braz Chem Soc. 2006;17:562–9.
dos Chaves PS, Ourique AF, Frank LA, Pohlmann AR, Guterres SS, Beck RCR. Carvedilol-loaded nanocapsules: mucoadhesive properties and permeability across the sublingual mucosa. Eur J Pharm Biopharm. 2017;114:88–95.
Santos SS, Lorenzoni A, Ferreira LM, Mattiazzi J, Adams AIH, Denardi LB, et al. Clotrimazole-loaded Eudragit® RS100 nanocapsules: preparation, characterization and in vitro evaluation of antifungal activity against Candida species. Mater Sci Eng C. 2013;33:1389–94.
Dillen K, Vandervoort J, Van den Mooter G, Ludwig A. Evaluation of ciprofloxacin-loaded Eudragit® RS100 or RL100/PLGA nanoparticles. Int J Pharm. 2006;314:72–82.
Mora-Huertas CE, Fessi H, Elaissari A. Polymer-based nanocapsules for drug delivery. Int J Pharm. 2010;385:113–42.
Anton N, Vandamme TF. Nano-emulsions and micro-emulsions: clarifications of the critical differences. Pharm Res. 2011;978–85. Springer.
Anton N, Benoit JP, Saulnier P. Design and production of nanoparticles formulated from nano-emulsion templates—a review. J Control Release. 2008;128:185–99.
Ghosh I, Schenck D, Bose S, Ruegger C. Optimization of formulation and process parameters for the production of nanosuspension by wet media milling technique: Effect of Vitamin e TPGS and nanocrystal particle size on oral absorption. Eur J Pharm Sci. 2012;47:718–28.
Wang Y, Zheng Y, Zhang L, Wang Q, Zhang D. Stability of nanosuspensions in drug delivery. J Control Release. 2013;172:1126–41.
Haidar I, Harding IH, Bowater IC, Eldridge DS, Charman WN. The role of lecithin degradation on the pH dependent stability of halofantrine encapsulated fat nano-emulsions. Int J Pharm. 2017;528:524–35.
Perugini P, Simeoni S, Scalia S, Genta I, Modena T, Conti B, Pavanetto F. Effect of nanoparticle encapsulation on the photostability of the sunscreen agent, 2-ethylhexyl-p-methoxycinnamate. Int J Pharm. 2002;246(1–2):37–45. https://doi.org/10.1016/S0378-5173(02)00356-3.
Ourique AF, Pohlmann AR, Guterres SS, Beck RCR. Tretinoin-loaded nanocapsules: preparation, physicochemical characterization, and photostability study. Int J Pharm. 2008;352(1–2):1–4. https://doi.org/10.1016/J.IJPHARM.2007.12.035.
Lopes E, Pohlmann AR, Bassani V, Guterres SS. Polymeric colloidal systems containing ethionamide: preparation and physico-chemical characterization. Pharmazie. 2000;55:527–30.
Jumaa M, Müller BW. In vitro investigation of the effect of various isotonic substances in parenteral emulsions on human erythrocytes. Eur J Pharm Sci. 1999;9:207–12.
Sharma A, Madhunapantula SV, Robertson GP. Toxicological considerations when creating nanoparticle-based drugs and drug delivery systems. Expert Opin Drug Metab Toxicol. 2012;47–69.
Fracasso R, Baierle M, Goëthel G, Barth A, Freitas F, Nascimento S, et al. Evaluation of potential acute cardiotoxicity of biodegradable nanocapsules in rats by intravenous administration. Toxicol Res (Camb). 2015;5:168–79.
Chen L, Zhang Y, Liu J, Wei L, Song B, Shao L. Exposure of the murine RAW 264.7 macrophage cell line to dicalcium silicate coating: assessment of cytotoxicity and pro-inflammatory effects. J Mater Sci Mater Med. 2016;27:1–11.
Selvaraj V, Bodapati S, Murray E, Rice KM, Winston N, Shokuhfar T, et al. Cytotoxicity and genotoxicity caused by yttrium oxide nanoparticles in HEK293 cells. Int J Nanomed. 2014;9:1379–91.
Söderberg TA, Johansson A, Gref R. Toxic effects of some conifer resin acids and tea tree oil on human epithelial and fibroblast cells. Toxicology. 1996;107:99–109.
Cassol-Jr OJ, Comim CM, Silva BR, Hermani FV, Constantino LS, Felisberto F, et al. Treatment with cannabidiol reverses oxidative stress parameters, cognitive impairment and mortality in rats submitted to sepsis by cecal ligation and puncture. Brain Res. 2010;1348:128–38.
