Abstract
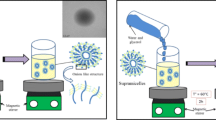
Two pegylated lipid nanocapsules for triamcinolone transdermal delivery were designed. Both present a size close to 50 nm and a single monomodal distribution in particle size (PI < 0.2), with a zeta potential of − 20 ± 2 and + 18 ± 1, respectively. The triamcinolone encapsulation efficacy varied between 68 and 80%. They proved to be stable under storage conditions (4 °C) for at least 6 months and at a physiological temperature, using different media, for 48 h. Also, they were shown not to affect cell viability at the concentrations used. For ex vivo transdermal experiments, newborn pig skin was used. With respect to the triamcinolone transdermal penetration, the nanocapsules were demonstrated to have an absorption promoting effect, both when the drug nanocapsules were in solution or loaded into the hydrogel, quantifying between 2 and 15 times more absorbed drug than the control. In addition, regarding the triamcinolone retained in the skin, it is observed that lipid nanocapsules act as triamcinolone promoters when the nanosystems were in solution and when they were included in the hydrogel. This vehicle showed a greater triamcinolone reservoir effect in comparison to the nanocapsules, proving to be a good vehicle to formulate triamcinolone transdermal delivery.
Graphical abstract






Similar content being viewed by others
Availability of data and materials
Not applicable.
References
Cunha S, Amaral MH, Lobo JMS, et al. Lipid nanoparticles for nasal/intranasal drug delivery [Review]. Crit Rev Ther Drug Carrier Syst. 2017;34(3):257–82.
Ickenstein LM, Garidel P. Lipid-based nanoparticle formulations for small molecules and RNA drugs. Expert Opin Drug Deliv. 2019;16(11):1205–1226.
Qin Z, Chen F, Chen D, et al. Transdermal permeability of triamcinolone acetonide lipid nanoparticles. Int J Nanomedicine. 2019;14:2485–95.
Formica ML, Legeay S, Bejaud J, et al. Novel hybrid lipid nanocapsules loaded with a therapeutic monoclonal antibody - bevacizumab - and triamcinolone acetonide for combined therapy in neovascular ocular pathologies. Mater Sci Eng C Mater Biol Appl. 2021;119(111398):22.
Chen J, Wei N, Lopez-Garcia M, et al. Development and evaluation of resveratrol, vitamin E, and epigallocatechin gallate loaded lipid nanoparticles for skin care applications. Eur J Pharm Biopharm. 2017;117:286–291.
Rabiei M, Kashanian S, Samavati SS, et al. Nanomaterial and advanced technologies in transdermal drug delivery. J Drug Target. 2020;28(4):356–367.
Beloqui A, Solinís M, Rodríguez-Gascón A, et al. Nanostructured lipid carriers: promising drug delivery systems for future clinics. Nanomedicine. 2016;12(1):143–61.
Takeuchi I, Shimamura Y, Kakami Y, et al. Transdermal delivery of 40-nm silk fibroin nanoparticles. Colloids Surf B Biointerfaces. 2019;175:564–568.
Formica ML, Ullio Gamboa GV, Tártara LI, et al. Triamcinolone acetonide-loaded lipid nanocapsules for ophthalmic applications. Int J Pharm. 2020;573(118795):1.
Vitorino C, Almeida A, Sousa J, et al. Passive and active strategies for transdermal delivery using co-encapsulating nanostructured lipid carriers: in vitro vs. in vivo studies. Eur J Pharm Biopharm. 2014;86(2):133–144.
Albash R, El-Nabarawi MA, Refai H, et al. Tailoring of PEGylated bilosomes for promoting the transdermal delivery of olmesartan medoxomil: in-vitro characterization, ex-vivo permeation and in-vivo assessment. Int J Nanomedicine. 2019;14:6555–74.
Rangsimawong W, Opanasopit P, Rojanarata T, et al. Terpene-containing PEGylated liposomes as transdermal carriers of a hydrophilic compound [Research Support, Non-U S Gov’t]. Biol Pharm Bull. 2014;37(12):1936–43.
Fantini A, Padula C, Nicoli S, et al. The role of vehicle metamorphosis on triamcinolone acetonide delivery to the skin from microemulsions. Int J Pharm. 2019;565:33–40.
Ishii H, Todo H, Sugibayashi K. Effect of sebum and ointment rubbing on the skin permeation of triamcinolone acetonide from white petrolatum ointment. Biol Pharm Bull. 2010;33(5):876–80.
Pradhan M, Singh D, Singh MR. Fabrication, optimization and characterization of triamcinolone acetonide loaded nanostructured lipid carriers for topical treatment of psoriasis: application of Box Behnken design, in vitro and ex vivo studies. J Drug Deliv Sci Technol. 2017;41:325–333.
