Abstract
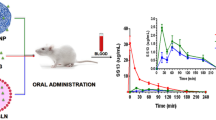
Linagliptin (LGP), a novel anti-diabetic drug, is a DPP-4 inhibitor used in the treatment of type II diabetes. One of the major disadvantages of LGP is its low oral bioavailability (29.5%) due to first-pass metabolism and P-gp efflux. In an attempt to increase the oral bioavailability, LGP solid lipid nanoparticles (LGP-SLNs) were developed with poloxamer 188 and Tween 80 as P-gp inhibitors. LGP-SLNs were formulated using palmitic acid, poloxamer 188 and Tween 80 as lipid, surfactant and co-surfactant, respectively, by hot homogenization ultrasonication method and optimized using 32 full factorial designs. Particle size, entrapment efficiency (%EE) and drug release at 24 h were evaluated as responses. An optimized batch of LGP-SLNs (L12) was evaluated for intestinal transport of LGP by conducting in situ single-pass intestinal perfusion (SPIP), everted gut sac and Caco-2 permeability study. The pharmacokinetic and pharmacodynamic evaluation of L12 was carried out in albino Wistar rats. The mean particle size, polydispersity index, zeta potential and %EE of L12 were found to be 225.96 ± 2.8 nm, 0.180 ± 0.034, − 5.4 ± 1.07 mV and 73.8 ± 1.73%, respectively. %CDR of 80.96 ± 3.13% was observed in 24 h. The permeability values of LGP-SLNs in the absorptive direction were 1.82-, 1.76- and 1.74-folds higher than LGP-solution (LGP-SOL) in SPIP, everted gut sac and Caco-2 permeability studies, respectively. LGP-SLNs exhibited relative bioavailability of 300% and better reduction in glucose levels in comparison with LGP-SOL in rats. The enhanced oral bioavailability exhibited by LGP-SLNs bioavailability may be due to P-gp efflux inhibition and lymphatic targeting. Improved bioabsorption can cause reduction in dose, dose-related side effects and frequency of administration. Thus, LGP-SLNs can be considered promising carriers for oral delivery but clinical studies are required to confirm the proof of concept.
Graphical abstract












Similar content being viewed by others
References
Nawaz MS, Shah KU, Khan TM, et al. Evaluation of current trends and recent development in insulin therapy for management of diabetes mellitus. Diabetes Metab Syndr. 2017;11(Suppl 2):S833–9.
Sepehri Z, Kiani Z, Afshari M, Kohan F, Dalvand A, Ghavami S. Inflammasomes and type 2 diabetes: an updated systematic review. Immunol Lett. 2017;192:97–103.
Pawlak R. Vegetarian diets in the prevention and management of diabetes and its complications. Diabetes Spectr. 2017;30(2):82–8.
Inzucchi S, Bergenstal R, Buse J, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2014;38(1):140–9.
Garber A, Abrahamson M, Barzilay J, Blonde L, Bloomgarden Z, Bush M, et al. AACE/ACE Comprehensive Diabetes Management Algorithm 2015. Endocr Pract. 2015;21(4):438–47.
Chahal H, Chowdhury T. Gliptins: a new class of oral hypoglycaemic agent. QJM. 2007;100(11):671–7.
Scheen A. DPP-4 inhibitors in the management of type 2 diabetes: a critical review of head-to-head trials. Diabetes Metab. 2012;38(2):89–101.
Barnett A, Patel S, Harper R, Toorawa R, Thiemann S, von Eynatten M, et al. Linagliptin monotherapy in type 2 diabetes patients for whom metformin is inappropriate: an 18-week randomized, double-blind, placebo-controlled phase III trial with a 34-week active-controlled extension. Diabetes Obes Metab. 2012;14(12):1145–54.
Del Prato S, Barnett A, Huisman H, Neubacher D, Woerle H, Dugi K. Effect of linagliptin monotherapy on glycaemic control and markers of β-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2011;13(3):258–67.
Kawamori R, Inagaki N, Araki E, Watada H, Hayashi N, Horie Y, et al. Linagliptin monotherapy provides superior glycaemic control versus placebo or voglibose with comparable safety in Japanese patients with type 2 diabetes: a randomized, placebo and active comparator-controlled, double-blind study. Diabetes Obes Metab. 2012;14(4):348–57.
