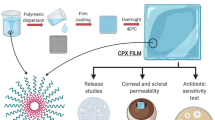
Abstract
The formulation and delivery of highly hydrophobic drugs in an optimized dosage form is challenging to formulation scientists. Posaconazole has shown promising action in case studies against fungal keratitis. Biological macromolecules like gellan gum would aid in enhancing the availability of such drugs by increasing the contact time of the formulation. Herein, we propose a transmucosal ocular delivery system of Posaconazole by developing a gellan gum–based in situ gelling nanosuspension. The HPLC method for Posaconazole was developed and validated as per ICH guidelines. The nanosuspension was prepared by microfluidization and optimized by Quality by Design. The gellan gum concentration selected was 0.4% w/v based on the viscosity and mucoadhesion measurements. A greater zone of inhibition of ~ 15 mm was observed for the prepared nanosuspension as compared to ~ 11 mm for the marketed itraconazole nanosuspension. A potential irritancy score of 0.85, considered to be non-irritant, was observed for the developed nanosuspension. Higher drug release of ~ 35% was noted for the nanosuspension compared to about ~ 10% for the coarse suspension. Ex vivo corneal retention studies on excised goat cornea demonstrated ~ 70% drug retention in the tissue.
Graphical abstract
Graphical abstract depicting the central hypothesis of the work.

Similar content being viewed by others
Introduction
Fungal keratitis is an inflammation of the cornea caused by fungi and is further classified as superficial and deep keratitis. Filamentous fungi and yeast are two medically important groups of fungi responsible for causing corneal infection [1]. Fungi are unable to penetrate an intact cornea and gain access via a prior corneal defect or injury. Once they enter the cornea, they can proliferate through the corneal channels and cause infection and inflammation. However, the fungal organisms are able to cross an intact Descemet membrane and can reach the anterior and posterior chambers of the eye [2]. Fungal infections are resistant and extremely difficult to treat, which may further progress to the entire eye or result in loss of vision. Males and people with a history of ocular damage or trauma are more liable to fungal keratitis [3]. Mucormycosis (also known as black fungus) is another fungal infection which is life-threatening and prevails in immunocompromised patients. It is commonly termed “black fungus” due to the development of a necrotic, black eschar leading to blackening or discoloration over the nose. Some of the cardinal signs of mucormycosis related to the eye are vision loss and ophthalmoplegia [4]. Increasing cases of mucormycosis were recently reported in patients infected with COVID-19. Researchers and doctors have predicted that the 50% mortality rate in patients with mucormycosis is further triggered in critically ill COVID-19 patients. Mucormycosis has such a severe effect on the eye that doctors had to surgically remove the eye to help save lives of people who were severely impacted by the disease [5, 6].
Fungal keratitis remains to be a deadly, fast progressing infection that is quite difficult to diagnose in its early stages because of which treatment becomes a major challenge. Fungal infections can be diagnosed by tissue sampling and culturing. The main disadvantage of the culturing method is a delay in diagnosis, which results in holdup of treatment. Treatment of fungal keratitis involves two modules, i.e., drug therapy and surgical intervention, of which the former is mostly preferred by patients and physicians alike. Most of the drugs available to treat fungal keratitis have major drawbacks including poor penetration, toxicity, and emergence of resistance [1]. Taking into account the adverse effects of various agents, Posaconazole emerges to be a potential agent with high efficacy and minimal adverse effects as studied in many clinical cases of recalcitrant fungal keratitis as well as mucormycosis [1, 4, 7, 8]. To achieve maximum efficacy, it is immensely important to provide maximum drug concentration at the site of action for prophylaxis as well as treatment. Posaconazole targets lanosterol-14a-demethylase, which is involved in the synthesis of ergosterol, a major lipid constituent of fungal cell wall. Posaconazole inhibits the oxidative demethylation of lanosterol, thus inhibiting the formation of ergosterol. This finally leads to ergosterol depletion and fungal cell death [9]. Posaconazole is also an inhibitor of human ether-a-go-go-related gene (hERG) channels that are overexpressed in cancer cells and thus halts tumor cell invasion [10]. Various studies have also been carried out to ascertain the use of Posaconazole in the prophylaxis of fungal infections in cancer patients after chemotherapy, which guarantees its use in a highly immunocompromised condition like cancer [11,12,13,14]. Hence, Posaconazole proves to be a potential candidate for use in patients with eye cancer for the prophylaxis as well as treatment of recalcitrant fungal keratitis and mucormycosis, which is resistant to conventional antifungal agents.
A nanosuspension aids in solving the issue of poor solubility and bioavailability and alters the pharmacokinetics of the drug. This leads to improvement in drug safety and efficacy. Nanosuspension is used as a formulation strategy for drugs which are insoluble in aqueous as well as organic phases [15]. Antifungal agents formulated as nanosuspensions are known to decrease toxicity and enhance efficacy in previously published literature [16, 17]. The term “in situ gel” implies gelling of the formulation at the site of action after application. The drug is delivered in a liquid form which then is converted into a gel at the site of action. In situ gelling systems combine the advantages of an ocular liquid formulation with a semisolid one, which results in increased contact time with the ocular mucosa and ease of formulation [18,19,20, 21, 22].
Gellan gum (GG) is an exocellular anionic water-soluble polysaccharide produced by Sphingomonas elodea with a repeating unit of tetrasaccharide composed of two residues of β-d-glucose, one residue of β-d-glucuronic acid, and one residue of α-l-rhamnose {[D-Glc(β1 → 4)D-GlcA(β1 → 4)D-Glc(β1 → 4)L-Rha(α1 → 3)]n} [23, 24]. GG has the property to undergo a solution to gel transition when it comes in contact with mono- and divalent cations. This results in the formation of a strong gel [25]. A plethora of studies have explored GG for topical administration of various agents aimed at enhancing efficacy of the formulation [25,26,27,28,29]. GG-based formulation would undergo gelation due to the presence of Na+, K+, Mg2+, and Ca2+ ions in tear fluid [30]. In the present work, we employed Kelcogel CG-LA, a low acyl grade of GG, used as a gelling agent which is known to give transparent and mechanically robust gels stable over a wide range of pH. Low acyl GG was chosen owing to its characteristic property of forming gels when in contact with cations [31]. In situ gelling with the help of low acyl grade of GG has demonstrated formation of clear, robust, stable, and soft gels along with potential results in pre-clinical and clinical studies [32,33,34,35]. Novelty of the present work lies in demonstrating enhanced tissue retention of Posaconazole which is of utmost importance in conditions like recalcitrant fungal keratitis. Retention is established by using GG, a naturally occurring polysaccharide, to induce gelation which is specific to ions present in the tear fluid. Herein, we explore the sol–gel transformation property of GG for enhancing the contact time and retention of Posaconazole nanosuspension which can tackle fungal keratitis. We thus hypothesize that a GG-based in situ gelling nanosuspension of Posaconazole would provide maximum drug concentration at the site of action for a prolonged time, thus achieving maximum efficacy.
Materials and methods
Materials
Posaconazole was obtained as a gift sample by Alkem Laboratories (Mumbai, India). Deacetylated gellan gum NF (Kelcogel CG-LA, low acyl grade) was obtained from Signet Chemicals (Mumbai, India). Tween 20 was purchased from Abitech (Mumbai, India). Pluronic F 127 was procured from BASF (Navi Mumbai, India). All other chemicals and excipients used were of analytical and pharmaceutical grade.