Andrades M, Ritter C, De Oliveira MR, Streck EL, Fonseca Moreira JC, Dal-Pizzol F. Antioxidant treatment reverses organ failure in rat model of sepsis: role of antioxidant enzymes imbalance, neutrophil infiltration, and oxidative stress. J Surg Res. 2011;167:e307–13.
Lomas-Neira J, Chung CS, Perl M, Gregory S, Biffl W, Ayala A. Role of alveolar macrophage and migrating neutrophils in hemorrhage-induced priming for ALI subsequent to septic challenge. Am J Physiol Lung Cell Mol Physiol. 2006;290:L51–8.
Lomas-Neira JL, Chung CS, Grutkoski PS, Miller EJ, Ayala A. CXCR2 inhibition suppresses hemorrhage-induced priming for acute lung injury in mice. J Leukoc Biol. 2004;76:58–64.
Iskander KN, Craciun FL, Stepien DM, Duffy ER, Kim J, Moitra R, et al. Cecal ligation and puncture-induced murine sepsis does not cause lung injury. Crit Care Med. 2013;41:159–70.
Coria-Avila GA, Gavrila AM, Ménard S, Ismail N, Pfaus JG. Cecum location in rats and the implications for intraperitoneal injections. Lab Anim (NY). 2007;36:25–30.
Caldefie-Chézet F, Fusillier C, Jarde T, Laroye H, Damez M, Vasson M, et al. Potential anti-inflammatory effects of Melaleuca alternifolia essential oil on human peripheral blood leukocytes. Phytother Res. 2006;20:364–70.
Türck P, Fraga S, Salvador I, Campos-Carraro C, Lacerda D, Bahr A, et al. Blueberry extract decreases oxidative stress and improves functional parameters in lungs from rats with pulmonary arterial hypertension. Nutrition. 2020;70:110579.
Banne AF, Amiri A, Pero RW. Reduced level of serum thiols in patients with a diagnosis of active disease. J Anti Aging Med. 2003;6:327–34.
Lee MT, Lin WC, Yu B, Lee TT. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals - a review. Asian Australas J Anim Sci. 2017;299–308.
Najafi-Taher R, Ghaemi B, Kharazi S, Rasoulikoohi S, Amani A. Promising antibacterial effects of silver nanoparticle-loaded tea tree oil nanoemulsion: a synergistic combination against resistance threat. AAPS PharmSciTech. 2017.
Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94:223–53.
Santos RCV, Lopes LQS, dos Santos Alves CF, Fausto VP, Pizzutti K, Barboza V, et al. Antimicrobial activity of tea tree oil nanoparticles against American and European foulbrood diseases agents. J Asia Pac Entomol. 2014;17:343–7.
Acknowledgements
The authors like to thank the LCME-UFSC for technical support during TEM analyses and the Laboratory of Applied Virology for performing the cytotoxicity assays. We would like to thank the Brazilian governmental agencies CNPq and CAPES for the financial support for this project and for the student scholarship, respectively.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Mariana Alves Battisti: conceptualization, methodology, writing—original draft, investigation, and data curation; Larissa Constantino: methodology and data curation; Debora Fretes Argenta: writing—review and editing; Flávio Henrique Reginatto and Felipe Dal Pizzol: Supervision; Thiago Caon: result discussion and writing—review and editing; Angela Machado Campos: conceptualization, supervision, methodology, writing—review and editing, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In vivo studies were performed according to the National Institutes of Health guidelines and approved by the local ethics committee (protocol number: PP00963).
Consent for publication
The authors declare that all authors have been actively involved in the work leading to this paper and all take responsibility for the published work.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Tea tree oil (TTO)-loaded nanocapsules were more stable than nanoemulsions.
• TTO-loaded nanocapsules were less cytotoxic than nanoemulsions.
• TTO-loaded NEs and NCs reduced neutrophil infiltration levels in peritoneal lavage.
• Both nanostructured systems reduced the bacterial growth in peritoneal lavage.
• Both nanoparticles increased group thiols in the kidney and lungs.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Battisti, M.A., Constantino, L., Argenta, D.F. et al. Nanoemulsions and nanocapsules loaded with Melaleuca alternifolia essential oil for sepsis treatment. Drug Deliv. and Transl. Res. 14, 1239–1252 (2024). https://doi.org/10.1007/s13346-023-01458-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-023-01458-w