Gomis R, Espadero R, Jones R, Woerle H, Dugi K. Efficacy and safety of initial combination therapy with linagliptin and pioglitazone in patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011;13(7):653–61.
Haak T, Meinicke T, Jones R, Weber S, Eynatten M, Woerle H. Initial combination of linagliptin and metformin improves glycaemic control in type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2012;14(6):565–74.
Forst T, Uhlig-Laske B, Ring A, Graefe-Mody U, Friedrich C, Herbach K, et al. Linagliptin (BI 1356), a potent and selective DPP-4 inhibitor, is safe and efficacious in combination with metformin in patients with inadequately controlled type 2 diabetes. Diabet Med. 2010;27(12):1409–19.
Taskinen M, Rosenstock J, Tamminen I, Kubiak R, Patel S, Dugi K, et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011;13(1):65–74.
Gallwitz B, Rosenstock J, Rauch T, Bhattacharya S, Patel S, von Eynatten M, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380(9840):475–83.
Ross S, Rafeiro E, Meinicke T, Toorawa R, Weber-Born S, Woerle H. Efficacy and safety of linagliptin 2.5 mg twice daily versus 5 mg once daily in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, placebo-controlled trial. Curr Med Res Opin. 2012;28(9):1465–74.
Lewin A, Arvay L, Liu D, Patel S, von Eynatten M, Woerle H. Efficacy and tolerability of linagliptin added to a sulfonylurea regimen in patients with inadequately controlled type 2 diabetes mellitus: an 18-week, multicenter, randomized, double-blind, placebo-controlled trial. Clin Ther. 2012;34(9):1909–1919.e15.
Owens D, Swallow R, Dugi K, Woerle H. Efficacy and safety of linagliptin in persons with type 2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: a 24-week randomized study 1. Diabet Med. 2011;28(11):1352–61.
Lehrke M, Marx N, Patel S, Seck T, Crowe S, Cheng K, et al. Safety and tolerability of Linagliptin in patients with type 2 diabetes: a comprehensive pooled analysis of 22 placebo-controlled studies. Clin Ther. 2014;36(8):1130–46.
Shaik M, Vanapatla SR. Enhanced oral bioavailability of linagliptin by the influence of gallic acid and ellagic acid in male Wistar albino rats: involvement of p-glycoprotein inhibition. Drug Metab Pers Ther. 2019;34(2):/j/dmdi.2019.34.issue-2/dmpt-2018-0020/dmpt-2018-0020.xml. https://doi.org/10.1515/dmpt-2018-0020.
Scheen AJ. Pharmacokinetics of dipeptidylpeptidase-4 inhibitors. Diabetes Obes Metab. 2010;12(8):648–58.
Bohannon N. Overview of the gliptin class (dipeptidyl peptidase-4 inhibitors) in clinical practice. Postgrad Med. 2009;121(1):40–5.
Deacon C, Holst J. Linagliptin, a xanthine-based dipeptidyl peptidase-4 inhibitor with an unusual profile for the treatment of type 2 diabetes. Expert Opin Investig Drugs. 2009;19:133–40.
Hüttner S, Graefe-Mody E, Withopf B, Ring A, Dugi K. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single Oral doses of BI 1356, an inhibitor of dipeptidyl peptidase 4, in healthy male volunteers. J Clin Pharmacol. 2008;48(10):1171–8.
Fuchs H, Binder R, Greischel A. Tissue distribution of the novel DPP-4 inhibitor BI 1356 is dominated by saturable binding to its target in rats. Biopharm Drug Dispos. 2009;30(5):229–40.
Fuchs H, Tillement J, Urien S, Greischel A, Roth W. Concentration-dependent plasma protein binding of the novel dipeptidyl peptidase 4 inhibitor BI 1356 due to saturable binding to its target in plasma of mice, rats and humans. J Pharm Pharmacol. 2009;61(1):55–62.
Heise T, Graefe-Mody E, Hüttner S, Ring A, Trommeshauser D, Dugi K. Pharmacokinetics, pharmacodynamics and tolerability of multiple oral doses of linagliptin, a dipeptidyl peptidase-4 inhibitor in male type 2 diabetes patients. Diabetes Obes Metab. 2009;11(8):786–94.