Posaconazole analytical method development
The HPLC method for Posaconazole was developed and validated as per ICH guidelines. Agilent 1200 series HPLC was used with a quaternary pump and a G 1314 A VW detector. The mobile phase used was methanol:water in a ratio of 75:25 [36]. Linearity was studied for a concentration range of 10–60 ppm. Furthermore, the method was validated in terms of accuracy, precision, and robustness. Forced degradation studies as per the ICH guidelines Q3B(R2) were carried out to study the degradation of Posaconazole at various conditions viz. acid, base, peroxide, thermal, and photodegradation [37]. In addition, limit of detection (LOD) and limit of quantitation (LOQ) were calculated [38].
The LOD was calculated by using the given equation:
where
- σ:
-
standard deviation
- S:
-
slope of the calibration curve
The LOQ was calculated by using the given equation:
where
- σ:
-
standard deviation
- S:
-
slope of the calibration curve
Pre-formulation studies
Solubility of Posaconazole in surfactants
Solubility of Posaconazole was determined in surfactants permitted for ophthalmic use. Selection of appropriate surfactants was carried out based on saturation solubility studies [39,40,41]. Aqueous solutions of surfactants, i.e., Tween 20, Tween 80, Propylene glycol, and Pluronic 127, as per their IIG limits, were used for studying the solubility of Posaconazole [42]. An excessive amount of drug was added to these surfactant solutions and placed for 24 h in a water bath shaker at 37 ± 2 °C. After 24 h, the drug-containing solutions were centrifuged and the supernatant was filtered and injected into the HPLC system to ascertain the quantity of drug dissolved in the respective surfactant.
Contact angle analysis
Contact angle is the estimate of the wettability of the surfactant mixture and the drug. The more the wetting of the drug with the surfactants chosen, the more is its ability to increase the suspension stability [43]. For the contact angle analysis, 6 surfactants (1% w/v solution) (Supplementary table S1) were selected based on their safety and use in ophthalmic formulations. The contact angle measurement system G10 KRUSS (Goniometer) was used to measure the contact angle [44]. A tablet press was used to make small tablets of pure Posaconazole, and a small drop of surfactant solution was placed with the help of a glass syringe on the surface of the tablet. The contact angle was resolved by observing through the eyepiece, the angle made by the surfactant drop with the tablet.
Formulation of Posaconazole nanosuspension using quality by design (QbD) approach
Quality by Design (QbD) was used to optimize the nanosuspension formulation. An appropriate design was selected based on the need for screening the excipients and optimizing the process. The number of factors and their respective levels also play an important role in the selection of an experimental design. An optimum design space was generated by the software wherein, if the experimental parameters are kept as per the domain generated, the results will fall in the desired range. Further validation was carried out by selecting a point in the design space and then carrying out that particular batch in triplicates. The response (i.e., particle size) was noted and checked whether it falls between the levels of the confidence intervals. The criteria of the response specified for the optimization purpose were 300 to 500 nm.
As per QbD optimization, 50 ml nanosuspension of Posaconazole was prepared by weighing the optimized amount of ingredients. A coarse suspension of Posaconazole in a solution of Tween 20 and Pluronic F127 was prepared with the help of an overhead stirrer. Benzalkonium chloride was added to this coarse suspension. After uniform dispersion of Posaconazole in the surfactant solution, the coarse suspension was passed through a microfluidizer and was sterilized by autoclaving at 121 °C for 20 min under a pressure of 15 psi.
Optimization of gellan gum (GG) for in situ gelling
Viscosity determination
Anton Paar rheometer (Physica MCR 301) was used to determine the viscosity of 0.2, 0.4, and 0.6% w/v GG solutions. The change in viscosity of these solutions when in contact with tear fluid was also studied [45]. The measurement assembly consists of a vertical probe which comes in contact with the formulation placed on a flat horizontal plate and measures the viscosity of the sample (Supporting figure S1). One milliliter of the respective concentrations (0.2, 0.4, and 0.6% w/v) of the GG solutions was placed below the vertical probe of the rheometer and the viscosity was measured at 25 different shear rates. All the measurements were carried out at 25 ± 0.5 °C. The concentration of GG which gives optimum viscosity before and after coming in contact with the tear fluid so as to provide optimum flow properties as well as gelation capacity was chosen. The aim was to study the trend of change in viscosity of the different concentrations of GG and to finalize the optimum viscosity required for the formulation.
Mucoadhesion testing
Mucoadhesion testing was done in order to determine mucoadhesion of various concentrations (0.2, 0.4, and 0.6% w/v solutions) of GG and optimize the same [46]. A texture analyzer (TA. XT plus Texture Analyzer, Stable Micro Systems, UK) was utilized to determine the mucoadhesion of GG on the mucous membrane of goat corneas. Goat eyeballs were procured from the slaughterhouse and corneas were excised carefully. After excising, they were stored in 0.9% saline solution to prevent them from drying out. Furthermore, 1 ml of the prepared formulation was allowed to come in contact with the epithelial surface of the cornea for 1 min before the testing at room temperature. The peak force used to detach the formulation from the membrane was determined with a probe which served as a measure of mucoadhesion. Table 1 specifies the parameters of the texture analyzer and the conditions maintained during the mucoadhesion testing:
Determination of particle size, drug content, and pH of the nanosuspension
Dynamic light scattering (DLS) analysis of the nanosuspension was done to determine the particle size and the polydispersity index (PDI) using the Zetasizer Nano ZS (Malvern Instruments Ltd., Worcestershire, UK). The instrument utilized a 4 mW He–Ne red laser at 633 nm. The light scattering was detected at 173° by non-invasive backscatter technology with a measuring range of 0.6 nm to 6 μm. Disposable polystyrene cuvettes (1 ml) were used for measurements. For determining the drug content of the nanosuspension, the quantity of the nanosuspension containing 40 ppm of Posaconazole was diluted in the mobile phase used for HPLC (methanol:water 75:25) in order to solubilize Posaconazole and this was injected into the HPLC system. The pH of the nanosuspension was analyzed using a calibrated pH meter (Eutech instruments, pH meter) at day 0, day 30, and day 60.
Ocular irritation studies (hen’s egg test–chorioallantoic membrane—HET-CAM)
Ex vivo ocular irritation and tolerance studies were performed using the HET-CAM; 8-day-old, incubated hen’s eggs were used for the same. Initially, one egg was cut open using a surgical sterile blade and checked for the development of CAM. The maturation of visible veins on the surface confirmed the development of CAM. Furthermore, the eggs were divided into 4 groups: positive control (10% w/v KOH; which is a known ocular irritant), negative control (0.9% saline; which does not cause irritation), Posaconazole in situ gelling nanosuspension, and a placebo group. After removing the egg surface, 0.3 ml of the test sample was applied to the CAM surface with the help of a micropipette ensuring that at least 50% of the CAM surface area was covered. Reactions on the CAM surface were observed for a period of 300 s. The time for the appearance of each of the endpoints (hemorrhage, vascular lysis, and coagulation) was monitored and recorded, in seconds [47, 48]. Based on the time required for the endpoints to develop, a potential irritancy (PI) score was calculated based on the following equation:
where
- h:
-
appearance time in seconds of hemorrhage
- v:
-
appearance time in seconds of vasoconstriction
- c:
-
appearance time in seconds of coagulation
In vitro drug release
In vitro drug release study was accomplished using a magnetic stirring procedure, wherein 2 ml coarse Posaconazole suspension and Posaconazole nanosuspension formulations were added to a dialysis bag and placed in an external media consisting of 20 ml artificial tear fluid (ATF) [49, 50]. Posaconazole being a BCS class II drug exhibits finite solubility which could be enhanced by the addition of a surfactant [51]. Two trials of the same were carried out. The first trial consisted of 0.1% w/v Tween 20 in the external media and the other without surfactant. Tween 20 was added to the media in order to study the difference in the release pattern of Posaconazole, a BCS class II drug. The release was carried out for 24 h and samplings were done at various time points, viz. 5 min, 15 min, 30 min, 45 min, 1 h, 2 h, 4 h, 8 h, 12 h, and 24 h. Samples were collected and injected into the HPLC system for measuring the amount of drug in solution form at each time point. Graph was plotted for % cumulative drug release v/s time.
