Abstract
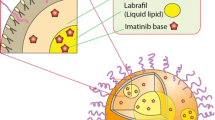
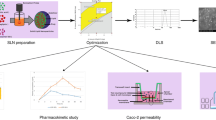
Ticagrelor (TCG), an antiplatelet agent, has low solubility and permeability; thus, there are many trials to apply the pharmaceutical technology for the enhancement of TCG solubility and permeability. Herein, we have developed the TCG high-loaded nanostructured lipid carrier (HL-NLC) and solidified the HL-NLC to develop the oral tablet. The HL-NLC was successfully fabricated and optimized with a particle size of 164.5 nm, a PDI of 0.199, an encapsulation efficiency of 98.5%, and a drug loading of 16.4%. For the solidification of HL-NLC (S-HL-NLC), the adsorbent was determined based on the physical properties of the S-HL-NLC, such as bulk density, tap density, angle of repose, Hausner ratio, Carr’s index, and drug content. Florite R was chosen because of its excellent adsorption capacity, excellent physical properties, and solubility of the powder after manufacturing. Using an S-HL-NLC, the S-HL-NLC tablet with HPMC 4 K was prepared, which is showed a released extent of more than 90% at 24 h. Thus, we have developed the sustained release tablet containing the TCG-loaded HL-NLC. Moreover, the formulations have exhibited no cytotoxicity against Caco-2 cells and improved the cellular uptake of TCG. In pharmacokinetic study, compared with raw TCG, the bioavailability of HL-NLC and S-HL-NLC was increased by 293% and 323%, respectively. In conclusion, we successfully developed the TCG high-loaded NLC tablet, that exhibited a sustained release profile and enhanced oral bioavailability.
Graphical abstract










Similar content being viewed by others
Data availability
Data sharing is not applicable to this article because no datasets were generated or analyzed during the current study.
References
Siller-Matula JM, Jilma B. Ticagrelor: from discovery to Phase III clinical trial. Future Cardiol. 2010;6:753–64.
Goto S, Huang C-H, Park S-J, Emanuelsson H, Kimura T. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome–randomized, double-blind, phase III PHILO study–. Circ J. 2015;79:2452–60.
Gao C-Z, Ma Q-Q, Wu J, Liu R, Wang F, Bai J, et al. Comparison of the effects of ticagrelor and clopidogrel on inflammatory factors, vascular endothelium functions and short-term prognosis in patients with acute ST-Segment Elevation Myocardial Infarction Undergoing Emergency Percutaneous Coronary Intervention: a pilot study. Cell Physiol Biochem. 2018;48:385–96.
Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–800.
Liu S, Wang Z, Hou L, Tian X, Zhang X, Cai W. Predicting the effect of tea polyphenols on ticagrelor by incorporating transporter-enzyme interplay mechanism. Chem Biol Interact. 2020;330:109228.
Huang J, Wang Q, Li T, Xia N, Xia Q. Nanostructured lipid carrier (NLC) as a strategy for encapsulation of quercetin and linseed oil: preparation and in vitro characterization studies. J Food Eng. 2017;215:1–12.
Shah NV, Seth AK, Balaraman R, Aundhia CJ, Maheshwari RA, Parmar GR. Nanostructured lipid carriers for oral bioavailability enhancement of raloxifene: Design and in vivo study. J Adv Res. 2016;7:423–34.
Tran TH, Ramasamy T, Truong DH, Choi H-G, Yong CS, Kim JO. Preparation and characterization of fenofibrate-loaded nanostructured lipid carriers for oral bioavailability enhancement. AAPS PharmSciTech. 2014;15:1509–15.
Tamjidi F, Shahedi M, Varshosaz J, Nasirpour AJ. Nanostructured lipid carriers (NLC): a potential delivery system for bioactive food molecules. Innov Food Sci Emerg Technol. 2013;19:29–43.
Liu D, Li J, Pan H, He F, Liu Z, Wu Q, et al. Potential advantages of a novel chitosan-N-acetylcysteine surface modified nanostructured lipid carrier on the performance of ophthalmic delivery of curcumin. Sci Rep. 2016;6:1–14.
Della Rocca J, Liu D, Lin WJN. Are high drug loading nanoparticles the next step forward for chemotherapy? Nanomedicine. 2012;7:303–5.
Jia L-J, Zhang D-R, Li Z-Y, Feng F-F, Wang Y-C, Dai W-T, et al. Preparation and characterization of silybin-loaded nanostructured lipid carriers. Drug Deliv. 2010;17:11–8.
Son G-H, Na Y-G, Huh HW, Wang M, Kim M-K, Han M-G, et al. Systemic design and evaluation of ticagrelor-loaded nanostructured lipid carriers for enhancing bioavailability and antiplatelet activity. Pharmaceutics. 2019;11:222.