Yang JH, Kim DK, Yun MY, et al. Transdermal delivery system of triamcinolone acetonide from a gel using phonophoresis [Comparative Study Research Support, Non-U S Gov’t]. Arch Pharm Res. 2006;29(5):412–7.
Liu W, Hu M, Liu W, et al. Investigation of the carbopol gel of solid lipid nanoparticles for the transdermal iontophoretic delivery of triamcinolone acetonide acetate. Int J Pharm. 2008;364(1):135–141.
Heurtault B, Saulnier P, Pech B, et al. A novel phase inversion-based process for the preparation of lipid nanocarriers. Pharm Res. 2002;19(6):875–880.
Hassan PA, Rana S, Verma G. Making sense of brownian motion: colloid characterization by dynamic light scattering. Langmuir. 2015;31(1):3–12.
Alvarez-Figueroa MJ, Abarca-Riquelme JM, González-Aramundiz JV. Influence of protamine shell on nanoemulsions as a carrier for cyclosporine-a skin delivery. Pharm Dev Technol. 2019;24(5):630–638.
Bussio JI, Molina-Perea C, González-Aramundiz JV. Lower-sized chitosan nanocapsules for transcutaneous antigen delivery. Nanomaterials (Basel). 2018;8(9):659.
Bhattacharjee S. DLS and zeta potential – what they are and what they are not? J Control Release. 2016;235:337–351.
Lee MH, Shin GH, Park HJ. Solid lipid nanoparticles loaded thermoresponsive pluronic–xanthan gum hydrogel as a transdermal delivery system. J Appl Polym Sci. 2018;135(11):46004.
Eiras F, Amaral MH, Silva R, et al. Characterization and biocompatibility evaluation of cutaneous formulations containing lipid nanoparticles. Int J Pharm. 2017;519(1):373–380.
Tichota DM, Silva AC, Sousa Lobo JM, et al. Design, characterization, and clinical evaluation of argan oil nanostructured lipid carriers to improve skin hydration. Int J Nanomedicine. 2014;9:3855–64.
Rampersad SN. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors (Basel, Switzerland). 2012;12(9):12347–60.
Alvarez-Figueroa MJ, Muggli-Galaz C, González PM. Effect of the aggregation state of bile salts on their transdermal absorption enhancing properties. J Drug Deliv Sci Technol. 2019;54:101333.
Abd E, Yousef SA, Pastore MN, et al. Skin models for the testing of transdermal drugs. Clin Pharmacol. 2016;8:163–76.
Shaker DS, Ishak RAH, Elhuoni MA, et al. Boosting transdermal delivery of atorvastatin calcium via o/w nanoemulsifying system: two-step optimization, ex vivo and in vivo evaluation. Int J Pharm. 2020;578:119073.
Alvarez-Figueroa MJ, Narváez-Araya D, Armijo-Escalona N, et al. Design of chitosan nanocapsules with Compritol 888 ATO® for imiquimod transdermal administration. Evaluation of their skin absorption by Raman microscopy. Pharm Res. 2020;37(10):195.
Sacha M, Faucon L, Hamon E, et al. Ex vivo transdermal absorption of a liposome formulation of diclofenac. Biomedicine & Pharmacotherapy. 2019;111:785–790.
Karabasz A, Szczepanowicz K, Cierniak A, et al. In vivo studies on pharmacokinetics, toxicity and immunogenicity of polyelectrolyte nanocapsules functionalized with two different polymers: poly-l-glutamic acid or PEG. Int J Nanomedicine. 2019;14:9587–602.
Ourique AF, Melero A, de Bona da Silva C, et al. Improved photostability and reduced skin permeation of tretinoin: development of a semisolid nanomedicine. Eur J Pharm Biopharm. 2011;79(1):95–101.
Wang W, Wat E, Hui PC, et al. Dual-functional transdermal drug delivery system with controllable drug loading based on thermosensitive poloxamer hydrogel for atopic dermatitis treatment [Research Support, Non-U S Gov't]. Sci Rep. 2016;6(24112).
Koroleva M, Nagovitsina T, Yurtov E. Nanoemulsions stabilized by non-ionic surfactants: stability and degradation mechanisms. Phys Chem Chem Phys. 2018;20(15):10369–77.
Wang Y, Zheng Y, Zhang L, et al. Stability of nanosuspensions in drug delivery. J Control Release. 2013;172(3):1126–1141.
Clogston JD, Patri AK. Zeta Potential measurement. In: McNeil SE, editor. Characterization of nanoparticles intended for drug delivery. Totowa, NJ: Humana Press; 2011. p. 63–70.