Ex vivo corneal retention study
Ex vivo corneal retention study was performed using Franz diffusion cells with an isolated goat cornea model in order to determine the amount of drug localized in the cornea to provide a sustained effect of Posaconazole post-instillation. We also determined the drug diffusing through the cornea in the receptor chamber and the amount of drug retained above the cornea [52]. Whole goat eyeballs were procured from a local butcher’s shop within an hour of slaughtering the animal. The cornea was carefully excised from the eyeball and stored in artificial tear fluid (ATF). The corneal membrane was mounted between the donor and receptor chamber of the Franz diffusion cell. The receptor chamber of the cell contained 10 ml artificial fluid while 1 ml coarse Posaconazole suspension and Posaconazole in situ gelling nanosuspension formulations were placed in the donor chamber of the cell. The corneal retention study was carried out for 24 h [52,53,54,55]. The absorbance was measured at 260 nm. At the end of 24 h, the sample remaining on the surface of the cornea was carefully scraped out and sonicated with methanol in order to solubilize the drug, and the absorbance of this solution was noted. The cornea was homogenized using a hand tissue homogenizer with methanol to extract the drug in the tissue. The absorbance of the extracted drug was measured at 260 nm and a graph was plotted to determine the fraction of drug permeated in the receptor chamber as well as that retained in the tissue and above the tissue.
Antifungal assay
Antifungal assay was carried out to determine the activity of the prepared formulation against Candida albicans (ATCC) using the standard agar cup diffusion method [56]. Sabouraud dextrose agar was used to culture and grow the fungi. The required apparatus and broth were autoclaved at 15 psi for 20 min. Sabouraud dextrose agar slants were prepared, and the slant surface was streaked with fungal strains followed by incubation at 37 °C in dark conditions for 24 h for the fungus to grow. After 24 h, the grown fungus was taken out from the slant and its suspension was prepared in 0.9% saline. A definite volume of the autoclaved agar broth was poured into each of the Petri plates and was allowed to solidify. Suspension of the strain was added in equal amounts on the agar surface and was spread uniformly using a spreader. A well was bored in the center of the plate and 140 μl of the formulation was added. The lid was closed, and the plates were incubated at 37 °C in dark conditions for 24 h, after which, the zone of inhibition (ZOI) was measured [57, 58]. The ZOIs were directly correlated with the activity of the respective formulations and compared with the marketed formulations.
Stability of the nanosuspension
Stability of the Posaconazole nanosuspension was examined both at room conditions and at accelerated conditions of temperature and % relative humidity (RH) according to the ICH Q1A (R2) guidelines [59]. This was done by placing the batches of Posaconazole nanosuspension in amber-colored glass vials having rubber closures at room conditions (30 °C/65% RH) and at accelerated conditions (40 °C/75% RH) in the respective stability chambers. The batches were kept in both the stability chambers for 1-month and 3-month stability testing. At the end of each time point, the batches were assessed for various critical parameters such as appearance, particle size, drug content, and pH.
Results and discussion
Posaconazole analytical method development
The analytical method for Posaconazole was developed and validated with regard to the ICH guidelines. The mobile phase and the mobile phase ratio for the analytical method development were chosen based on published studies [36]. The mobile phase consisted of methanol:water at a ratio of 75:25 v/v. Other system parameters for the same are mentioned in “Posaconazole analytical method development.” Supporting figure S2 represents a typical chromatogram of Posaconazole with a retention time of 6.16 ± 0.3 min. The retention time obtained for Posaconazole was comparable to that reported in other studies [60, 61]. Figure 1 gives the chromatographic peaks for the forced degradation study. No significant peaks were observed in any of the forced degradation conditions. As seen in Supporting table S2, minor degradation was observed in acidic, basic, and thermal conditions. Thus, Posaconazole can be well tolerated in the mentioned conditions without any significant amount of degradation. Determining the drug degradation profile for actives is a crucial determinant of the conditions used for formulation development and manufacturing. The developed analytical method should be able to differentiate and detect the presence of chromatographic peaks in the case of drug degradation. Thus, the method developed herein is a simple, efficient, and practical analytical method for the detection of Posaconazole.
Linearity
Linearity in response to the developed HPLC method with change in concentration of the analyte (i.e., Posaconazole) was studied around the concentration range of 10 to 60 ppm. The method was observed to be linear in the concentration range of 10–60 ppm (Supporting figure S3). Establishing linearity of the developed analytical method is imperative to its use in determining the drug content during formulation analysis. This aspect makes the analytical method reliable and sensitive. In the graph plotted for concentration (ppm) against AUC (Supporting figure S3), the coefficient of determination (R2) was found to be 0.999 and the regression equation was found to be:
Since the value of the coefficient of determination (R2) was found to be ~ 1; we concluded that the method was linear with a proportionate increase in the absorbance with increasing concentration of the analyte.
Limit of detection (LOD) and limit of quantitation (LOQ)
Detection limit tells us whether the analyte is able to be detected and quantified with an acceptable level of confidence. The least analyte concentration that can be detected in the sample is called LOD, whereas, LOQ is the least concentration of the analyte in the sample that can be determined quantitatively [62]. These detection limits are often associated with obtaining the correct result with a 95% probability [63]. LOD and LOQ of Posaconazole were observed to be 0.18 µg/ml and 0.57 µg/ml, respectively. In any circumstances, if the concentration of Posaconazole were to fall below 0.18 µg/ml or below 0.57 µg/ml, then the proposed HPLC method will not be able to detect or quantify it, respectively.
Precision and accuracy
In analytical terms, precision can be defined as the closeness of values to each other in a set of replicate experiments. Whereas accuracy is the closeness of experimental measurements to a specific or pre-defined value [64]. Intraday and interday precision were carried out three times on the same day and for three consecutive days, respectively. Relative standard deviation (RSD) was calculated and found to be less than 2% (%RSD of 0.003 and 0.006 for intraday and interday precision, respectively). A lower value of the %RSD elucidates the higher precision of the developed HPLC method. For determining the accuracy of the method, a standard of 40 ppm was spiked to 80 and 120% with Posaconazole (32 and 48 ppm, respectively), and the percent recovery was calculated (Supporting table S3). % Recovery was calculated in terms of the injected concentration of Posaconazole which was compared to the output AUC given by the HPLC system. This was then back-calculated to the concentration in terms of ppm. In totality, lower %RSD coupled with good recovery values of Posaconazole ascertain the precision and accuracy of the developed method.
Robustness
Robustness elucidates the reliability of the developed method. It is the capacity of the method to remain unaffected by deliberate, small changes in method parameters [65]. The robustness of the developed HPLC method was studied by varying the analyst, column temperature, and injection volume. The %RSD of the variations implemented on the method was found to be below 2% (Supporting table S4). %RSD values for analyst change and temperature change were 0.18% and 1.7%, respectively. A low value of %RSD indicates that the developed method is robust and would give un-biased results even with small variations in method parameters. The % increase or decrease in the mean AUC was found to be proportionate with respect to iterations in the injection volume. 50% (10 µl) and 150% (30 µl) injection volume change resulted in a ~ 47% and ~ 147% change in the mean AUC post-HPLC analysis.