Thomas L, Eckhardt M, Langkopf E, Tadayyon M, Himmelsbach F, Mark M. (R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydro-purine-2,6-dione (BI 1356), a novel xanthine-based dipeptidyl peptidase 4 inhibitor, has a superior potency and longer duration of action compared with other dipeptidyl peptidase-4 inhibitors. J Pharmacol Exp Ther. 2008;325(1):175–82.
Retlich S, Withopf B, Greischel A, Staab A, Jaehde U, Fuchs H. Binding to dipeptidyl peptidase-4 determines the disposition of linagliptin (BI 1356) - investigations in DPP-4 deficient and wildtype rats. Biopharm Drug Dispos. 2009;30(8):422–36.
Fuchs H, Runge F, Held H. Excretion of the dipeptidyl peptidase-4 inhibitor linagliptin in rats is primarily by biliary excretion and P-gp-mediated efflux. Eur J Pharm Sci. 2012;45(5):533–8.
Patel M, Patel R. Linagliptin loaded solid-SMEEDS for enhanced solubility and dissolution: formulation development and optimization by D-optimal design. J Drug Deliv Ther. 2019;9(2):47–56.
Veni DK, Gupta NV. Development and evaluation of Eudragit coated environmental sensitive solid lipid nanoparticles using central composite design module for enhancement of oral bioavailability of linagliptin. Int J Polym Mater Polym Biomater. 2019;69(7):407–18.
Xie S, Wang S, Zhao B, Han C, Wang M, Zhou W. Effect of PLGA as a polymeric emulsifier on preparation of hydrophilic protein-loaded solid lipid nanoparticles. Colloids Surf B: Biointerfaces. 2008;67(2):199–204.
Mehnert W. Solid lipid nanoparticles production, characterization and applications. Adv Drug Deliv Rev. 2001;47(2–3):165–96.
Müller R. Solid lipid nanoparticles (SLN) for controlled drug delivery -a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–77.
Zhang Z, Bu H, Gao Z, Huang Y, Gao F, Li Y. The characteristics and mechanism of simvastatin loaded lipid nanoparticles to increase oral bioavailability in rats. Int J Pharm. 2010;394(1–2):147–53.
Müller R, Runge S, Ravelli V, Mehnert W, Thünemann A, Souto E. Oral bioavailability of cyclosporine: solid lipid nanoparticles (SLN®) versus drug nanocrystals. Int J Pharm. 2006;317(1):82–9.
Pandey R, Sharma S, Khuller G. Oral solid lipid nanoparticle-based antitubercular chemotherapy. Tuberculosis. 2005;85(5–6):415–20.
Han C, Qi C, Zhao B, Cao J, Xie S, Wang S, et al. Hydrogenated castor oil nanoparticles as carriers for the subcutaneous administration of tilmicosin: in vitro and in vivo studies. J Vet Pharmacol Ther. 2009;32(2):116–23.
Wang X, Zhang S, Zhu L, Xie S, Dong Z, Wang Y, et al. Enhancement of antibacterial activity of tilmicosin against Staphylococcus aureus by solid lipid nanoparticles in vitro and in vivo. Vet J. 2012;191(1):115–20.
Shah H, Patel R. Statistical modeling of zaltoprofen loaded biopolymeric nanoparticles: characterization and anti-inflammatory activity of nanoparticles loaded gel. Int J Pharm Investig. 2015;5(1):20–7.
Zhang L, Hao W, Xu L, Gao Y, Wang X, Zhu D, et al. A pH-sensitive methenamine mandelate-loaded nanoparticle induces DNA damage and apoptosis of cancer cells. Acta Biomater. 2017;62:246–56.
Tantra R, Schulze P, Quincey P. Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology. 2010;8(3):279–85.
Sahle FF, Balzus B, Gerecke C, Kleuser B, Bodmeier R. Formulation and in vitro evaluation of polymeric enteric nanoparticles as dermal carriers with pH-dependent targeting potential. Eur J Pharm Sci. 2016;92:98–109.