Beloqui A, Solinís MÁ, Gascón AR, del Pozo-Rodríguez A, des Rieux A, Préat V. Mechanism of transport of saquinavir-loaded nanostructured lipid carriers across the intestinal barrier. J Control Rel. 2013;16:115–23.
Hu F-Q, Jiang S-P, Du Y-Z, Yuan H, Ye Y-Q, Zeng S. Preparation and characteristics of monostearin nanostructured lipid carriers. Int J Pharm. 2006;314:83–9.
Sun B, Luo C, Li L, Wang M, Du Y, Di D, et al. Core-matched encapsulation of an oleate prodrug into nanostructured lipid carriers with high drug loading capability to facilitate the oral delivery of docetaxel. Colloids Surf B Biointerfaces. 2016;143:47–55.
Battaglia L, Gallarate M. Lipid nanoparticles: state of the art, new preparation methods and challenges in drug delivery. Expert Opin Drug Deliv. 2012;9:497–508.
Iqbal MA, Md S, Sahni JK, Baboota S, Dang S, Ali J. Nanostructured lipid carriers system: recent advances in drug delivery. J Drug Target. 2012;20:813–30.
Kobierski S, Ofori-Kwakye K, Müller RH, Keck CM. Resveratrol nanosuspensions: Interaction of preservatives with nanocrystal production. Pharmazie. 2011;66:942–7.
Xia D, Shrestha N, van de Streek J, Mu H, Yang M. Spray drying of fenofibrate loaded nanostructured lipid carriers. Asian J Pharm Sci. 2016;11:507–15.
Tian Z, Yi Y, Yuan H, Han J, Zhang X, Xie Y, et al. Solidification of nanostructured lipid carriers (NLCs) onto pellets by fluid-bed coating: preparation, in vitro characterization and bioavailability in dogs. Powder Technol. 2013;247:120–7.
Souto E, Mehnert W, Müller R. Polymorphic behaviour of Compritol® 888 ATO as bulk lipid and as SLN and NLC. J Microencapsul. 2006;23:417–33.
Shete H, Patravale V. Long chain lipid based tamoxifen NLC. Part I: preformulation studies, formulation development and physicochemical characterization. Int J Pharm. 2013;454:573–83.
Salminen H, Helgason T, Aulbach S, Kristinsson B, Kristbergsson K, Weiss J, et al. Influence of co-surfactants on crystallization and stability of solid lipid nanoparticles. J Colloid Interface Sci. 2014;426:256–63.
Lopes MA, Abrahim-Vieira B, Oliveira C, Fonte P, Souza AM, Lira T, et al. Probing insulin bioactivity in oral nanoparticles produced by ultrasonication-assisted emulsification/internal gelation. Int J Nanomedicine. 2015;10:5865.
Müller RH, Jacobs C. Buparvaquone mucoadhesive nanosuspension: preparation, optimisation and long-term stability. Int J Pharm. 2002;237:151–61.
Shevalkar G, Vavia P. Solidified nanostructured lipid carrier (S-NLC) for enhancing the oral bioavailability of ezetimibe. J Drug Deliv Sci Technol. 2019;53:101211.
Emami J, Mohiti H, Hamishehkar H, Varshosaz J. Formulation and optimization of solid lipid nanoparticle formulation for pulmonary delivery of budesonide using Taguchi and Box-Behnken design. Res Pharm Sci. 2015;10:17.
Okolie JA, Epelle EI, Nanda S, Castello D, Dalai AK, Kozinski JA. Modeling and process optimization of hydrothermal gasification for hydrogen production: a comprehensive review. J Supercrit Fluids. 2021;173:105199.
Sweed NM, Fayez AM, El-Emam SZ, Dawoud MHS. Response surface optimization of self nano-emulsifying drug delivery system of rosuvastatin calcium for hepatocellular carcinoma. J Pharm Investig. 2021;51:85–101.
Rohmah M, Raharjo S, Hidayat C, Martien R. Application of response surface methodology for the optimization of β-carotene-loaded nanostructured lipid carrier from mixtures of palm stearin and palm olein. J Am Oil Chem Soc. 2020;97:213–23.
Anwar W, Dawaba HM, Afouna MI, Samy AM, Rashed MH, Abdelaziz AE. Enhancing the oral bioavailability of candesartan cilexetil loaded nanostructured lipid carriers: In vitro characterization and absorption in rats after oral administration. Pharmaceutics. 2020;12:1047.
Kan S, Lu J, Liu J, Wang J, Zhao Y. A quality by design (QbD) case study on enteric-coated pellets: Screening of critical variables and establishment of design space at laboratory scale. Asian J Pharm Sci. 2014;9:268–78.
Soni G, Yadav KS, Gupta MK. QbD based approach for formulation development of spray dried microparticles of erlotinib hydrochloride for sustained release. J Drug Deliv Sci Technol. 2020;57:101684.