Thus, the HPLC method for the routine analysis of Posaconazole was successfully validated for various parameters, and %RSD of all the tested parameters was found to be within the specified range of 2%.
Solubility and contact angle analysis of Posaconazole in surfactants
Developing a suspension system requires the use of a surfactant as a stabilizer to prevent aggregation of the colloidal dispersion [41]. Optimizing an ideal surfactant which caters the needs of the active and provides it with enhanced stability is of utmost importance. The first step in selecting an appropriate surfactant was to study its solubility and wetting capacity with Posaconazole. Secondly, being an ophthalmic formulation, keeping an eye on the surfactant concentration which goes in the final formulation is a crucial checkpoint to avoid irritation and increase its tolerability. Hence, we decided to use surfactants at their respective strengths based on previously identified values which are considered to be safe for ophthalmic use according to the inactive ingredients guide (IIG) [66]. The next step was to study the solubility of Posaconazole in the shortlisted surfactants. The solubility of Posaconazole was studied in various surfactants based on their safety in ophthalmic preparations per IIG limits. Since we were not aiming to solubilize Posaconazole and rather just suspend it, we aimed for surfactants with minimal solubilization capacity. Supporting table S1 gives the solubility in ppm of Posaconazole in the surfactant solutions prepared as per the IIG limits of the surfactants permitted for ocular instillation. Whenever an interface between a solid and liquid is formed, contact angle plays an imperative role. The contact angle is the angle formed between the liquid surface and the outline of the contact surface which dictates the wetting capacity of a solid by a liquid. The lower the contact angle, the better is the wetting [67]. Contact angle measurements were performed to ascertain the wetting tendency of the surfactant solutions with Posaconazole.
As seen from Supporting tables S1 and S5, Posaconazole is minimally soluble and has the least contact angle with Tween 20 and Pluronic F 127. In order to enhance the stability of the nanosuspension, it is necessary that the suspended particles have good wetting and minimum solubility in the surfactant. To achieve this, the selected surfactant should minimally solubilize the drug and provide maximum wetting. Thus, surfactants in which Posaconazole had the least solubility and minimum contact angle were chosen. Hence, Tween 20 and Pluronic F 127 were chosen as surfactants to stabilize the formulation.
QbD approach for formulating the nanosuspension
Microfluidization technology was utilized for the formulation of a nanosuspension. Microfluidizer (Microfluidics M-110P) is a high shear fluid processor which is unique in its ability to achieve uniform particle size reduction. Advantages of this emerging technology over other existing methods of nanosizing include improved stability of the active, optimized characteristics, and efficient delivery of lipophilic drugs to their target. Numerous studies have developed and optimized nanoparticulate formulations by using microfluidization as a top-down approach [68,69,70,71]. A stable nanosuspension can be prepared by optimizing various formulation and instrument parameters. A suitable product and process optimization design was selected based on the number of factors and their respective levels mentioned below.
The target product profile (TPP) consists of the quality attributes of the final product which play a crucial role in governing its safety and efficacy [72]. The TPP of Posaconazole in situ gelling system was selected based on prior knowledge available of the active and similar products thereof in the market. A critical quality attribute (CQA) of the final pharmaceutical product is a chemical, microbiological, physical, or biological attribute that must fall within a range to ensure the desired quality of the product [73]. The TPP and CQAs for Posaconazole in situ gelling nanosuspension are summarized in Supporting tables S6 and S7, respectively. The next step after identifying the CQAs was the scientific correlation and impact analysis of the formulation components, i.e., critical process parameters (CPPs) on the identified CQAs. CPPs are the parameters used for processing the final product which have an impact on the CQAs. This was established by constructing a risk assessment matrix and ranking the effects of CPPs on CQAs as low, medium, and high risk [74].
The CPPs include process attributes, i.e., number of cycles, the drug to surfactant ratio, and homogenization pressure. The selection of an experimental design is postulated on factors and their respective levels. Factors, in this case, are the CPPs and their respective levels are the ranges of their operation. The factors and their levels are listed in the Supporting table S8.
The response which is checked for is the particle size of the final nanosuspension. As seen from the Supporting table S8, there are 3 factors and 3 levels for each factor. Box-Behnken design (BBD) was used as the optimization design in the Design expert software (DoE). Table 2 summarizes the batches suggested by DoE and the particle size response obtained as measured.
Scrupulous selection of excipients, the drug to excipient ratio, and the CPPs have a major role in affecting the CQAs. This in turn affects the quality and efficacy of the final dosage form. Supporting table S9 gives the relationship between CPPs and the magnitude of the effect they have on the CQAs of the final product. The drug to surfactant ratio, homogenization pressure, and number of cycles play a vital role in determining the particle size and release of the final formulation. Based on these correlations, the effects of CPPs on the product CQAs are ranked as low, medium, and high risk. The goal of QbD was to convert high-risk factors into medium and low risk.
The fit summary given by the software scans the data and studies the correlation of the factors with the pattern of response obtained. The p-value for the linear model was found to be less than 0.05 which implies that there exists a linear correlation between the factors and response. The p-value for the lack of fit test was found to be greater than 0.05 which suggests the suitability of the model applied as suggested by the software.
The final equation in terms of coded factors is expressed as follows:
where
- A:
-
drug:surfactant (D:S) ratio
- B:
-
homogenization pressure
- C:
-
number of cycles
The contour plot (Supporting figure S4) is a two-dimensional (2D) plot where the response is plotted against a combination of factors and/or mixture components, thus portraying their relationship. The contour plot in Supporting figure S4 demonstrates the combined effect of the drug:surfactant ratio and the number of cycles of the microfluidizer on the particle size of the nanosuspension. As the drug:surfactant ratio and the number of cycles increase, the particle size decreases [color gradient from green (larger particle size) to blue (smaller particle size)]. The 3D surface plot (Supporting figure S5) conveys the same information as the contour plot (it is a projection of the contour plot giving shape in addition to the color and contour). Both the graphs give an effect of the number of cycles and the D:S ratio on the response, when homogenization pressure is kept at a constant value of 15,000 psi. Both the plots demonstrate that as the D:S ratio and number of cycles increase, the graph moves color gradation shifts from green to blue, indicating inverse correlation.
The graphical optimization or an overlay graph (Fig. 2) indicates a “sweet spot” (yellow patch) where the response falls within the set criteria. The bright yellow color indicates the settings that are acceptable, whereas the grey color defines unacceptable parameter settings. The parameter settings include the CPPs which fall within the “acceptable” criteria when the response (particle size) is between 300 and 500 nm. The numerical optimization solution (white flag) represents the factor settings with the predicted response (particle size). Figure 2 gives the design space (in yellow) with a flag which corresponds to the parameters with which the validation batch is taken. The results of the batch along with the confidence intervals are specified in Supporting table S10. Validation batch results shown for a selected batch in the design space as generated by the DoE demonstrate that the response falls well within the range.
The model validation was successfully carried out and the design space was thus optimized. Figure 2 demonstrates that when the homogenization pressure is kept at a constant value of 15,000 psi coupled with varying the drug:surfactant ratio and the number of cycles such that they fall within the range highlighted by the yellow patch, the particle size will fall between 300 and 500 nm. By using this approach, we can reduce the highly variable impact of the listed CPPs on the particle size (Supporting table S9). Thus, the factors which were high risk in the risk assessment matrix, i.e., homogenization pressure, number of cycles, and D:S ratio, were optimized to a set range and converted to low risk as summarized in Supporting table S11.