Andrade DFD, Zuglianello C, Pohlmann AR, Guterres SS, Beck RCR. Assessing the in vitro drug release from lipid-core nanocapsules: a new strategy combining dialysis sac and a continuous-flow system. AAPS PharmSciTech. 2015;16(6):1409–17.
Manoochehri S, Darvishi B, Kamalinia G, Amini M, Fallah M, Ostad SN, et al. Surface modification of PLGA nanoparticles via human serum albumin conjugation for controlled delivery of docetaxel. DARU J Pharm Sci. 2013;21(1):58.
Naik DR, Raval JP. Amorphous polymeric binary blend pH-responsive nanoparticles for dissolution enhancement of antiviral drug. J Saudi Chem Soc. 2016;20:167–77.
Kalhapure RS, Sikwal DR, Rambharose S, Mocktar C, Singh S, Bester L, et al. Enhancing targeted antibiotic therapy via pH responsive solid lipid nanoparticles from an acid cleavable lipid. Nanomedicine. 2017;13(6):2067–77.
Khaira R, Sharma J, Saini V. Development and characterization of nanoparticles for the delivery of gemcitabine hydrochloride. Sci World J. 2014;2014:1–6.
Salphati L, Childers K, Pan L, Tsutsui K, Takahashi L. Evaluation of a single-pass intestinal-perfusion method in rat for the prediction of absorption in man. J Pharm Pharmacol. 2001;53(7):1007–13.
Athukuri BL, Neerati P. Enhanced oral bioavailability of metoprolol with gallic acid and ellagic acid in male Wistar rats: involvement of CYP2D6 inhibition. Drug Metab Pers Ther. 2016;31(4):229–34.
Hanafy A, Mahgoub H. A validated HPLC method for the determination of linagliptin in rat plasma. Application to a pharmacokinetic study. J Chromatogr Sci. 2016;54(9):1–5.
Chalikwar SS, Belgamwar VS, Talele VR, Surana SJ, Patil MU. Formulation and evaluation of nimodipine-loaded solid lipid nanoparticles delivered via lymphatic transport system. Colloids Surf B: Biointerfaces. 2012;97:109–16.
Guideline ICH. Stability testing of new drug substances and products. Q1A (R2), Current Step. 2003.
Alex MA, Chacko A, Jose S, Souto E. Lopinavir loaded solid lipid nanoparticles (SLN) for intestinal lymphatic targeting. Eur J Pharm Sci. 2011;42(1–2):11–8.
Hussain N. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv Drug Deliv Rev. 2001;50(1–2):107–42.
Seju U, Kumar A, Sawant K. Development and evaluation of olanzapine loaded PLGA nanoparticles for nose-to-brain delivery: in vitro and in vivo studies. Acta Biomater. 2011;7(12):4169–76.
Alam T, Pandit J, Vohora D, Aqil M, Ali A, Sultana Y. Optimization of nanostructured lipid carriers of lamotrigine for brain delivery: in vitro characterization and in vivo efficacy in epilepsy. Expert Opin Drug Deliv. 2015;12(2):181–94.
Subedi RK, Kang KW, Choi H-K. Preparation and characterization of solid lipid nanoparticles loaded with doxorubicin. Eur J Pharm Sci. 2009;37(3–4):508–13.
Lalani J, Patil S, Kolate A, Lalani R, Misra A. Protein-functionalized PLGA nanoparticles of lamotrigine for neuropathic pain management. AAPS PharmSciTech. 2015;16(2):413–27.
Dalencon F, Amjaud Y, Lafforgue C, Derouin F, Fessi H. Atovaquone and rifabutine-loaded nanocapsules: formulation studies. Int J Pharm. 1997;153(1):127–30.
Ammar HO, Ghorab MM, Mahmoud AA, Higazy IM. Lamotrigine loaded poly-ɛ-(d, l-lactide-co-caprolactone) nanoparticles as brain delivery system. Eur J Pharm Sci. 2018;115:77–87.
Sawant KK, Dodiya SS. Recent advances and patents on solid lipid nanoparticles. Recent Pat Drug Deliv Formul. 2008;2(2):120–35.
Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine. 2010;6(6):714–29.
Jia L, Shen J, Li Z, Zhang D, Zhang Q, Liu G, et al. In vitro and in vivo evaluation of paclitaxel-loaded mesoporous silica nanoparticles with three pore sizes. Int J Pharm. 2013;445(1–2):12–9.