Nandini PT, Doijad RC, Shivakumar HN, et al. Formulation and evaluation of gemcitabine-loaded solid lipid nanoparticles. Drug Delivery. 2015;22(5):647–651.
Lollo G, Vincent M, Ullio-Gamboa G, et al. Development of multifunctional lipid nanocapsules for the co-delivery of paclitaxel and CpG-ODN in the treatment of glioblastoma. Int J Pharm. 2015;495(2):972–980.
Jokerst JV, Lobovkina T, Zare RN, et al. Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond). 2011;6(4):715–28.
Ita KB. Prodrugs for transdermal drug delivery – trends and challenges. J Drug Target. 2016;24(8):671–678.
Heurtault B, Saulnier P, Pech B, et al. Interfacial stability of lipid nanocapsules. Colloids Surf B Biointerfaces. 2003;30(3):225–235.
Huynh NT, Passirani C, Saulnier P, et al. Lipid nanocapsules: a new platform for nanomedicine. Int J Pharm. 2009;379(2):201–209.
Tatke A, Dudhipala N, Janga KY, et al. In situ gel of triamcinolone acetonide-loaded solid lipid nanoparticles for improved topical ocular delivery: tear kinetics and ocular disposition studies. Nanomaterials. 2018;9(1).
Łuczyński J, Frąckowiak R, Włoch A, et al. Gemini ester quat surfactants and their biological activity. Cell Mol Biol Lett. 2013;18(1):89–101.
Elnaggar YSR, El-Refaie WM, El-Massik MA, et al. Lecithin-based nanostructured gels for skin delivery: an update on state of art and recent applications. J Control Release. 2014;180:10–24.
Fiume Z. Final report on the safety assessment of lecithin and hydrogenated lecithin [Review]. Int J Toxicol. 2001;1:21–45.
Pitto-Barry A, Barry NPE. Pluronic® block-copolymers in medicine: from chemical and biological versatility to rationalisation and clinical advance. Polym Chem. 2014;5(10):3291–3297. https://doi.org/10.1039/C4PY00039K.
Yamaguchi K, Mitsui T, Aso Y, et al. Structure–permeability relationship analysis of the permeation barrier properties of the stratum corneum and viable epidermis/dermis of rat skin. J Pharm Sci. 2008;97(10):4391–4403.
Abdel-Mottaleb MMA, Moulari B, Beduneau A, et al. Surface-charge-dependent nanoparticles accumulation in inflamed skin. J Pharm Sci. 2012;101(11):4231–4239.
Sala M, Diab R, Elaissari A, et al. Lipid nanocarriers as skin drug delivery systems: properties, mechanisms of skin interactions and medical applications. Int J Pharm. 2018;535(1):1–17.
Zhai Y, Zhai G. Advances in lipid-based colloid systems as drug carrier for topic delivery. J Control Release. 2014;193:90–99.
Lin H, Xie Q, Huang X, et al. Increased skin permeation efficiency of imperatorin via charged ultradeformable lipid vesicles for transdermal delivery. Int J Nanomedicine. 2018;13:831–42.
Gillet A, Compère P, Lecomte F, et al. Liposome surface charge influence on skin penetration behaviour. Int J Pharm. 2011;411(1):223–231.
Sinico C, Manconi M, Peppi M, et al. Liposomes as carriers for dermal delivery of tretinoin: in vitro evaluation of drug permeation and vesicle-skin interaction [Comparative Study]. J Control Release. 2005;103(1):123–36.
Flaten GE, Palac Z, Engesland A, et al. In vitro skin models as a tool in optimization of drug formulation. Eur J Pharm Sci. 2015;75:10–24.
Haltner-Ukomadu E, Sacha M, Richter A, et al. Hydrogel increases diclofenac skin permeation and absorption. Biopharm Drug Dispos. 2019;40(7):217–24.
Funding
This work was supported by the Fondo Nacional de Desarrollo Científico y Tecnológico de Chile (FONDECYT 1201482 to J.V. González-Aramundiz) and Programa de Equipamiento Científico y Tecnológico (FONDEQUIP EQM160042 and FONDEQUIP EQM130032).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. María Javiera Alvarez-Figueroa, Diego A. Alarcón, and José Vicente González-Aramúndiz performed material preparation, data collection, and analysis. María Javiera Alvarez-Figueroa and José Vicente González-Aramúndiz wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethics and Biosecurity Committee of the Pontificia Universidad Católica de Chile approved the ex vivo animal study protocol.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alvarez-Figueroa, M.J., Alarcón, D.A. & González-Aramúndiz, J.V. Effect of zeta potential of innovative lipid nanocapsules on triamcinolone transdermal delivery. Drug Deliv. and Transl. Res. 12, 2740–2750 (2022). https://doi.org/10.1007/s13346-022-01134-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01134-5