Optimization of GG concentration for in situ gelling
Viscosity determination
Anton Paar rheometer was used to determine the viscosity of 0.2, 0.4, and 0.6% w/v GG solutions alone and when in contact with artificial tear fluid. Figure 3 gives viscosity measurements of the prepared nanosuspension with viscosity represented on the X axis, shear rate on the Y axis, and shear stress on the Z axis. The rate at which the probe rotates was termed as shear rate and the stress applied by the rotating probe on the GG solution was termed as shear stress. As seen in Fig. 3a and c, 0.4% w/v GG solution shows higher viscosity compared to 0.2% GG solution. Also, GG solution when in contact with ATF (Fig. 3b, d, f) demonstrates higher viscosity when compared to GG solution in the absence of ATF (Fig. 3a, c, e). As a general trend, the viscosity was observed to decrease as the shear rate increased. Also, the shear stress applied on the substance by the probe increases as the shear rate increases. This corroborates the fact that cross-linking of GG occurs when it comes in contact with sodium and calcium ions present in the ATF which leads to increased viscosity in the presence of ATF [75]. Also, viscosity increases as the concentration of GG increases. This phenomenon would lead to cross-linking and gelling of GG when in contact with natural tear film, which would give rise to the enhanced retention time of the formulated eye drops when instilled.
Mucoadhesion testing
Texture analyzer was used to measure the mucoadhesion of the prepared formulations (with 0.2, 0.4, and 0.6% w/v solutions of GG) with freshly excised goat lens mucosa as the membrane. Numerous studies have utilized excised goat cornea to test the mucoadhesion of the prepared nanoformulation [46, 54, 76, 77]. Mucoadhesion is measured in terms of the peak force (N) required by the probe to detach the formulation from the mucous membrane and the work of adhesion, i.e., the inter-particulate force of adhesion between the particles of the formulation.
As seen from Supporting table S12 and Supporting figure S6, peak force (N) and the work of adhesion (N.s) were 0.00866 N and 0.14 N.s for the 0.4% w/v GG ATF which therefore showed the maximum mucoadhesion. As per the viscosity results, 0.6% w/v GG ATF had maximum viscosity, i.e., it forms a more viscous system when in contact with the tear fluid. Although, when we studied the ease with which the solutions of various concentrations of GG were dispensed by an eye dropper bottle, we found that plain 0.6% w/v GG alone formed a very viscous system which restricted its smooth flow from the eye dropper bottle. Hence, 0.4% w/v was chosen as the optimized concentration of GG for in situ gelling. Higher mucoadhesion gives rise to enhanced retention of the formulation and more contact time with the cornea. Similarly, an optimum value of the mucoadhesion is a balance between its ease to come out of the dropper bottle without any resistance to flow and enhancement in the contact time.
Ocular irritation studies (hen’s egg test–chorioallantoic membrane—HET-CAM)
HET-CAM is a widely used test to determine the irritancy potential of the developed formulations or test substances. Many studies have reported its use in studying the eye irritancy potential of pharmaceutical formulations [78,79,80]. HET-CAM was performed in order to study the ocular irritation of the prepared formulations which is dependent on the active as well as the excipients used at their respective concentrations. The potential irritancy score, which gives the intensity of irritation caused by the test substance, was calculated using the time required for the endpoints (hemorrhage, vascular lysis, and protein coagulation) to develop on the CAM surface based on the equation stated earlier. A PI score of 0–0.9 is deemed to be non-irritant; a score of 1–4.9 is termed to be slightly irritant; a score of 5–8.9 causes moderate irritation; and a score of 9–12 causes severe irritation [81]. Table 4 gives the scores for the respective groups and the inferences based on them.
As seen in Table 3, it is evident from the PI score that the positive control shows severe irritation, and the negative control shows no irritation indicating that the CAM surface is responsive to the tested substances and that the assay is reliable. Also, Posaconazole nanosuspension is found to be non-irritant with a PI score of 0.85. This study gives us a proof of concept that the developed formulation would be well tolerated by the eye at the respective quantities of the active and excipients used.
In vitro drug release
In vitro drug release was studied for the developed nanosuspension with and without the surfactant Tween 20 in the external media (simulated artificial tear fluid). Tween 20 was added in order to facilitate the release and dissolution of Posaconazole which is a BCS class II drug [51]. Various other studies have reported the use of Tweens as surfactants to facilitate the in vitro release and dissolution of BCS class II dugs [82,83,84]. Figure 5a, b denote the % cumulative release v/s. time for both the suspension and the in situ gelling nanosuspension with and without Tween 20. Herein, we wanted to compare the in vitro drug release from the prepared Posaconazole in situ gelling nanosuspension against the Posaconazole coarse suspension as the control. The aim was to obtain a discriminating release pattern to differentiate the release profiles of both the formulations being tested. Our second aim was to determine whether Posaconazole formulated as an in situ gelling nanosuspension (nanosized Posaconazole) increases its release and dissolution as compared to its coarse (non-size reduced Posaconazole) counterpart. Ideally, particle size reduction is known to increase the dissolution and thus the bioavailability of the drug by increasing its surface area available for solvation [85].
As seen in Fig. 4a, it is evident that in the absence of the surfactant, the drug release is comparatively less (~ 2% for the coarse suspension and ~ 10% for the in situ gelling nanosuspension); however, a discriminating release pattern is observed between the coarse suspension and the in situ gelling nanosuspension. From Fig. 4b, it can be concluded that with the addition of a surfactant, the release is enhanced but the release pattern is not discriminating (~ 10% for the coarse suspension and ~ 25% for the in situ gelling nanosuspension). The reason being that the surfactant increases the dissolution of the drug from both the coarse suspension and the in situ gelling nanosuspension at a similar rate. It should also be noted that we observed a tenfold and a threefold increase in the dissolution of Posaconazole in both the scenarios, i.e., with and without the addition of Tween 20 in the external media respectively, by the in situ gelling nanosuspension as compared to the coarse suspension. This observation further corroborates the finding that nanosizing aids in increasing the dissolution of a poorly soluble BCS class II drug.
Ex vivo corneal retention study
Franz diffusion cell assembly was used to determine the localization of Posaconazole in various chambers of the diffusion cell. This was done to simulate in vivo conditions to study whether Posaconazole would be retained in the corneal tissue, above the tissue, or in the receptor chamber (penetrates from the tissue into the receptor chamber). Our major aim was to determine the corneal retention of Posaconazole to serve as a drug depot and hence provide sustained effect in conditions like fungal keratitis. To our advantage, albumin is one of the major components of the corneal tissue and Posaconazole predominantly binds to albumin [86, 87]. This essentially implies that Posaconazole would preferentially bind to the cornea. Firstly, retention of Posaconazole above the cornea would imply that under in vivo conditions, it would be cleared off via tear drainage. This would ultimately result in the loss of the drug. Secondly, if Posaconazole was found to traverse the cornea (isolated goat cornea in this case) and reach the receptor chamber, it would possibly mimic the same route in in vivo conditions and be localized in the posterior chamber of the eye. In the third scenario, Posaconazole could be localized in the cornea. We aimed at achieving this condition, wherein, maximum localization of Posaconazole in the corneal tissue would give efficacious corneal clearing in fungal keratitis in both prophylactic and treatment conditions. The percentage amount of drug permeated in the receptor chamber, in the tissue, and above the tissue was calculated and a graph was plotted denoting the % distribution of the drug. Figure 5 denotes the retention of the drug in the coarse suspension and the in situ gelling nanosuspension. The majority of the drug from the nanosuspension (~ 70%) is retained in the tissue followed by retention of drug above the tissue (~ 20%) and a minimum amount of the drug permeated in the receptor chamber (< 10%). The imperative function of the prepared formulation is prophylaxis and treatment of fungal keratitis. In order to serve this purpose, the prepared formulation should deposit the majority of the drug in the cornea so as to inhibit the fungus from penetrating it. Deposition of the drug above the cornea would also aid the intended function; however, due to the high clearance rate of the cornea, establishing an enhanced drug concentration would be a problem. Also, permeation of the drug in the receptor chamber would imply permeation inside the cornea into the posterior chamber in vivo. This would also not serve the desired property of the prepared formulation acting as a prophylactic aid.