Jia L-J, Zhang D-R, Li Z-Y, Feng F-F, Wang Y-C, Dai W-T, et al. Preparation and characterization of silybin-loaded nanostructured lipid carriers. Drug Deliv. 2010;17(1):11–8.
Peppas NA. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 1985;60:110–1.
Kim D-H, Martin DC. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials. 2006;27(15):3031–7.
Venkateswarlu V, Manjunath K. Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles. J Control Release. 2004;95(3):627–38.
Cavalli R. Sterilization and freeze-drying of drug-free and drug-loaded solid lipid nanoparticles. Int J Pharm. 1997;148(1):47–54.
Dai H, Li X, Li X, Bai L, Li Y, Xue M. Coexisted components of Salvia miltiorrhiza enhance intestinal absorption of cryptotanshinone via inhibition of the intestinal P-gp. Phytomedicine. 2012;19:1256–62.
Hubatsch I, Ragnarsson EGE, Artursson P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat Protoc. 2007;2(9):2111–9.
Guan Y, Huang J, Zuo L, Xu J, Si L, Qiu J, et al. Effect of pluronic P123 and F127 block copolymer on P-glycoprotein transport and CYP3A metabolism. Arch Pharm Res. 2011;34(10):1719–28.
Acknowledgements
The authors would like to acknowledge Dr. Tarjani Sheth, Department of English, Uka Tarsadia University, for the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Caco-2 permeability test
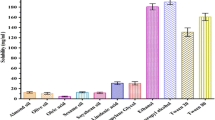
The Caco-2 cell monolayer model bears close resemblance to human intestinal barrier properties. Therefore, this model finds widespread applications in prediction of in vivo permeability and absorption. Bidirectional (AP → BL and BL → AP) transport analysis using the Caco-2 cell monolayer model is the gold standard to evaluate substrates and inducers/inhibitors of P-gp transporters [72]. In order to compare the intestinal permeability, LGP-SOL and LGP-SLNs were evaluated using the Caco-2 cell monolayers (Fig. 9). The cells were cultured in the Transwell® for 21 days for monolayer formation, which was confirmed by a TEER cut-off value of 250 Ω cm2. As shown in Fig. 9, the observed mean Papp (AP → BL) values of LGP-SOL and LGP-SLNs were 0.87 × 10−6 and 2.09 × 10−6 cm/s, respectively.
Absorptive permeability Papp (AP → BL) of LGP from LGP-SLNs was significantly (p < 0.01) increased (2.4-fold) than LGP-SOL. The possible explanation for the enhancement of transepithelial permeability of LGP by LGP-SLNs formulation is due to poloxamer 188 and Tween 80, both of which are known P-gp inhibitors and hence assisted in increasing the intestinal permeability of LGP [73] .
The observed mean Papp (BL → AP) values of LGP-SOL and LGP-SLNs were 1.59 × 10−6 and 2.04 × 10−6 cm/s, respectively. The secretory permeability of LGP-SOL was 1.83-folds higher than absorptive permeability, suggesting higher efflux permeability compared with its influx component. The ratio of Papp (BL → AP) (secretion component) and Papp (AP → BL) (absorption component) is defined as efflux ratio (ER). The ER of LGP-SOL and LGP-SLNs were 1.828 and 0.976, respectively. An ER higher than unity suggested a net efflux transport, whereas ER near to unity indicated that LGP excretion from LGP-SLNs is reduced and the amount absorbed is increased. Thus, it was confirmed that the addition of poloxamer 188 and Tween 80 in the preparation of LGP-SLNs increased the intestinal permeability of the LGP. These results are in confirmation with our findings of single-pass perfusion and everted sac studies.
Conflict of interest
The authors declare that they have no conflicts of interest.
Animal studies
All institutional and national guidelines for the care and use of laboratory animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shah, P., Chavda, K., Vyas, B. et al. Formulation development of linagliptin solid lipid nanoparticles for oral bioavailability enhancement: role of P-gp inhibition. Drug Deliv. and Transl. Res. 11, 1166–1185 (2021). https://doi.org/10.1007/s13346-020-00839-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00839-9