Waghule T, Rapalli VK, Singhvi G, Manchanda P, Hans N, Dubey SK, et al. Voriconazole loaded nanostructured lipid carriers based topical delivery system: QbD based designing, characterization, in-vitro and ex-vivo evaluation. J Drug Deliv Sci Technol. 2019;52:303–15.
Nayak AP, Tiyaboonchai W, Patankar S, Madhusudhan B, Souto EB. Curcuminoids-loaded lipid nanoparticles: novel approach towards malaria treatment. Colloids Surf B Biointerfaces. 2010;81:263–73.
Suriyanon N, Punyapalakul P, Ngamcharussrivichai C. Mechanistic study of diclofenac and carbamazepine adsorption on functionalized silica-based porous materials. Chem Eng J. 2013;214:208–18.
Saker A, Cares-Pacheco M-G, Marchal P, Falk V. Powders flowability assessment in granular compaction: what about the consistency of Hausner ratio? Powder Technol. 2019;354:52–63.
Patel MM, Patel RJ. Linagliptin loaded solid-SMEEDS for enhanced solubility and dissolution: formulation development and optimization by D-optimal design. J Drug Deliv Ther. 2019;9:47–56.
Qi S, McAuley WJ, Yang Z, Tipduangta P. Physical stabilization of low-molecular-weight amorphous drugs in the solid state: a material science approach. Ther Deliv. 2014;5:817–41.
Park J-B, Choi B-K, Kang C-Y. Effects of absorbent materials on a self-emulsifying drug delivery system for a poorly water soluble drug. J Pharm Investig. 2015;45:529–39.
Khatri P, Katikaneni P, Desai D, Minko T. Evaluation of Affinisol® HPMC polymers for direct compression process applications. J Drug Deliv Sci Technol. 2018;47:461–7.
Ghori MU, Ginting G, Smith AM, Conway BR. Simultaneous quantification of drug release and erosion from hypromellose hydrophilic matrices. Int J Pharm. 2014;465:405–12.
Vrbanac H, Krese A. The influence of different mechanical stress on the release properties of HPMC matrix tablets in sucrose-NaCl media. J Drug Deliv Sci Technol. 2019;54:101246.
Maincent J, Williams R. Sustained-release amorphous solid dispersions. Drug Deliv Transl Res. 2018;8:1714–25.
Jain AK, Söderlind E, Viridén A, Schug B, Abrahamsson B, Knopke C, et al. The influence of hydroxypropyl methylcellulose (HPMC) molecular weight, concentration and effect of food on in vivo erosion behavior of HPMC matrix tablets. J Control Release. 2014;187:50–8.
Tahara K, Yamamoto K, Nishihata T. Overall mechanism behind matrix sustained release (SR) tablets prepared with hydroxypropyl methylcellulose 2910. J Control Release. 1995;35:59–66.
Na Y-G, Pham TMA, Byeon J-J, Kim M-K, Han M-G, Baek J-S, et al. Development and evaluation of TPGS/PVA-based nanosuspension for enhancing dissolution and oral bioavailability of ticagrelor. Int J Pharm. 2020;581:119287.
Kim S-J, Lee H-K, Na Y-G, Bang K-H, Lee H-J, Wang M, et al. A novel composition of ticagrelor by solid dispersion technique for increasing solubility and intestinal permeability. Int J Pharm. 2019;555:11–8.
Na Y-G, Byeon J-J, Wang M, Huh HW, Kim M-K, Bang K-H, et al. Statistical approach for solidifying ticagrelor loaded self-microemulsifying drug delivery system with enhanced dissolution and oral bioavailability. Mater Sci Eng C. 2019;104:109980.
Acknowledgements
This work was supported by the Technology Development Program of Small and Medium Enterprises (S3119289) through the Korea Technology & Information Promotion Agency for SMEs. Also, this work was supported by the National Research Council of Science & Technology (NST) grant by the Korea government (MSIT) (grant no. CRC21021).
Funding
This work was supported by the Technology Development Program of Small and Medium Enterprises (S3119289) through the Korea Technology & Information Promotion Agency for SMEs. Also, this work was supported by the National Research Council of Science & Technology (NST) grant by the Korea government (MSIT) (grant no. CRC21021).
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology, M Jung, M Jin; formal analysis, W-J Jeon, HS Lee; software, H Kim; validation, J-H Won; visualization, H Yoo, H-W Bai; data curation, S-C Han; Resources and methodology, H Suh, KU Kang; conceptualization and writing—original draft preparation, H–K Lee; writing—review, editing and supervision, Cheong-Weon Cho.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jung, M., Jin, M., Jeon, WJ. et al. Development of a long-acting tablet with ticagrelor high-loaded nanostructured lipid carriers. Drug Deliv. and Transl. Res. 13, 1212–1227 (2023). https://doi.org/10.1007/s13346-022-01205-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01205-7