Antifungal assay
Antifungal assay was carried out using the optimized formulation and the activity was compared with the available marketed formulation. The agar cup diffusion method was used to find the antifungal efficacy of the prepared formulation. This was done by measuring the zone of inhibition (ZOI) of the Posaconazole nanosuspension against Candida albicans. Studies have reported the use of the agar cup diffusion method to determine the antifungal efficacy by the zone of inhibition method [88,89,90]. The antifungal efficacy of the formulation was calculated in terms of the ZOI (mm).
0.9% NaCl and placebo were used as controls along with a widely used marketed ophthalmic nanosuspension of itraconazole (0.5% w/v). We compared our Posaconazole nanosuspension at the same concentration as the marketed itraconazole preparation, i.e., 0.5% w/v. The ZOI is a circular area surrounding the cup, in which when the antifungal is instilled, the fungi do not grow. Hence, the higher the ZOI higher is the susceptibility of the fungi to the antifungal formulation [91]. As seen in Table 4, the ZOI of the Posaconazole nanosuspension was greater as compared to that of the marketed itraconazole nanosuspension. It can be deduced that the prepared Posaconazole nanosuspension has a greater efficacy against C. albicans as compared to that of the marketed antifungal nanosuspension available for ocular use.
Stability of the nanosuspension
We further performed stability studies on the developed Posaconazole in situ gelling nanosuspension. We checked for parameters like appearance, drug content, particle size, and pH of the nanosuspension for a period of 3 months on storage at the mentioned conditions. Table 5 represents the stability data for 1 month, 2 months, and 3 months of the Posaconazole nanosuspension which was kept at room conditions (30 °C/65% RH) and at accelerated conditions (40 ºC/75% RH) of temperature and humidity. The nanosuspension was observed to be stable for a period of 3 months at both room and accelerated stability conditions.
Conclusion
A stable in situ gelling nanosuspension of Posaconazole was developed using the QbD approach with microfluidization as the size reduction mechanism. The in situ gelling nanosuspension was tested for its efficacy and ocular tolerability. A robust analytical method for the routine analysis of Posaconazole was developed and validated. The in situ gelling polymer used was GG which uses ion-induced gelling as the gelation mechanism as evident in the changes in viscosity. Ocular irritation studies were carried out using the HET-CAM method in order to ascertain the ophthalmic tolerability of the prepared formulations. Ex vivo ocular retention study confirmed the localization of Posaconazole in the corneal tissue for up to 24 h. The prepared formulation was tested for stability as per ICH guidelines for short-term, long-term, and accelerated stability at 30 °C/65% RH and 40 °C/75% RH conditions.
The future scope with respect to Posaconazole as an API in treating fungal infections is very strong considering the potency of the moiety. The challenge lies in the formulation of this active being a BCS class II molecule; various oily type formulations could be explored for different fungal conditions. Also, intraocular formulations could be studied to treat fungal infections manifesting in the posterior chamber of the eye.
Availability of data and materials
All data generated or analyzed during this study are reported in this article (and its Supplementary information files) and the datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ATF:
-
Artificial tear fluid
- GG:
-
Gellan gum
- PF 127:
-
Pluronic F 127
- HPLC:
-
High-pressure liquid chromatography
- HET-CAM:
-
Hens egg test–chorioallantoic membrane test
- BCS:
-
Biopharmaceutical classification system
- PI:
-
Potential irritancy score
- API:
-
Active pharmaceutical ingredient
- RH:
-
Relative humidity
- ICH:
-
International Conference on Harmonization
- QbD:
-
Quality by Design
- DoE:
-
Design of experiments
- ZOI:
-
Zone of inhibition
- CQA:
-
Critical quality attributes
- CMA:
-
Critical material attributes
- CPP:
-
Critical process parameters
- BBD:
-
Box-Behnken design
- MIC:
-
Minimum inhibitory concentration
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantitation
- AUC:
-
Area under the curve
- IST:
-
Isothermal stress testing
- hERG:
-
Human ether-a-go-go-related gene
- PDI:
-
Polydispersity index
References
Ansari Z, Miller D, Galor A. Current thoughts in fungal keratitis: diagnosis and treatment. 2013. p. 209–18.
Thomas PA. Fungal infections of the cornea. 2003. p. 852–62.
Rosa RH, Miller D, Alfonso EC. The changing spectrum of fungal keratitis in South Florida. 1994. p. 1005–13.
Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. 2005. p. 556–69.
Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Elsevier Inc.; 2021. p. 264.e5-.e8.
Maini A, Tomar G, Khanna D, Kini Y, Mehta H, Bhagyasree V, et al. Sino-orbital mucormycosis in a COVID-19 patient: a case report. Elsevier Ltd; 2021. p. 105957.
Matsuo H, Tomita G, Araie M, Suzuki Y, Kaji Y, Obata H, Tanaka S. Histopathological findings in filtering blebs with recurrent blebitis. British journal of ophthalmology. 2002 Jul 1;86(7):827.
Tu EY, McCartney DL, Beatty RF, Springer KL, Levy J, Edward D, et al. Successful treatment of resistant ocular fusariosis with Posaconazole (SCH-56592). 2007.
Hof H. A new, broad-spectrum azole antifungal: Posaconazole - mechanisms of action and resistance, spectrum of activity. 2006. p. 2–6.
Fortunato P, Pillozzi S, Tamburini A, Pollazzi L, Franchi A, La Torre A, et al. Irresponsiveness of two retinoblastoma cases to conservative therapy correlates with up-regulation of hERG1 channels and of the VEGF-A pathway. 2010.
Kontoyiannis DP, Lewis RE. Posaconazole prophylaxis in hematologic cancer. N Eng J Med. 2007;356(21):2214-8.
Mattiuzzi G, Yilmaz M, Kantarjian H, Borthakur G, Konopleva M, Jabbour E, et al. Pharmacokinetics of Posaconazole prophylaxis of patients with acute myeloid leukemia. J Infect Chemother. 2015;21(9):663–7.
Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007(356):348–59.
Robenshtok E, Gafter-Gvili A, Goldberg E, Weinberger M, Yeshurun M, Leibovici L, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. 2007. p. 5471–89.
Sutradhar KB, Khatun S, Luna IP. Increasing possibilities of nanosuspension. 2013.
Kayser O, Olbrich C, Yardley V, Kiderlen AF, Croft SL. Formulation of amphotericin B as nanosuspension for oral administration. 2003. p. 73–5.
Trotta M, Gallarate M, Carlotti ME, Morel S. Preparation of griseofulvin nanoparticles from water-dilutable microemulsions. 2003. p. 235–42.
Gupta H, Jain S, Mathur R, Mishra P, Mishra AK, Velpandian T, et al. Sustained ocular drug delivery from a temperature and pH triggered novel in situ gel system. 2007. p. 507–15.
Cao Y, Zhang C, Shen W, Cheng Z, Yu LL, Ping Q, et al. Poly(N-isopropylacrylamide)-chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. 2007. p. 186–94.
Gupta S, Samanta MK, Raichur AM. Dual-drug delivery system based on in situ gel-forming nanosuspension of forskolin to enhance antiglaucoma efficacy. 2010. p. 322–35.
Rokade M. In situ gel- sustained nasal drug delivery 2015. p. 4958–66.
Moghimipour E, Salimi A, Yousefvand T. Preparation and evaluation of celecoxib nanoemulsion for ocular drug delivery. 2017. p. S543-S50.
Nitta Y, Nishinari K. Gelation and gel properties of polysaccharides gellan gum and tamarind xyloglucan. 2005.
Vilela J, Cunha R. High acyl gellan as an emulsion stabilizer. 2015.
Mahdi MH, Conway BR, Mills T, Smith AM. Gellan gum fluid gels for topical administration of diclofenac. Elsevier B.V.; 2016. p. 535–42.
Novac O, Lisa G, Profire L, Tuchilus C, Popa MI. Antibacterial quaternized gellan gum based particles for controlled release of ciprofloxacin with potential dermal applications. 2014. p. 291–9.
Morsi N, Ibrahim M, Refai H, El Sorogy H. Nanoemulsion-based electrolyte triggered in situ gel for ocular delivery of acetazolamide. Elsevier; 2017. p. 302–14.
Ter Horst B, Moakes RJA, Chouhan G, Williams RL, Moiemen NS, Grover LM, et al. A gellan-based fluid gel carrier to enhance topical spray delivery. Acta Materialia Inc.; 2019. p. 166–79.
Janga KY, Tatke A, Dudhipala N, Balguri SP, Ibrahim MM, Maria DN, et al. Gellan gum based sol-to-gel transforming system of natamycin transfersomes improves topical ocular delivery. 2019. p. 814–22.
Tseng RC, Chen CC, Hsu SM, Chuang HS. Contact-lens biosensors. 2018.
Kelco CP. US I. KELCOGEL® gellan gum. 2007. p. 30.
Rupenthal I, Green C, Alany R. Comparison of ion-activated in situ gelling systems for ocular drug delivery. Part 1: Physicochemical characterisation and in vitro release. 2011. p. 69–77.
Mandal S, Thimmasetty M, Prabhushankar G, Geetha M. Formulation and evaluation of an in situ gel-forming ophthalmic formulation of moxifloxacin hydrochloride. 2012. p. 78–82.
Meng Y-C, Hong L, Jin J. A study on the gelation properties and rheological behavior of gellan gum. 2013. p. 20–4.
Kotreka U, Davis V, Adeyeye M. Development of topical ophthalmic in situ gel-forming estradiol delivery system intended for the prevention of age-related cataracts. 2017. p. e0172306.
Garcia CV, Costa GR, Mendez ASL. Stability-indicating HPLC method for Posaconazole bulk assay. 2012. p. 317–27.
Research USFaDACfBEa. Guidance for industry Q3B(R2) impurities in new drug products. 2006. p. 1–8.
FDA. Validation of analytical procedures: text and methodology Q2(R1). 2005.
Abbott PS. Solubility. Available from: https://www.stevenabbott.co.uk/practical-surfactants/solubility.php. Accessed 22 Aug 2018.
Aghrbi I, Fülöp V, Jakab G, Kállai-Szabó N, Balogh E, Antal I. Nanosuspension with improved saturated solubility and dissolution rate of cilostazol and effect of solidification on stability. J Drug Delivery Sci Technol 2021;61.
Patel V, Agrawal Y. Nanosuspension: an approach to enhance solubility of drugs. 2011. p. 81–7.
Lachman L, Lieberman HA, Kanig JL. Theory and practice of industrial pharmacy.pdf. 1991.
Kwok DY, Neumann AW. Contact angle measurement and contact angle interpretation. 1999. p. 167–249.
Fule R, Amin P. Hot melt extruded amorphous solid dispersion of Posaconazole with improved bioavailability: investigating drug-polymer miscibility with advanced characterisation. 2014.
Ghorbani Gorji S, Ghorbani Gorji E, Mohammadifar MA, Zargaraan A. Complexation of sodium caseinate with gum tragacanth: effect of various species and rheology of coacervates. Elsevier B.V.; 2014. p. 503–11.
Akhter S, Anwar M, Siddiqui MA, Ahmad I, Ahmad J, Ahmad MZ, et al. Improving the topical ocular pharmacokinetics of an immunosuppressant agent with mucoadhesive nanoemulsions: formulation development, in-vitro and in-vivo studies. Elsevier B.V.; 2016. p. 19–29.
Gilleron L, Coecke S, Sysmans M, Hansen E, Van Oproy S, Marzin D, et al. Evaluation of a modified HET-CAM assay as a screening test for eye irritancy. 1996. p. 431–46.
Steiling W, Bracher M, Courtellemont P, De Silva O. The HET-CAM, a useful in vitro assay for assessing the eye irritation properties of cosmetic formulations and ingredients. 1999. p. 375–84.
Shazly G, Nawroth T, Langguth P. Comparison of dialysis and dispersion methods for in vitro release determination of drugs from multilamellar liposomes. 2008. p. 7–10.
Phan CM, Bajgrowicz-Cieslak M, Subbaraman LN, Jones L. Release of moxifloxacin from contact lenses using an in vitro eye model: impact of artificial tear fluid composition and mechanical rubbing. 2016. p. 1–10.
Bhakay A, Rahman M, Dave RN, Bilgili E. Bioavailability enhancement of poorly water-soluble drugs via nanocomposites: formulation–processing aspects and challenges. 2018.
Dave V, Paliwal S, Yadav S, Sharma S. Effect of in vitro transcorneal approach of aceclofenac eye drops through excised goat, sheep, and buffalo corneas. 2015.
Aslam M, Imam SS, Aqil M, Sultana Y, Ali A. Levofloxacin loaded gelrite-cellulose polymer based sustained ocular drug delivery: formulation, optimization and biological study. J Polym Eng. 2016;36(8):761–9.
Gade S, Patel KK, Gupta C, Anjum MM, Deepika D, Agrawal AK, et al. An ex vivo evaluation of moxifloxacin nanostructured lipid carrier enriched in situ gel for transcorneal permeation on goat cornea. J Pharm Sci. 2019;108(9):2905–16.
Patidar N, Rathore MS, Sharma DK, Middha A, Gupta VB. Transcorneal permeation of ciprofloxacin and diclofenac from marketed eye drops. Indian J Pharm Sci. 2008;70(5):651.
Balouiri M, Sadiki M, Saad I. Methods for in vitro evaluating antimicrobial activity: A review. 2015.
Chandrasekaran M, Venkatesalu V. Antibacterial and antifungal activity of Syzygium jambolanum seeds. 2004. p. 105–8.
Kaur P, Thakur R, Choudhary A. An in vitro study of the antifungal activity of silver/chitosan nanoformulations against important seed borne pathogens. 2012. p. 83–6.
FDA. Guidance for Industry: Q1A(R2) Stability testing of new drug substances and products, U.S. Department of Health and Human Services, Food and Drug Administration. 2003. p. 1–22.
Cendejas-Bueno E, Forastiero A, Rodriguez-Tudela JL, Cuenca-Estrella M, Gomez-Lopez A. HPLC/UV or bioassay: two valid methods for posaconazole quantification in human serum samples. Clin Microbiol Infect. 2012;18(12):1229–35.
Tang P. Determination of Posaconazole in plasma/serum by high-performance liquid chromatography with fluorescence detection. Separations. 2017;4(2).
Research CfDEaR, Center for Biologics Evaluation and. Guidance for industry Q2B validation of analytical procedures: Methodology. 1996. p. 301–827.
Holst-Jensen A. Sampling, detection, identification and quantification of genetically modified organisms (GMOs). 2007. p. 231–68.
Sackett D. The rational clinical examination. A primer on the precision and accuracy of the clinical examination. 1992. p. 2638–44.
Heyden Y, Nijhuis A, Smeyers-Verbeke J, Vandeginste BGM, Massart D. Guidance for robustness/ruggedness tests in method validation. 2001. p. 723–53.
FDA U. Inactive ingredient search for approved drug products U.S. Department of Health and Human Services 2022. Available from: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm. Accessed 15 Dec 2018.
Dwivedi C, Pandey I, Pandey H, Ramteke P, Pandey A, Mishra DSB, et al. Electrospun nanofibrous scaffold as a potential carrier of antimicrobial therapeutics for diabetic wound healing and tissue regeneration. 2017. p. 147–64.
Mhatre S, Naik S, Patravale V. Exploring green and industrially scalable Microfluidizer™ technology for development of barium sulphate nanosuspension for enhanced contrasting. J Drug Delivery Sci Technol 2021;64.
Kwon SS, Nam YS, Lee JS, Ku BS, Han SH, Lee JY, Chang IS. Preparation and characterization of coenzyme Q10-loaded PMMA nanoparticles by a new emulsification process based on microfluidization. Colloids Surf A: Physicochem Eng Asp. 2002;210(1):95-104.
Verma S, Gokhale R, Burgess DJ. A comparative study of top-down and bottom-up approaches for the preparation of micro/nanosuspensions. Int J Pharm. 2009;380(1–2):216–22.
Verma S, Lan Y, Gokhale R, Burgess DJ. Quality by design approach to understand the process of nanosuspension preparation. Int J Pharm. 2009;377(1–2):185–98.
Fukuda I, Pinto C, Moreira C, Saviano A, Lourenço F. Design of experiments (DoE) applied to pharmaceutical and analytical quality by design (QbD). 2018.
Holm P, Allesø M, Bryder MC, Holm R. Q8 (R2) Pharmaceutical Development. ICH quality guidelines: an implementation guide. 2017 Sep 27:535–77.
Rawal M, Singh A, Amiji MM. Quality-by-design concepts to improve nanotechnology-based drug development. Pharm Res. 2019;36(11):153.
Vilela JA, Cavallieri ÂL, Da Cunha RL. The influence of gelation rate on the physical properties/structure of salt-induced gels of soy protein isolate–gellan gum. Food Hydrocoll. 2011;25(7):1710-8.
Gratieri T, Gelfuso GM, Rocha EM, Sarmento VH, de Freitas O, Lopez RF. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur J Pharm Biopharm. 2010;75(2):186–93.
Kanoujia J, Kushwaha PS, Saraf SA. Evaluation of gatifloxacin pluronic micelles and development of its formulation for ocular delivery. Drug Deliv Transl Res. 2014;4(4):334–43.
Schrage A, Gamer AO, van Ravenzwaay B, Landsiedel R. Experience with the HET-CAM method in the routine testing of a broad variety of chemicals and formulations. ATLA. 2010;38(1):39-52.
Rajpal Deshmukh G, Hema Kumar K, Suresh Reddy PV, Srinivasa Rao B, Venkata Satish Kumar C. Experience with the HET-CAM method in the routine testing of a broad variety of chemicals and formulations. ATLA. 2010 Mar;38(1):39-52.
Tavaszi J, Budai P. The use of HET-CAM test in detecting the ocular irritation. Commun Agric Appl Biol Sci. 2007;72:137–41.
Barile FA. Validating and troubleshooting ocular in vitro toxicology tests. 2010. p. 136–45.
Akbari J, Saeedi M, Morteza-Semnani K, Kelidari HR, Sadegh Moghanlou F, Zareh G, et al. The effect of Tween 20, 60, and 80 on dissolution behavior of sprionolactone in solid dispersions prepared by PEG 6000. Adv Pharm Bull. 2015;5(3):435–41.
Hoppe K, Sznitowska M. The effect of polysorbate 20 on solubility and stability of candesartan cilexetil in dissolution media. AAPS PharmSciTech. 2014;15(5):1116–25.
Raval A, Bahadur P, Raval A. Effect of nonionic surfactants in release media on accelerated in-vitro release profile of sirolimus eluting stents with biodegradable polymeric coating. J Pharm Anal. 2018;8(1):45–54.
Khadka P, Ro J, Kim H, Kim I, Kim JT, Kim H, et al. Pharmaceutical particle technologies: an approach to improve drug solubility, dissolution and bioavailability. Asian J Pharm Sci. 2014;9(6):304–16.
Nees DW, Fariss RN, Piatigorsky J. Serum albumin in mammalian cornea: implications for clinical application. Invest Ophthalmol Vis Sci. 2003;44(8):3339–45.
Yanjun Li UT, Cornelius J. Clancy, M. Hong Nguyen and Hartmut Derendorf. Pharmacokinetic/pharmacodynamic profile of Posaconazole. Clinical Pharmacokinetics. 2010;49:379–96.
Bhalodia NR, Shukla VJ. Antibacterial and antifungal activities from leaf extracts of Cassia fistula l.: an ethnomedicinal plant. J Adv Pharm Technol Res. 2011;2(2):104–9.
Desai D, Kukreja G, Dhundale V, Rokade S, Hajare S. Identification of potential antifungal metabolite producing Pseudomonas tolaasii strain GD76 obtained from contaminated agar plate. Res J Microbiol. 2017;13:21–7.
Peano A, Pasquetti M, Tizzani P, Chiavassa E, Guillot J, Johnson E. Methodological issues in antifungal susceptibility testing of Malassezia pachydermatis. J Fungi (Basel). 2017;3(3).
Bhargav HS, Shastri SD, Poornav SP, Darshan KM, Nayak MM, editors. Measurement of the zone of inhibition of an antibiotic. 2016 IEEE 6th International Conference on Advanced Computing (IACC); 2016.
Acknowledgements
The authors would like to sincerely thank MicrofluidicsTM for providing the facility to perform microfluidization on Microfluidics M-110P. The authors would also like to thank Prof. S. S. Bhagwat, Department of Chemical Engineering, ICT for providing the facility to use the G10 KRUSS system for contact angle measurement.
Author information
Authors and Affiliations
Contributions
Purva Khare: methodology; validation; investigation; writing—original draft; visualization. Manasi M. Chogale: methodology, formal analysis, supervision. Pratik Kakade: methodology, supervision. Vandana B. Patravale: conceptualization, resources, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experiments reported herein did not involve any human or animal subjects. The experiments performed herein comply with the current laws of the country.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khare, P., Chogale, M.M., Kakade, P. et al. Gellan gum–based in situ gelling ophthalmic nanosuspension of Posaconazole. Drug Deliv. and Transl. Res. 12, 2920–2935 (2022). https://doi.org/10.1007/s13346-022-01155-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01155-0